Abstract
Purpose
To provide a descriptive report of mortality and morbidity in the first 30 days of diagnosis of urosepsis. Secondary aim is to identify risk factors of unfavourable outcomes.
Methods
Prospective observational multicentre cohort study conducted from September 2014 to November 2018 in European hospitals. Adult patients (≥ 18 years) diagnosed with acute urosepsis according to Sepsis-2 criteria with confirmed microbiological infection were included. Outcomes were classified in one of four health states: death, multiple organ failure, single organ failure, and recovery at day 30 from onset of urosepsis. Descriptive statistics and ordinal logistic regression analysis was performed.
Results
Three hundred and fifty four patients were recruited, and 30-day mortality rate was 2.8%, rising to 4.6% for severe sepsis. All patients who died had a SOFA score of ≥ 2 at diagnosis. Upon initial diagnosis, 79% (n = 281) of patients presented with OF. Within 30 days, an additional 5% developed OF, resulting in a total of 84% affected. Charlson score (OR 1.14 CI 1.01–1.28), patients with respiratory failure at baseline (OR 2.35, CI 1.32–4.21), ICU admission within the past 12 months (OR 2.05, CI 1.00–4.19), obstruction causative of urosepsis (OR 1.76, CI 1.02–3.05), urosepsis with multi-drug-resistant(MDR) pathogens (OR 2.01, CI 1.15–3.53), and SOFA baseline score ≥ 2 (OR 2.74, CI 1.49–5.07) are significantly associated with day 30 outcomes (OF and death).
Conclusions
Impact of comorbidities and MDR pathogens on outcomes highlights the existence of a distinct group of patients who are prone to mortality and morbidity. These findings underscore the need for the development of pragmatic classifications to better assess the severity of UTIs and guide management strategies.
Study registration: Clinicaltrials.gov registration number NCT02380170.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urosepsis, a commonly occurring form of sepsis resulting from urinary tract infections (UTIs), poses threat to life and can lead to long-term morbidity. UTIs are one of the most common sources of sepsis, with estimates ranging from 20 to 40% of all sepsis cases [1, 2]. Despite urosepsis having relatively low mortality rates, it remains an area that requires further research [2]. Our understanding of predictors of mortality and morbidity in urosepsis is limited, but identifying patient factors associated with urosepsis at the time of diagnosis holds promise for improving patient care and outcomes.
Urosepsis outcomes vary among populations and are influenced by factors like severity, patient fitness, frailty, and age [1,3,4]. Recent studies suggest that clinical findings can improve prognostic tools and aid decision-making [5]. While common risk factors for urosepsis include indwelling catheters, obstructive uropathy, tissue necrosis, abscess, urinary tract interventions, and urological functional impairments, their precise impact on outcomes remains uncertain [6].
Guidelines now favour a risk-based approach over using the Systemic Inflammatory Response Syndrome (SIRS) criteria for sepsis diagnosis and prognosis [4,5,6]. The low specificity of the SIRS criteria have contributed to potential inclusion of non-infectious conditions and overdiagnosis of sepsis [5]. Despite poor specificity, SIRS remains widely used due to its simplicity. However, performance programs for sepsis have improved early detection and outcomes [7]. Furthermore, the rise in antimicrobial resistance (AMR) and multi-drug resistant (MDR) pathogens compromises effectiveness of antibiotics and adds complexity to our understanding of urosepsis risk factors [8,9,10].
The aim of this study is to provide a descriptive report of mortality and morbidity in the first 30 days after the diagnosis of urosepsis. We used the SIRS criteria, clinical findings, and microbiological confirmation of urinary tract infection as the underlying cause of sepsis. In addition, we aim to explore the risk factors for unfavourable outcomes expressed as organ failure and death by means of a comprehensive analysis of registered variables.
Methods
This multi-center study assessed urosepsis in 34 European hospitals. Diagnosis required meeting at least two SIRS criteria, with the urinary tract identified as the sepsis source. Patients were followed for 30 days, with data collected at baseline and on days 3, 7, 9, and 30.
Sequential Organ Failure Assessment (SOFA) score domains were used to assess organ failure at predefined time points. Organ failure (OF) was defined as sustained impairment of normal organ function, hindering its physiological role. SOFA scores greater than 1 or an increase of 1 point from baseline indicated organ failure. Patients undergoing invasive supportive treatment (mechanical ventilation, vasopressor support, or renal replacement therapy) were also monitored at specified time points to track organ function.
Study oversight and design
This prospective observational cohort study was conducted from September 2014 to November 2018 after ethical approval and registration. (Justus Liebig University, Giessen, Germany Ethical Board (AZ: 77/14) on 15/05/2014) (NCT02380170). The study, conducted from September 2014 to November 2018, was a prospective observational cohort study. It was approved and registered by the Justus Liebig University in Germany. Recruitment was monitored biweekly, and issues related to recruitment were managed between site investigators and the study management group (TEBJ, FW and ZT). The centers were selected from the Global Prevalence of Infections in Urology study [11].
Adult patients (≥ 18 years) diagnosed with acute urosepsis according to Sepsis-2 criteria were included in the study [12]. Patients were recruited from various healthcare settings, including emergency units, urology wards, other wards, outpatient clinics, and community care referrals. Urinary tract infection was confirmed through positive urine and blood cultures before antibiotic treatment. Patients with sepsis from other sites were excluded. Enrolled patients were categorized as non-severe or severe sepsis, including septic shock, based on Sepsis-2 definitions. Further study design details can be found in Supplement II [12].
Data collection
Data were collected using an online case report form, with patient characteristics and physiological variables found in Supplement-III. SOFA items and additional clinical findings were gathered at diagnosis and follow-ups. Initial treatment details were recorded within the first 24 h of urosepsis onset, and follow-up assessments included outcomes and treatments. Data collection semi-automatic controlled, with any inconsistencies resolved by SMG to determine CRF eligibility (supplement IV).
Causative pathogens and their susceptibility profile were identified according to local practice, which included Clinical & Laboratory Standards Institute (CLSI) or European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria [13, 14]. Pathogens were classified as MDR or extensively drug-resistant according to the European Centre for Disease Prevention and Control (ECDC) and Centers for Disease Control and Prevention (CDC) joint initiative definitions [15].
Statistical analysis
Anonymized data were analysed using the statistical package “R” version 3.2. Descriptive analysis summarized key information, including patient demographics, clinical variables, mortality rates, and rates of organ failure as a measure of morbidity. OF was categorized into single organ failure and multiple organ failure (MOF). The outcomes were one of four health states: death, MOF, single organ failure, and recovery.
Ordinal logistic regression analysis was used to evaluate the relationship between each risk factor and outcomes. A comprehensive analysis was conducted, incorporating all measured risk factors into the model, assessing the combined influence of these factors on outcomes.
Results
Diagnostic criteria and initial management
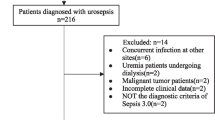
Patient population. The analysis included 354 patients meeting sepsis-2 criteria with identified pathogens (see patient case disposition in Supplementary Fig. 4) and completed 30-day follow-up. Median age was 65.1 years (IQR 51.1–74.1), with 45% females (n = 183). See Table 1 for patient details.
Clinical presentation
3.4% of patients (n = 12) presented with septic shock, while 45.5% (n = 161) had severe sepsis. These two groups were combined and categorized as severe sepsis (n = 173), with the remaining cases classified as non-severe sepsis (n = 181). Additional information regarding baseline SIRS criteria is available in the supplementary materials (Table 1 in the Supplement).
Microbiological findings
Positive urine cultures were obtained in 338 patients, while positive blood cultures were obtained in 189 patients. Gram-negative bacteria were the predominant pathogens, accounting for 82% of urine cultures and 56.6% of blood cultures. S-Table 2 details pathogen frequencies in different cultures. MDR pathogens were observed in 28.5% of urine cultures and 21% of blood cultures (Fig. 5 in Supplement).
Initial management
All patients received antibiotics, either as a single agent (70%) or in combination (30%). The median time from diagnosis to the first antibiotic dose was 60 min (IQR 15–180 min). Antibiotic susceptibility data were available for 73% of cases (n = 260), and in 89% of these cases (n = 233), the causative pathogens were sensitive to the administered antibiotics. Urinary tract obstruction was relieved in 39.8% of patients (n = 141), with 72% of these cases (n = 101) addressed within 24 h of urosepsis diagnosis. Infected tissue debridement was performed in 6.5% of patients (n = 23), and among them, 56.5% (n = 13) underwent the procedure within the first 3 days after diagnosis.
Mortality and organ failure
Mortality
Mortality rate within 30 days was 2.8% (n 10). Mean time to death from the point of diagnosis of urosepsis was 8.4 days (min 3.6–max 25.2). All patients who died presented with OF at diagnosis. The mortality rate was higher in patients who presented with kidney (5% vs no kidney failure: 0, p = 0.01), respiratory (8% vs no respiratory failure: 1%, p = 0.00), and cardiovascular system failure (7% vs no failure: 2%, p = 0.04).
Dynamics of organ failure
Initially, 79% (n = 281) of patients developed OF, with an additional 5% developing within 30 days, affecting 84%. The rate decreased to 24% (n = 85) by the end of the 30-day period. Initially, 48% (n = 170) of the cohort had MOF, which decreased to 7% (n = 25) on day 30 (Fig. 1a, b). Time trends of the OF sites are explained in Fig. 1c.
Details of organ failure over 30 days follow-up. a Proportion of patients with multiple organ failure. b Proportion of patients that require invasive supportive treatment and (c) proportion of patients with sites affected. c Among the cases of OF, kidney failure (red line) was most common at the time of diagnosis (57%) until day 3 (37%). However, by day 7, both kidney (22%) and respiratory failure (yellow line) (22%) were the leading causes. This trend persisted on day 15 (kidney: 13% and respiratory: 19%) and day 30 (kidney: 13% and respiratory: 16%), where respiratory failure surpassed kidney failure as the predominant cause of OF
Impact of comorbidity and risk factors
Sepsis severity was greater in patients who died or had OF on day 30 (p = 0.00). Details of individual variables and their distribution among outcome groups at day 30 are available in Table 1. Among patients with MDR pathogens detected in either urine or blood cultures, 33% (n = 31) had organ failure at day 30, while only 20% (n = 52) of patients without MDR pathogens exhibited organ failure (p = 0.03).
The logistic ordinal regression analysis populated with all risk factors identified Charlson score (OR 1.16 CI 1.03–1.30), patients with respiratory failure at baseline (OR 3.50, CI 2.01–6.12), history of UTIs and its frequency (OR 1.74, CI 1.11–2.74), urosepsis with MDR pathogens (OR 1.66, CI 1.12–2.74), and sepsis severity (OR 2.33, CI 1.38–3.95) are significantly associated with day 30 outcomes (organ failure and death).
Impact of SOFA score
A total of 200 (56%) patients had a SOFA score of ≥ 2 at diagnosis and patients who died were all within this group. SOFA score of ≥ 2 at diagnosis was significantly associated with both single OF and MOF at day 30 (p = 0.00) (OR 4.38, CI 2.48–7.74) (Table 2).
The logistic ordinal regression analysis repeated with SOFA baseline categories instead of SIRS severity, indicates that Charlson score (OR 1.14, CI 1.01–1.28), patients with respiratory failure at baseline (OR 2.35, CI 1.32–4.21), ICU admission within the past 12 months (OR 2.05, CI 1.00–4.19), obstruction causative of urosepsis (OR 1.76, CI 1.02–3.05), urosepsis with MDR pathogens (OR 2.01, CI 1.15–3.53) and SOFA baseline score ≥ 2 (OR 2.74, CI 1.49–5.07) are significantly associated with day 30 outcomes (OF and death).
Discussion
Our study aimed to investigate the mortality and morbidity outcomes of urosepsis and identify important risk factors associated with them.
Main findings
The 30-day mortality rate for urosepsis meeting SIRS criteria and confirmed microbiologically was 2.8%, rising to 4.6% for severe sepsis. Higher Charlson scores, respiratory failure at urosepsis diagnosis, and urosepsis caused by MDR pathogens were associated with ongoing OF after 30 days, increased severity of OF, and higher mortality rates. Patients with recent health events (ICU admissions, UTIs) and higher UTI frequency had a greater risk of OF at 30 days. Urinary tract obstruction was also related to negative outcomes. A baseline SOFA score ≥ 2 was a significant predictor of death, with all deaths occurring within this group.
Findings compared with other studies
The sepsis mortality rate varies, commonly reported around 10%, depending on factors like sepsis source, population studied, local AMR prevalence, and management protocols [1,2,3,4]. However, our study found a lower mortality rate due to lower proportion of severe sepsis cases, a favourable Charlson score (0–1) in 60% of the cohort and timely management [16, 17]. Still, severe sepsis cases had a notable 4.6% mortality rate.
The importance of risk factors
Studies show AMR's potential to increase sepsis mortality at the population level [8, 10, 18]. For example, a recent study suggests AMR's association with pyelonephritis progressing to sepsis [18]. Our findings also indicate MDR pathogens as significant predictors of urosepsis mortality and organ failure. In a Swedish retrospective study community onset urosepsis with blood stream infection (2019 and 2020), low AMR rates were observed and inappropriate empirical treatment was associated with mortality [16]. In our study in 89% of cases, the pathogens were susceptible to the antibiotics given. However, achieving a high rate of appropriate antibiotic administration involved the use of reserve antibiotics. Specifically, 25% of patients (n: 87) received Carbapenem group antibiotics (results not presented).
We found that, a history of UTI within the past 12 months increased the risk of mortality and morbidity, potentially due to the presence of MDR pathogens in persistent infection sites. Further analysis revealed higher rates of MDR pathogens in patients with UTI history (37%—n = 56) compared to those without (19%, n = 38; p = 0.00). Similar patterns were observed for other risk factors, such as previous ICU admission (49% MDR rate vs. 23% without admission) and the presence of indwelling catheters (35% MDR rate vs. 21% without catheter). However, poorer outcomes cannot be solely attributed to MDR pathogens as factors like impaired immune responses, frailty, and functional disorders may also contribute [19]. The complex pathogenesis of urosepsis necessitates a differentiated management approach, with consideration of urological risk in guiding empirical treatment.
Clinical implications
All deaths in our study were observed exclusively in patients with a baseline SOFA score of ≥ 2, leading to a mortality rate of 5% within this specific subgroup. Furthermore, our research reveals that 62.4% of patients with a SOFA < 2 experienced progression to develop OF, with 13% still experiencing OF at day 30. (s-Table 3). This is in comparison to 33% of patients with a SOFA ≥ 2 (Table 2). Our study supports the use of the SOFA score in confirming the diagnosis of urosepsis. It highlights that timely identification and immediate intervention result in improved results, underscoring the significance of further evaluation for risk classification, escalating or de-escalating treatment.
Weaknesses
The SERPENS study, which was carried out by urology teams, was initially powered by an anticipated mortality rate of over 10%. Nevertheless, the death rate recorded in the research was considerably lower, suggesting that the study may have lacked sufficient statistical power. The observed phenomenon can be attributed to a selection bias that impacted by discrepancies in patient treatment among diverse healthcare jurisdictions.
Methodology
In our study, we used ordinal categories to measure mortality and organ dysfunction severity, with a potential alternative methodology using organ failure-free days [20]. This method provides a comprehensive understanding of organ functionality and quantitative evaluation of urosepsis' influence on organ failure and recovery. Future research could use this methodology to gain additional perspectives on urosepsis outcomes.
Conclusions
This study provides new information about the mortality and morbidity patterns associated with contemporary urosepsis. Analysis of risk factors emphasizes the impact of comorbidities and MDR pathogens on outcomes and highlights the existence of a distinct group of patients who do not initially present with organ failure but may progress to a more severe state. These findings underscore the need for the development of pragmatic classifications to better assess the severity of UTIs and guide management strategies.
Data availability
Data will be made available upon request to the corresponding author and will be subject to approval by the study coordinators for research purposes.
References
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S et al (2020) Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. The Lancet 395(10219):200–211
Leligdowicz A, Dodek PM, Norena M, Wong H, Kumar A, Kumar A (2014) Association between source of infection and hospital mortality in patients who have septic shock. Am J Respir Crit Care Med 189(10):1204–1213
Liu V, Escobar GJ, Greene JD, Soule J, Whippy A, Angus DC, Iwashyna TJ (2014) Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 312(1):90–92
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, Mcintyre L, Ostermann M, Prescott HC et al (2021) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med 49(11):e1063–e1143
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM et al (2016) The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315(8):801–810
NICE (2016) Sepsis: recognition, diagnosis and early management NG51. In: NICE (ed). UK: National Institute for Health and Care Excellence
Damiani E, Donati A, Serafini G, Rinaldi L, Adrario E, Pelaia P, Busani S, Girardis M (2015) Effect of performance improvement programs on compliance with sepsis bundles and mortality: a systematic review and meta-analysis of observational studies. PLoS ONE 10(5):e0125827
CDC (2021) Antibiotic resistance threats in the United States, 2019. In: Prevention (ed). CfDCa
Madrazo M, López-Cruz I, Piles L, Viñola S, Alberola J, Eiros JM, Artero A (2023) Risk factors and the impact of multidrug-resistant bacteria on community-acquired urinary sepsis. Microorganisms 11(5):1278
Busani S, Serafini G, Mantovani E, Venturelli C, Giannella M, Viale P, Mussini C, Cossarizza A, Girardis M (2019) Mortality in patients with septic shock by multidrug resistant bacteria: risk factors and impact of sepsis treatments. J Intensive Care Med 34(1):48–54
Wagenlehner F, Tandogdu Z, Bartoletti R, Cai T, Cek M, Kulchavenya E, Köves B, Naber K, Perepanova T, Tenke P et al (2016) The global prevalence of infections in urology study: a long-term, worldwide surveillance study on urological infections. Pathogens (Basel, Switzerland) 5(1):10
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 29(4):530–538
Humphries RM, Ambler J, Mitchell SL, Castanheira M, Dingle T, Hindler JA, Koeth L, Sei K, Development CM, Standardization Working Group of the Subcommittee on Antimicrobial Susceptibility T (2018) CLSI methods development and standardization working group best practices for evaluation of antimicrobial susceptibility tests. J Clin Microbiol 56(4):e01934-e11917
Leclercq R, Canton R, Brown DF, Giske CG, Heisig P, MacGowan AP, Mouton JW, Nordmann P, Rodloff AC, Rossolini GM et al (2013) EUCAST expert rules in antimicrobial susceptibility testing. Clin Microbiol Infect 19(2):141–160
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B et al (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18(3):268–281
Holmbom M, Andersson M, Grabe M, Peeker R, Saudi A, Styrke J, Aljabery F (2022) Community-onset urosepsis: incidence and risk factors for 30-day mortality—a retrospective cohort study. Scand J Urol 56(5–6):414–420
Schuttevaer R, Boogers W, Brink A, Dijk WV, Steenwinkel JD, Schuit S, Verbon A, Lingsma H, Alsma J (2022) Predictive performance of comorbidity for 30-day and 1-year mortality in patients with bloodstream infection visiting the emergency department: a retrospective cohort study. BMJ Open 12(4):e057196
Kozyrakis D, Kratiras Z, Soukias G, Chatzistamou SE, Zarkadas A, Perikleous S, Kateris D, Katsaros I, Skriapas K, Karagiannis D (2020) Clinical outcome and prognostic factors of sepsis, septic shock and prolonged hospitalization, of patients presented with acute obstructive pyelonephritis. J Endourol 34(4):516–522
Cinel I, Dellinger RP (2007) Advances in pathogenesis and management of sepsis. Curr Opin Infect Dis 20(4):345–352
REMAP-CAP Investigators (2021) Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med 384(16):1491–1502
Acknowledgements
SERPENS Investigators: Michael Samarinas, Dr., Univeristy Hospital of Larissa, Greece. Bugra Cetin, Dr., Assoc. Prof., Gaziosmanpasa Taksim Teaching Hospital, Istanbul, Turkey. Maria Štefkovičová, Dr., Teaching Hospital Trenčín, Slovakia. Maria Kopilec Garabasov, Dr. PhD, Teaching Hospital Trenčín, Slovakia. Ester Illiano, Dr., Ospedale Santa Maria della Misericordia di Perugia, Italy. Bruno Pereira, Dr., Centro Hospitalar Cova de Beira, Lisbon, Portugal. Fiona Wu, Dr., National University Hospital, Singapore (NCIS), Singapore. Przemyslaw Adamczyk, Nicolaus Copernicus City Hospital Specjalistyczny Szpital Miejski, Torun, Poland. Yiloren Tanidir, Assoc. Prof., Marmara University School of Medicine, Department of Urology, Istanbul, Turkey. Mustafa Bazardzanovic, Dr., University Clinical Center Tuzla, Bosnia and Herzegovina. Hansjörg Keller, Sana Klinikum Hof GmbH, Germany. Razvan-Vasile Dican, Sana Klinikum Hof GmbH, Germany. Carlos Oliviera, Dr., Hospital de Braga, Portugal. Andrea Cocci, San Luca Nuovo Padiglione, Florence, Italy. Juan Gomez Rivas, Department of Urology. Hospital Clínico San Carlos. Madrid-Spain. Francesco Beniamin, Ospedale Ca’ Foncello di Treviso, Treviso, Italy. Peter Tenke, Prof., South Pest Teaching Hospital, Budapest, Hungary. Seydali Eredjepov, EstiMed, Tashkent, Uzbekistan. Alexey A. Shevyrin, European Association of Urology Section of Infections in Urology. Branka Terzic,Dr., Clinical Center of Serbia, Serbia. Senad Bajramovic, Dr., Clinical University Center Sarajevo, Sarajevo, Bosnia and Hercegovina. Peter Hasan, Dr., European Association of Urology Section of Infections in Urology. Tommaso Brancato, Prof., Regina Apostolorum Hospital S. Evyenio, Rome, Italy. Baris Nuhoglu, Prof., Gaziosmanpasa Taksim Teaching Hospital, Istanbul, Turkey
Funding
This research was supported by the European Association of Urology Research Foundation (EAU-RF). The EAU-RF received an unconditional grant from Cubist Ltd in 2015 for research in urosepsis. The EAU-RF or Cubist had no role in the study design, data collection, analysis and interpretation; or preparation of the report.
Author information
Authors and Affiliations
Consortia
Contributions
Conception and design: Zafer Tandogdu, Truls Erik Bjerklund Johansen, Florian Wagenlehner. Acquisition of data: Zafer Tandogdu, Truls Erik Bjerklund Johansen, Florian Wagenlehner, Bela Koves, Slobodan Ristovski, Mustafa Bahadir Can Balci, Kristin Rennesund, Stavros Gravas, DjordJe Nale, José Medina-Polo, Mária Kopilec Garabášová, Elisabetta Costantini, Jorge Cano-Valasco, Maja Sofronievska Glavinova, Baris Nuhoglu, Franck Bruyere, Tamara Perepanova, Ekaterina Kulchavenya, Mete Cek, Bugra Cetin, Maria Štefkovičová, Maria Kopilec Garabasov, Ester Illiano, Bruno Pereira,. Fiona Wu,. Przemyslaw Adamczyk, Yiloren Tanidir, Mustafa Bazardzanovic, Hansjörg Keller, Razvan-Vasile Dican, Carlos Oliviera,. Andrea Cocci, Juan Gomez Rivas, Francesco Beniamin. Analysis and interpretation of data: Zafer Tandogdu, Truls Erik Bjerklund Johansen, Florian Wagenlehner. Drafting of the manuscript: Zafer Tandogdu Truls Erik Bjerklund Johansen, Florian Wagenlehner. Critical revision of the manuscript: Zafer Tandogdu, Truls Erik Bjerklund Johansen, Florian Wagenlehner, Bela Koves, Slobodan Ristovski, Mustafa Bahadir Can Balci, Kristin Rennesund, Stavros Gravas, DjordJe Nale, José Medina-Polo, Mária Kopilec Garabášová, Elisabetta Costantini, Jorge Cano-Valasco, Maja Sofronievska Glavinova, Baris Nuhoglu, Franck Bruyere, Tamara Perepanova, Ekaterina Kulchavenya, Mete Cek, Bugra Cetin, Maria Štefkovičová, Maria Kopilec Garabasov, Ester Illiano, Bruno Pereira,. Fiona Wu,. Przemyslaw Adamczyk, Yiloren Tanidir, Mustafa Bazardzanovic, Hansjörg Keller, Razvan-Vasile Dican, Carlos Oliviera,. Andrea Cocci, Juan Gomez Rivas, Francesco Beniamin. Statistical analysis: Zafer Tandogdu. Obtaining funding: Zafer Tandogdu, Truls Erik Bjerklund Johansen, Florian Wagenlehner.
Corresponding author
Ethics declarations
Conflict of interests
Authors declare that they have no conflict of interest in keeping with the current research and manuscript.
Ethical approval
This study was conducted in accordance with the principles outlined in the Declaration of Helsinki. Ethical approval for the research protocol was obtained from Justus Liebig University, Giessen, Germany ethical board (AZ: 77/14) on 15/05/2014. Confidentiality and privacy of participant data were strictly maintained, and all data were anonymized before analysis. The authors affirm their commitment to transparency, accuracy, and ethical conduct throughout the research process.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the SERPENS Investigators are listed in Acknowledgements.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tandogdu, Z., Koves, B., Ristovski, S. et al. Urosepsis 30-day mortality, morbidity, and their risk factors: SERPENS study, a prospective, observational multi-center study. World J Urol 42, 314 (2024). https://doi.org/10.1007/s00345-024-04979-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00345-024-04979-2