Abstract
Purpose
To present our initial experience with periurethral adjustable continence therapy (ACT™) for urinary incontinence due to intrinsic sphincter deficiency (ISD) in children.
Methods
This is an approved prospective non-randomized pilot study (NCT03351634) aiming to treat children born with spinal dysraphism (SD) or exstrophy epispadias complex (EEC) with ACT™. Endpoints were patient-reported changes in daily pad count, 24-h Pad test and complications.
Results
Since April 2018, 13 children (six girls, seven boys) were implanted at the median age of 12 years (5–16). The etiology of incontinence was neurogenic ISD (7/13, 54%) and EEC (6/13, 46%).
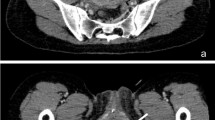
After ACT™ implantation, continence (no pad or 1 security pad/day) was achieved in 9(69%) patients (5/7 SD, 4/6 EEC). Additionally, two (15%) patients had a significant improvement (decreasing Pad test from 1049 to 310 g at 3 months). One patient (7%) had no improvement. Results were stable at 21 months (6–43) of follow-up. Mean final balloon volume was 2.89 ml (± 0.85) with a median of 3 fillings to obtain continence.
We had four revisions due to cutaneous port erosion (n = 3) and balloon migration (n = 1) and two definitive explantations. PinQ score was significantly improved (47 vs 40.5 with balloon, p = ns). Neither degradation of the upper urinary tract nor cystomanometric changes have been observed at 6 and 12 months postoperatively.
Conclusion
Urinary incontinence due to ISD owing to EEC or SD can be successfully treated with ACT™ periurethral balloons. Given the minimal invasiveness of this therapy, it might be a first‐line option treatment in children with complex stress urinary incontinence.

Similar content being viewed by others
Data Availability
Raw data are available through the clinical research direction of Marseille Public University Hospitals (APHM) who was the study instigator.
References
Rawashdeh YF, Austin P, Siggaard C et al (2012) International Children’s Continence Society’s recommendations for therapeutic intervention in congenital neuropathic bladder and bowel dysfunction in children. Neurourol Urodyn 31(5):615–620. https://doi.org/10.1002/nau.22248
Guys JM, Hery G, Haddad M, Borrionne C (2011) Neurogenic bladder in children: basic principles, new therapeutic trends. Scand J Surg 100(4):256–263. https://doi.org/10.1177/145749691110000405
Ludwikowski BM, Bieda JC, Lingnau A, González R (2019) Surgical management of neurogenic sphincter incompetence in children. Front Pediatr 7:97. https://doi.org/10.3389/fped.2019.00097
De Vocht TF, Chrzan R, Dik P, Klijn AJ, De Jong TPVM (2010) Long-term results of bulking agent injection for persistent incontinence in cases of neurogenic bladder dysfunction. J Urol 183(2):719–723. https://doi.org/10.1016/j.juro.2009.10.044
Maruf M, Manyevitch R, Michaud J et al (2020) Urinary continence outcomes in classic bladder exstrophy: a long-term perspective. J Urol 203(1):200–205. https://doi.org/10.1097/JU.0000000000000505
Johnston AW, Wiener JS, Todd PJ (2020) Pediatric neurogenic bladder and bowel dysfunction: will my child ever be out of diapers? Eur Urol Focus 6(5):838–867. https://doi.org/10.1016/j.euf.2020.01.003
Faure A, Hery G, Mille E et al (2017) Long-term efficacy of young-Dees bladder neck reconstruction: role of the associated bladder neck injection for the treatment of children with urinary incontinence. Urology 108:166–170. https://doi.org/10.1016/j.urology.2017.06.005
Alova I, Margaryan M, Bernuy M, Lortat-Jacob S, Lottmann HB (2012) Long-term effects of endoscopic injection of dextranomer/hyaluronic acid based implants for treatment of urinary incontinence in children with neurogenic bladder. J Urol 188(5):1905–1909. https://doi.org/10.1016/j.juro.2012.07.016
Castellan M, Gosalbez R, Labbie A, Ibrahim E, Disandro M (2005) Bladder neck sling for treatment of neurogenic incontinence in children with augmentation cystoplasty: long-term followup. J Urol 173(6):2128–2131. https://doi.org/10.1097/01.ju.0000157688.41223.d2. (discussion 2131)
Noordhoff TC, van den Hoek J, Yska MJ, Wolffenbuttel KP, Blok BFM, Scheepe JR (2019) Long-term follow-up of bladder outlet procedures in children with neurogenic urinary incontinence. J Pediatr Urol 15(1):35.e1-35.e8. https://doi.org/10.1016/j.jpurol.2018.08.018
Simeoni J, Guys JM, Mollard P et al (1996) Artificial urinary sphincter implantation for neurogenic bladder: a multi-institutional study in 107 children. Br J Urol 78(2):287–293. https://doi.org/10.1046/j.1464-410x.1996.06126.x
Castera R, Podestá ML, Ruarte A, Herrera M, Medel R (2001) 10-year experience with artificial urinary sphincter in children and adolescents. J Urol 165(6 Pt 2):2373–2376. https://doi.org/10.1097/00005392-200106001-00039
Herndon CDA, Rink RC, Shaw MBK et al (2003) The Indiana experience with artificial urinary sphincters in children and young adults. J Urol 169(2):650–654. https://doi.org/10.1097/01.ju.0000047320.28201.9d. (discussion 654)
Mehnert U, Bastien L, Denys P et al (2012) Treatment of neurogenic stress urinary incontinence using an adjustable continence device: 4-year followup. J Urol 188(6):2274–2280. https://doi.org/10.1016/j.juro.2012.07.131
Kaboré FA, Gaillet S, Blanc J et al (2014) Initial results of adjustable periurethral balloons (ACT™ and pro ACT™) in the treatment of adult stress urinary incontinence with intrinsic sphincter deficiency. Prog Urol 24(17):1132–1138. https://doi.org/10.1016/j.purol.2014.08.004
Phé V, Nguyen K, Rouprêt M, Cardot V, Parra J, Chartier-Kastler E (2014) A systematic review of the treatment for female stress urinary incontinence by ACT® balloon placement (Uromedica, Irvine, CA, USA). World J Urol 32(2):495–505. https://doi.org/10.1007/s00345-013-1117-0
Decter RM, Roth DR, Fishman IJ, Shabsigh R, Scott FB, Gonzales ET (1988) Use of the AS800 device in exstrophy and epispadias. J Urol 140(5 Pt 2):1202–1203. https://doi.org/10.1016/s0022-5347(17)42002-7
Chartier-Kastler E, Costa P, Ben Naoum K, Cour F, Le Normand L, Haab F (2007) French multicentre prospective study of the use of ACT balloons (Uromedica, Inc., Plymouth, Min, U.S.A.; Medtronic, Minneapolis, U.S.A.) for the treatment of female stress urinary incontinence. Prog Urol 17(7):1372–1377. https://doi.org/10.1016/s1166-7087(07)78580-9
Demeestere A, de Guerry ML, Bergot C et al (2022) Adjustable continence therapy (ACT®) balloons to treat neurogenic and non-neurogenic female urinary incontinence. Neurourol Urodyn 41(1):313–322. https://doi.org/10.1002/nau.24822
Paret F, Leclair MD, Karam G, Rigaud J, Baron M, Perrouin-Verbe MA (2023) Long-term results of artificial urinary sphincter implantation for urinary incontinence due to intrinsic sphincter deficiency in children. Neurourol Urodyn 42(1):355–365. https://doi.org/10.1002/nau.25106
Ruiz E, Puigdevall J, Moldes J et al (2006) 14 years of experience with the artificial urinary sphincter in children and adolescents without spina bifida. J Urol 176(4 Pt 2):1821–1825. https://doi.org/10.1016/j.juro.2006.05.024
Ronzi Y, Le Normand L, Chartier-Kastler E et al (2019) Neurogenic stress urinary incontinence: is there a place for Adjustable Continence Therapy (ACT™ and ProACT™, Uromedica, Plymouth, MN, USA)? A retrospective multicenter study. Spinal Cord 57(5):388–395. https://doi.org/10.1038/s41393-018-0219-3
Ammirati E, Manassero A, Giammò A, Carone R (2017) Management of male and female neurogenic stress urinary incontinence in spinal cord injured (SCI) patients using adjustable continence therapy. Urologia 84(3):165–168. https://doi.org/10.5301/uj.5000242
Musco S, Padilla-Fernández B, Del Popolo G et al (2018) Value of urodynamic findings in predicting upper urinary tract damage in neuro-urological patients: a systematic review. Neurourol Urodyn 37(5):1522–1540. https://doi.org/10.1002/nau.23501
Catti M, Lortat-Jacob S, Morineau M, Lottmann H (2008) Artificial urinary sphincter in children–voiding or emptying? An evaluation of functional results in 44 patients. J Urol 180(2):690–693. https://doi.org/10.1016/j.juro.2008.04.039. (discussion 693)
Bajeot AS, Brierre T, Beauval JB et al (2021) Survival analysis of adjustable continence therapy device (ACT®/proACT®): a new message for patients. Prog Urol 31(4):215–222. https://doi.org/10.1016/j.purol.2020.10.005
Acknowledgements
We wish to thank Timothy C. Cook who provided ten implantable devices units free of charge
Author information
Authors and Affiliations
Contributions
AF and GK designed the study and data collection tools, collected data for the study, wrote the statistical analysis plan, cleaned and analyzed the data, and drafted and revised the paper. MH collected data. JP, TM, JMG and FM revised the paper.
Corresponding author
Ethics declarations
Conflict of interest
AF, MH, JP, TM, JMG, FM declare that they have no conflicts of interest. GK has been proctor for Uromedica ACT/proACT and is investigator in an adult female ACT study supported by Uromedica.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (2016-AO1974-47) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was registered on ClinicalTrials.gov, and the registration number was NCT03351634.
Informed consent
Informed consent was submitted for each patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Faure, A., Haddad, M., Pinol, J. et al. Initial experience with ACT™ periurethral adjustable balloons to treat urinary incontinence due to intrinsic sphincter deficiency in the pediatric population. World J Urol 41, 2767–2774 (2023). https://doi.org/10.1007/s00345-023-04550-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-023-04550-5