Abstract
Background
Current potential living kidney donor’s assessment includes functional and anatomical evaluation. Scintigraphy is recommended in some cases and some centers include this test in the donor’s protocol. Recent studies advocate for the avoidance of this test as CT or MRI volumetry showed to accurately assess donor’s renal function.
Objective
To summarize scientific evidence on image tests for pre-donation and/or post-nephrectomy renal function evaluation.
Evidence acquisition
This review followed the guidelines set by the European Association of Urology and adhered to PRISMA 2020 recommendations. The protocol was registered in PROSPERO on 10th December 2022 (ID: CRD42022379273).
Evidence synthesis
Twenty-one studies met the inclusion criteria after thorough screening and eligibility assessment. According to QUADAS-2, patient selection and flow/timing domains showed a predominant low risk of bias.
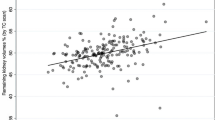
The correlation between split renal function (SRF) using CT and scintigraphy varied from weak (r = 0.21) to remarkably strong (r = 0.949). Bland–Altman agreement demonstrated moderate to excellent results, with mean differences ranging from -0.06% to 1.76%. The correlation between split renal volume (CT) and estimated glomerular filtration rate (eGFR) at 6 months or 1 year after nephrectomy showed a moderate correlation, with coefficients ranging from 0.708 to 0.83.
The correlation between SRF (MRI) and renal scintigraphy reported a moderate correlation, with correlation coefficients of 0.58 and 0.84. MRI and scintigraphy displayed a good agreement, with a 66% agreement observed and mean differences of ± 0.3%.
Conclusions
Despite study heterogeneity, MRI or CT-based renal volumetry appears promising compared to scintigraphy, with favorable correlations and agreement.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Living donor kidney transplantation (LDKT) is the most valuable source of organs for kidney transplantation (KT) worldwide. It has been promoted during the last decades as a crucial strategy to increase the number of organs and try to reach the increasing demand. In addition, LDKT has demonstrated superior outcomes in terms of organ survival, morbidity, and mortality compared to cadaveric donor KT [1]. This could be related to the shorter waiting time (or even the avoidance of dialysis) and the better quality of the organs with reduced ischemia time.
Living kidney donor needs a multidisciplinary evaluation to ensure that neither the donor nor the recipient assume excessive risks with the interventions. Historically, preoperative donor work-up includes renal function (creatinine clearance) and anatomical assessment (CT angiography). However, the SRF (defined as the proportion of total RF contributed by each kidney) should be measured by combining 51Cr EDTA and 99mTc DMSA in concrete occasions, such as when there is a size disparity between the two kidneys, the renal function (RF) is close to the acceptable threshold for the donation, or there is anatomical abnormality or complexity [2]. Moreover, to assess the potential donor’s RF carefully, some centers protocols include performing both imaging tests (CT and scintigraphy). In contrast, recent studies advocate for the avoidance of renal scintigraphy and instead suggest CT or MRI volumetry, as non-invasive, easy-to-perform, resource-saving, and reliable alternatives. Different studies indicate that pre-donation renal volumetry could accurately assess SRF and post-donation kidney function (PDKF). Cohort studies in healthy patients with an average glomerular filtration rate have demonstrated that renal volume is very similar in both kidneys [3,4,5,6]. Despite these promising results, there is still lack of evidence regarding the most appropriate preoperative differential RF assessment for kidney donor candidates.
To fill this gap, we conducted a systematic review of the available scientific evidence pertaining to imaging modalities for pre-donation RF evaluation of potential donors and/or for the PDKF evaluation.
Material and methods
Evidence acquisition
This systematic review was conducted following the principles highlighted by the European Association of Urology guidelines [7] and following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2020 recommendations [8]. The protocol was registered in the International Prospective Register of Ongoing Systematic Reviews (PROSPERO; http://www.crd.york.ac.uk/prospero) on 10th December 2022 (ID:CRD42022379273).
Search strategy
A systematic literature screening was conducted by two authors (ALA and TP) using the Cochrane Library, MEDLINE/Pubmed and Embase databases. The search was limited to articles published in the English language between January 2000 and January 2023. The search strategy incorporated both free-text and MeSH terms (Appendix 1). Furthermore, the reference lists of the initially selected articles were manually scrutinized to identify additional studies of relevance. The final list of selected articles underwent comprehensive review and approval by all co-authors.
Inclusion and exclusion criteria
A specific population (P), intervention (I), comparator (C), outcome (O), and study design (S) (PICOS) framework was assessed to define the study eligibility.
The PICOS framework for this review was as follows:
-
-(P): adult (age > 18 years) kidney donor candidates.
-
- (I): living donor nephrectomy.
-
- (C): comparative studies.
-
- (O): donor’s SRF and/or PDKF measured by eGFR.
-
- (S): prospective or retrospective studies published in English-language between January 2000 and January 2023, reporting pre-donation and/or PDKF comparison between an image test (volumetry calculation) and the current gold standard, scintigraphy.
Study selection and data extraction
Two members of the research team (ALA and TP) independently conducted a systematic search of the previous mentioned databases, following the PICOS framework as listed. The screening of titles and abstracts was performed using Rayyan (Rayyan Systems, Cambridge,MA, USA). Subsequently, the full texts of the remaining studies were examined by all co-authors after the exclusion process.
Data extraction was carried out using a pre-defined spreadsheet developed in advance and managed by two members of the team (ALA and TP). In instances where discrepancies arose regarding study selection or data extraction, resolution was achieved by another author (RC).
The following data were extracted for each study:
-
Study identification: authors, publication year, country.
-
Methods: study design, study period, predicted outcome (evaluate SRF, predict PDKF, eGFR), eGFR formula used, follow-up timing.
-
Participant characteristics: number of patients, number of events (pre-donation RF, PDKF, SRF), inclusion and exclusion criteria.
-
Imaging test characteristics:
-
Imaging test type:CT or MRI
-
CT: technique, contrast medium used, slice thickness, volume calculation
-
Scintigraphy: nuclear GFR tracer
-
-
Statistical analysis data: agreement (between tests, interobserver agreement), correlation, interobserver concordance.
Risk of bias (RoB) assessment
RoB assessment was performed independently by two authors (ALA and TP) using the Quality Assessment tool for Diagnostic Accuracy Studies (QUADAS-2) [9]. QUADAS-2 tool was used to assess the RoB over four domains, patient selection, index test(s), reference standard and flow and timing. The disagreement was solved by a third party (RC).
Data synthesis
The methodological and clinical heterogeneity of the included studies implied that meta-analysis was inappropriate. Therefore, a narrative synthesis of the data was performed. The primary outcomes of interest pertained to the comparison of SRF between the different tests. Additionally, secondary outcomes focused on the evaluation of PDKF.
Evidence synthesis
Literature search and study characteristics
The literature search initially included 695 papers. After screening and eligibility assessment, twenty-one studies met the inclusion criteria (Fig. 1).
Only three of the studies included were prospective design [3, 10, 11]. The samples sizes varied considerably, ranging from 12 patients [12] to 835 patients [13]. Furthermore, the timeframe for the included cohorts varied widely, ranging from 1997–2001 [14] to 2017–2020 [11]. A comprehensive breakdown of the main characteristics of the included studies is provided in Table 1.
Regarding the imaging modalities, three studies reported results from MRI [3, 10, 15] while eighteen from CT. As part of the inclusion criteria, all the studies compare their main outcomes with the gold standard, scintigraphy. Each included study underwent a correlation and agreement analysis between the two tests comparing SRF and/or PDKF. In addition, eight studies evaluate RF at 6 months or 1 year after surgery [12, 13, 16,17,18,19,20,21].
Functional and image tests outcomes
CT vs scintigraphy
SRF outcomes
The SRF correlation between CT and scintigraphy was assessed in 94.44% (17/18) of the studies. The correlation exhibited considerable variability, spanning from studies that reported a weak correlation (r = 0.21 [22]) to those demonstrating a remarkably strong correlation (r = 0.949 [17]). However, when considering the individual kidney comparison, the observed correlations improved significantly, ranging from 0.52 [23] to 0.955 [11].
Bland–Altman agreement was calculated in 72.22% (13/18) of the studies and found to be moderate to excellent, from a mean of −0.06% [24] to 1.76% [13]. Notably, Lal et al. demonstrated a perfect agreement of 0% in the individual comparison between the right and left kidney [11].
PDKF outcomes
PDKF was reported in 22.22% (4/18) of the studies [16, 18, 21, 25] but only one of them reported the predicted postoperative eGFR [23]. Furthermore, the correlation between split renal volume and eGFR at 6 months or 1 year after nephrectomy was assessed in 9 studies [6, 12, 13, 16,17,18,19,20,21]. The results showed a moderated level of correlation, with correlation coefficients ranging from 0.708 [19] to 0.83 [6] and coefficient of determination (R2) values spanning from 0.55 [18] to 0.74 [12]. This level of correlation is similar to that obtained when comparing scintigraphy-derived results to eGFR measurements (r from 0.634 to 0.85; R2 from 0.554 to 0.69).
MRI vs scintigraphy
SRF outcomes
The SRF correlation between MRI and renal scintigraphy was evaluated in 66.67% (2/3) of the comparative studies. The findings indicated a moderate correlation in both studies, with correlation coefficients of 0.58 and 0.84, respectively [10, 15].
Regarding the agreement analysis, different approaches were employed. One study utilized a plot method for evaluation, which proved challenging due to the absence of means and cut-offs provided by the authors [10]. In contrast, the other two studies applied the “agreement observed” between the two tests [15] and the Bland–Altman test [3]. These latter methods showed good agreement between MRI and scintigraphy, with a 66% agreement observed and mean differences of −0.3% in the right kidney and 0.3% in the left kidney, respectively.
PDKF outcomes
In the studies included, there was no assessment of correlation with PDKF.
RoB assessment and generalizability
RoB was evaluated according to QUADAS-2 assessment tool, as shown in Fig. 2 and Supplementary Table 1. This tool revealed a predominant low RoB in patient selection and flow and timing domains and a majority low concern of applicability in all the domains studied. Only four studies [12, 24,25,26] indicated that the outcomes were blinded for the researchers or radiologists.
In three studies [18, 20, 21] the authors not only assessed living donors but also collected data on recipients. Nonetheless, the outcomes of donors and recipients were reported separately, allowing for a thorough and independent analysis of each group.
Discussion
Donor’s evaluation is fundamental both for the receipt and the donor. In addition to anatomy and compatibility considerations, the potential donor’s assessment must prioritize the minimization of both short and long-term risks associated with nephrectomy. It is important to emphasize that donors are healthy individuals, willingly undergoing a nephrectomy, which is a high-risk surgical procedure [27], driven by altruistic intentions to "gift" their kidney, often to a relative.
There is an active debate on the donor’s optimal study pre-donation as recent studies support the option of performing only a volumetry test which can assess SRF and even PDKF instead of the actual (but not universalized) functional and anatomical evaluation or scintigraphy and CT.
Unfortunately, the availability of literature on SRF assessment through imaging tests remains limited, with only twenty-one studies being included in this review. Summarizing outcomes proved challenging due to the heterogeneity observed among the included studies, mainly concerning the methods employed for study design, scintigraphy tracer, CT characteristics, and statistical analysis. This heterogeneity rendered meta-analysis unfeasible. Furthermore, the absence of an established consensus makes direct comparisons arduous. Despite these challenges, the QUADAS-2 assessment revealed an acceptable RoB (Fig. 2).
Focusing on scintigraphy tracers, the studies included in this review used different agents, such as DTPA, MAG3, and DMSA, sometimes even within the same study [21, 26]. The choice of the radiopharmaceutical was dependent on the type of renal scan performed [30]. DTPA and MAG3 have proven useful in measuring GFR and evaluating flow through the pyelocalyceal system and bladder. These tracers are typically employed for dynamic imaging, while DMSA is primarily used for static imaging and cortical anatomy assessment, particularly for pathologies like renal ectopia or renal scarring [30]. There is currently lack of scientific evidence published on the optimal nuclear tracer for evaluating RF in a healthy population, such as potential kidney donor. Consequently, we can only speculate that the results obtained from different tracers yield similar outcomes. However, it is crucial to acknowledge that no concrete evidence supports this hypothesis.
In terms of CT characteristics, the studies reviewed employed various techniques (such as multidetector, conventional, and CT angiography), thickness (from 0.5 [4] to 5 mm [28]), and volume reconstruction calculation methods (automatic 3D reconstruction, area multiplied by thickness, modified ellipsoid…). This diversity complicates direct comparisons between studies. Regarding CT thickness, recent studies have yielded similar findings in assessing kidney volume, demonstrating low interobserver variability [29]. Focusing on volume reconstruction, there is currently no consensus on the method for the calculation of renal volumetry.
Our review revealed robust correlations between CT and scintigraphy, with good correlation coefficients ranging from 0.9 to 1 [14, 17, 21, 28], contrasting with Wahba et al. [20] lower correlations rate. Notably, these correlations held validity across studies with both small and large cohort populations, as evidenced in smaller studies [14, 28] compared those with larger cohort populations [17, 21]. Moreover, the correlation between MRI and scintigraphy also demonstrated favorable results, with a coefficient of 0.84 reported in the study conducted by Artunc et al.[10].
Regarding the agreement between tests, CT studies consistently showed the best correlations, as shown by the findings of different studies [11, 24, 26, 28]. Another small cohort study [3] also obtained excellent agreement between MRI and scintigraphy. Nevertheless, it is important to note that this level of agreement was not consistently observed in other MRI studies, particularly those with larger cohort populations [15].
The main limitation of this systematic review stems from the substantial heterogeneity observed in the literature due to the lack of robust evidence. However, these limitations serve as an initial catalyst for enhancing awareness and prompting improvements in study design, ultimately benefiting decision-making and diagnostic practices. Despite these challenges, our review demonstrated an acceptable RoB. Nevertheless, our systematic review provides valuable insights into the current state of evidence concerning imaging tests for assessing kidney function in potential donors.
Conclusions
Living donor nephrectomy holds significant importance for clinicians, patients, their families, and transplant surgeons alike. However, the current state of evidence is marred by a notable lack of robust data, presenting a significant challenge to informed decision-making in clinical practice. Nevertheless, promising results have emerged from studies assessing renal volumetry through MRI or CT demonstrating favorable correlation and agreement compared to the established gold standard, scintigraphy. It is imperative to prioritize improvements in study design and initiate prospective registries dedicated to this area. Our systematic review represents the first step to acknowledge these limitations and pave the way for multidisciplinary prospective studies involving nephrologists and urologists.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and in the supplementary material.
Abbreviations
- eGFR:
-
Estimated glomerular filtration rate
- KT:
-
Kidney transplantation
- LDKT:
-
Living donor kidney transplantation
- PDKF:
-
Post-donation kidney function
- SRF:
-
Split renal function
- RF:
-
Renal function
- RoB:
-
Risk of bias
References
Hariharan S, Israni AK, Danovitch G (2021) Long-term survival after kidney transplantation. N Engl J Med 385(8):729–743
British Transplantation Society (2018) Guidelines for living donor kidney transplantation. United Kingdom Guidelines, London
Krumm P, Hupka T, Haußmann F, Dittmann H, Mühlbacher T, Nadalin S et al (2021) Contrast-enhanced MRI for simultaneous evaluation of renal morphology and split renal function in living kidney donor candidates. Eur J Radiol 142:109864
Weinberger S, Baeder M, Scheurig-Muenkler C, Steffen IG, Magheli A, Miller K et al (2016) Optimizing scintigraphic evaluation of split renal function in living kidney donors using the geometric mean method: a preliminary retrospective study. J Nephrol 29(3):435–441
Nakamura N, Aoyagi C, Matsuzaki H, Furuya R, Irie S, Matsuoka H et al (2019) Role of computed tomography volumetry in preoperative donor renal function evaluation of living related kidney transplantation. Transplant Proc 51(5):1314–1316
Diez A, Powelson J, Sundaram CP, Taber TE, Mujtaba MA, Yaqub MS et al (2014) Correlation between CT-based measured renal volumes and nuclear-renography-based split renal function in living kidney donors. Clinical diagnostic utility and practice patterns. Clin Transplant 28(6):675–682
Knoll T, Omar MI, Maclennan S, Hernández V, Canfield S, Yuan Y et al (2018) Key steps in conducting systematic reviews for underpinning clinical practice guidelines: methodology of the european association of urology. Eur Urol 73(2):290–300
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 29:n71
Whiting PF (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155(8):529
Artunc F, Yildiz S, Rossi C, Boss A, Dittmann H, Schlemmer HP et al (2010) Simultaneous evaluation of renal morphology and function in live kidney donors using dynamic magnetic resonance imaging. Nephrol Dial Transplant 25(6):1986–1991
Lal H, Singh A, Prasad R, Yadav P, Akhtar J, Barai S et al (2021) Determination of split renal function in voluntary renal donors by multidetector computed tomography and nuclear renography How well do they correlate? South Afr J Radiol 25:1
Patankar K, Low RST, Blakeway D, Ferrari P (2014) Comparison of computer tomographic volumetry versus nuclear split renal function to determine residual renal function after living kidney donation. Acta Radiol 55(6):753–760
Eum SH, Lee H, Ko EJ, Cho HJ, Yang CW, Chung BH (2022) Comparison of CT volumetry versus nuclear renography for predicting remaining kidney function after uninephrectomy in living kidney donors. Sci Rep 12(1):5144
Nilsson H, Wadström J, Andersson L-G, Raland H, Magnusson A (2004) Measuring split renal function in renal donors: can computed tomography replace renography? Acta Radiol 45(4):474–480
Lange D, Helck A, Rominger A, Crispin A, Meiser B, Werner J et al (2018) Renal volume assessed by magnetic resonance imaging volumetry correlates with renal function in living kidney donors pre- and postdonation: a retrospective cohort study. Transpl Int 31(7):773–780
Mitsui Y, Sadahira T, Araki M, Wada K, Tanimoto R, Ariyoshi Y et al (2018) The assessment of renal cortex and parenchymal volume using automated CT volumetry for predicting renal function after donor nephrectomy. Clin Exp Nephrol 22(2):453–458
Lee HH, Han WK, Kang SK, Huh KH, Kim MS, Kim SI et al (2017) Usefulness of multi-detector computed tomography scanning as a replacement for diethylenetriamine pentaacetic acid. Transplant Proc 49(5):1023–1026
Almeida M, Pereira PR, Ramos M, Carneiro D, Mandaleno M, Silva F et al (2022) CT volumetry performs better than nuclear renography in predicting estimated renal function one year after living donation. Int Urol Nephrol 55(3):553–562
Yanishi M, Kinoshita H, Yoshida T, Takayasu K, Yoshida K, Kawa G et al (2015) Comparison of renal scintigraphy and computed tomographic renal volumetry for determining split renal function and estimating post-transplant renal function. Transplant Proc 47(9):2700–2702
Wahba R, Franke M, Hellmich M, Kleinert R, Cingöz T, Schmidt MC et al (2016) Computed tomography volumetry in preoperative living kidney donor assessment for prediction of split renal function. Transplantation 100(6):1270–1277
Halleck F, Diederichs G, Koehlitz T, Slowinski T, Engelken F, Liefeldt L et al (2013) Volume matters: CT-based renal cortex volume measurement in the evaluation of living kidney donors. Transpl Int 26(12):1208–1216
Yokoyama N, Ishimura T (2015) Usefulness of three-dimensional computerized tomographic volumetry for determining split renal function in donors for living-related kidney transplantation. Transplant Proc 47(3):588–590
Barbas AS, Li Y, Zair M, Van JA, Famure O, Dib MJ et al (2016) CT volumetry is superior to nuclear renography for prediction of residual kidney function in living donors. Clin Transplant 30(9):1028–1035
Summerlin AL, Lockhart ME, Strang AM, Kolettis PN, Fineberg NS, Smith JK (2008) Determination of split renal function by 3D reconstruction of CT angiograms: a comparison with gamma camera renography. Am J Roentgenol 191(5):1552–1558
Harper KC, Salameh JP, Akhlaq N, McInnes MDF, Ivankovic V, Beydoun MH et al (2021) The impact of measuring split kidney function on post-donation kidney function: a retrospective cohort study. PLoS ONE 16(7):e0253609
Soga S, Britz-Cunningham S, Kumamaru KK, Malek SK, Tullius SG, Rybicki FJ (2012) Comprehensive comparative study of computed tomography-based estimates of split renal function for potential renal donors: modified ellipsoid method and other CT-based methods. J Comput Assist Tomogr 36(3):323–329
Giessing M (2012) Living donor nephrectomy—quantifying the risk for the donor. Transplant Proc 44(6):1786–1789
Kato F, Kamishima T, Morita K, Muto NS, Okamoto S, Omatsu T et al (2011) Rapid estimation of split renal function in kidney donors using software developed for computed tomographic renal volumetry. Eur J Radiol 79(1):15–20
Houbois C, Haneder S, Merkt M, Holz JA, Morelli J, Kiel A et al (2020) Semiautomated renal cortex volumetry in multislice computed tomography: effect of slice thickness and iterative reconstruction algorithms. J Comput Assist Tomogr 44(2):236–241
Banker H, Sheffield EG, Cohen HL (2023) Nuclear renal scan. StatPearls. StatPearls Publishing, Treasure Island, FL
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement. None.
Author information
Authors and Affiliations
Contributions
Conception and design: RC. Acquisition of data: RC, ALA, AP. Analysis and interpretation of data: ALA, TP, MB. Drafting of the manuscript: ALA, TP. Critical revision of the manuscript for important intellectual content: RB, MB, AP, MID, BBM, SS, AT, RC.
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical statement
The authors certify that this document complies with the current ethical standards and that no individual data have been used.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
López-Abad, A., Prudhomme, T., Pecoraro, A. et al. Can CT or MRI volumetry substitute scintigraphy in living kidney donor evaluation? A systematic review. World J Urol 42, 382 (2024). https://doi.org/10.1007/s00345-024-05024-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00345-024-05024-y