Abstract
Introduction
High fluid temperatures have been seen in both in vitro and in vivo studies with laser lithotripsy, yet the thermal distribution within the renal parenchyma has not been well characterized. Additionally, the heat-sink effect of vascular perfusion remains uncertain. Our objectives were twofold: first, to measure renal tissue temperatures in response to laser activation in a calyx, and second, to assess the effect of vascular perfusion on renal tissue temperatures.
Methods
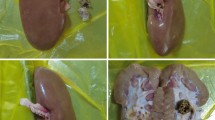
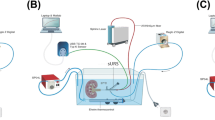
Ureteroscopy was performed in three porcine subjects with a prototype ureteroscope containing a temperature sensor at its tip. A needle with four thermocouples was introduced percutaneously into a kidney with ultrasound guidance to allow temperature measurement in the renal medulla and cortex. Three trials of laser activation (40W) for 60 s were conducted with an irrigation rate of 8 ml/min at room temperature in each subject. After euthanasia, three trials were repeated without vascular perfusion in each subject.
Results
Substantial temperature elevation was observed in the renal medulla with thermal dose in two of nine trials exceeding threshold for tissue injury. The temperature decay time (t½) of the non-perfused trials was longer than in the perfused trials. The ratio of t½ between them was greater in the cortex than the medulla.
Conclusion
High-power laser settings (40W) can induce potentially injurious temperatures in the in vivo porcine kidney, particularly in the medullary region adjacent to the collecting system. Additionally, the influence of vascular perfusion in mitigating thermal risk in this susceptible area appears to be limited.



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aldoukhi AH, Roberts WW, Hall TL et al (2017) Holmium laser lithotripsy in the new stone age: dust or bust? Front Surg 4:57
Pietropaolo A, Mani M, Hughes T et al (2022) Role of low- versus high-power laser in the treatment of lower pole stones: prospective non-randomized outcomes from a university teaching hospital. Ther Adv Urol 14:17562872221097344
Aldoukhi AH, Ghani KR, Hall TL et al (2017) Thermal response to high-power holmium laser lithotripsy. J Endourol 31(12):1308–1312
Aldoukhi AH, Hall TL, Ghani KR et al (2018) caliceal fluid temperature during high-power holmium laser lithotripsy in an in vivo porcine model. J Endourol 32(8):724–729
Hein S, Petzold R, Schoenthaler M et al (2018) Thermal effects of Ho: YAG laser lithotripsy: real-time evaluation in an in vitro model. World J Urol 36(9):1469–1475
Maxwell AD, MacConaghy B, Harper JD et al (2019) Simulation of laser lithotripsy-induced heating in the urinary tract. J Endourol 33(2):113–119
Wollin DA, Carlos EC, Tom WR et al (2018) Effect of laser settings and irrigation rates on ureteral temperature during holmium laser lithotripsy, an in vitro model. J Endourol 32(1):59–63
Hein S, Petzold R, Suarez-Ibarrola R et al (2020) Thermal effects of Ho:YAG laser lithotripsy during retrograde intrarenal surgery and percutaneous nephrolithotomy in an ex vivo porcine kidney model. World J Urol 38(3):753–760
Marom R, Dau JJ, Hall TL et al (2023) Thermal safety boundaries for laser power and irrigation rate during ureteroscopy: in-vivo porcine assessment with a Ho:YAG laser. Urology. https://doi.org/10.1016/j.urology.2023.07.014
Wang X-K, Jiang Z-Q, Tan J et al (2019) Thermal effect of holmium laser lithotripsy under ureteroscopy. Chin Med J 132(16):2004–2007
U.S. Food & Drug Administration (FDA). Class 2 Device Recall Olympus. 2021. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfres/res.cfm?id=188172. Accessed 3 Jul 2023.
Okhunov Z, Jiang P, Afyouni AS et al (2021) Caveat emptor: the heat is “ON”-an in vivo evaluation of the thulium fiber laser and temperature changes in the porcine kidney during dusting and fragmentation modes. J Endourol 35(11):1716–1722
Marom R, Dau JJ, Hall TL et al (2023) Effect of outflow resistance on intrarenal pressure at different irrigation rates during ureteroscopy: in vivo evaluation. Urolithiasis 51(1):98
Sapareto SA, Dewey WC (1984) Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 10(6):787–800
Yarmolenko PS, Moon EJ, Landon C et al (2011) Thresholds for thermal damage to normal tissues: an update. Int J Hyperthermia 27(4):320–343
Aldoukhi AH, Dau JJ, Majdalany SE et al (2021) Patterns of laser activation during ureteroscopic lithotripsy: effects on caliceal fluid temperature and thermal dose. J Endourol 35(8):1217–1222
Louters MM, Dau JJ, Hall TL et al (2022) Laser operator duty cycle effect on temperature and thermal dose: in-vitro study. World J Urol 40(6):1575–1580
Dalal R, Bruss ZS, Sehdev JS (2022) Physiology, renal blood flow and filteration. StatPearls Publishing, Florida
Goldberg SN, Hahn PF, Tanabe KK et al (1998) Percutaneous radiofrequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J Vasc Interv Radiol 9(1 Pt 1):101–111
Pallone TL, Edwards A, Mattson DL (2012) Renal medullary circulation. Compr Physiol 2(1):97–140
Funding
Funding for this study was provided, in part, by a Faculty Catalyst Award, Department of Urology, University of Michigan, and, in part, by a research grant from Boston Scientific.
Author information
Authors and Affiliations
Contributions
R Marom: Project development, Data Collection, Manuscript writing, Data analysis. WW Roberts: Manuscript writing/editing, Data analysis, Critical Revision, Study management. JJ Dau: Manuscript editing. KR Ghani: Manuscript editing. TL Hall: Manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
K.R.G. has consulting relationships with Boston Scientific, Ambu, Olympus, Coloplast, and Karl Storz. W.W.R. has a consulting relationship with Boston Scientific. R.M., J.J.D., and T.L.H. have no disclosures.
Ethical approval
All procedures performed in the study involving animal subjects were in accordance with the ethical standards of the institutional research committee and were approved by the University of Michigan’s Institutional Animal Care and Use Committee (IACUC).
Disclaimer
The prototype ureteroscope used in this study was a concept device/technology, which was not available for sale at the time the study was conducted. Pre-clinical study results may not necessarily be indicative of clinical performance.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marom, R., Dau, J.J., Ghani, K.R. et al. Assessing renal tissue temperature changes and perfusion effects during laser activation in an in vivo porcine model. World J Urol 42, 197 (2024). https://doi.org/10.1007/s00345-024-04896-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00345-024-04896-4