Abstract
Purpose
There is conflicting evidence on the association between asbestos exposure and bladder cancer. We performed a systematic review and meta-analysis to provide evidence on occupational asbestos exposure and the risk of mortality and incidence of bladder cancer.
Methods
We searched three relevant electronic databases (Pubmed, Scopus, and Embase) from inception to October 2021. The methodological quality of included articles was evaluated using the US National Institutes of Health tool. Standardized incidence ratios (SIRs) and standardized mortality ratios (SMRs) for bladder cancer, as well as respective 95% confidence intervals (CIs), were extracted or calculated for each included cohort. Main and subgroup meta-analyses according to first year of employment, industry, sex, asbestos type, and geographic region were performed.
Results
Fifty-nine publications comprising 60 cohorts were included. Bladder cancer incidence and mortality were not significantly associated with occupational asbestos exposure (pooled SIR: 1.04, 95% CI: 0.95–1.13, P = 0.000; pooled SMR: 1.06, 95% CI: 0.96–1.17, P = 0.031). Bladder cancer incidence was higher among workers employed between 1908 and 1940 (SIR: 1.15, 95% CI: 1.01–1.31). Mortality was elevated in asbestos workers cohorts (SMR: 1.12, 95% CI: 1.06–1.30) and in the subgroup analysis for women (SMR: 1.83, 95% CI: 1.22–2.75). No association was found between asbestos types and bladder cancer incidence or mortality. We observed no difference in the subgroup analysis for countries and no direct publication bias evidence.
Conclusion
There is evidence that workers with occupational asbestos exposure have a bladder cancer incidence and mortality similar to the general population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term asbestos comprises a group of natural minerals that form long, thin fibers when they crystallize [1]. Asbestos fibers tend to possess good strength properties (e.g. high tensile strength, wear and friction characteristics), flexibility (e.g. the ability to be woven), excellent thermal properties (e.g. heat stability and thermal, electrical and acoustic insulation), absorption capacity and resistance to chemical, thermal and biological degradation. Owing to its properties asbestos has been widely used worldwide and the story of this mineral was one of progressive commercial success until the mid-twentieth century [2]. The range of applications in which asbestos has been used includes roofing, thermal and electrical insulation, concrete pipes and sheets, flooring, gaskets, friction materials, coating and compounds, plastics, textiles, paper, mastics, thread, fiber jointing, and millboard. As the health risks associated with asbestos became increasingly recognized, its use began to decline. Despite widespread knowledge of the hazards of asbestos and bans on any use of asbestos in many countries, an estimated 1 million tons of this mineral was used around the world in 2020 [3].
In addition to the well-known association of asbestos with mesothelioma [4] and lung cancer [5], asbestos minerals have also been associated with ovarian [6], laryngeal [7] and gastrointestinal tract cancers [8]. Regarding other cancer sites, some epidemiological studies have reported an association between occupational exposure to asbestos and increased incidence of and mortality from bladder cancer [9,10,11]. Furthermore, asbestos fibers have been detected in tissue samples of bladder cancer patients affected by pulmonary asbestosis [12].
To our knowledge, no previous systematic reviews have been conducted on occupational asbestos exposure and the risk of mortality and incidence of bladder cancer. Thus, we aimed to perform a systematic review and meta-analysis to investigate this association.
Materials and methods
This systematic review and meta-analysis was performed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [13].
Search strategy
Three different databases were searched: Medline (PubMed), Scopus and Ovid (Embase). Firstly, a comprehensive search strategy was created using the following terms: "Neoplasms”, “Carcinoma”, “Asbestos”, “Amosite", “Crocidolite”, “Amphibole”, “Serpentine”, “Asbestosis". Next, a specific search strategy was performed adding the term “Bladder”. Results were restricted to studies conducted on humans, while no limits were applied for language. The databases were searched from inception to October 20, 2020. The resulting papers were hand screened and relevant references were evaluated to find any further relevant papers. Database searches were conducted with the aid of an expert librarian to ensure completeness and rigor. The search strategies for each database are given in Online Information 1.
Inclusion and exclusion criteria
Only full articles published in peer-reviewed journals were considered. Cohort-studies and nested case-control studies of workers with a respiratory occupational exposure to asbestos in all industries and occupations were included. Cohorts of workers who were exposed to asbestos through ingestion only and had doubtful exposure to asbestos were excluded. We included all studies conducted on workers employed in industries or occupations in which asbestos exposure was considered substantial, such as asbestos workers, miners and millers, and shipyards. For studies of workers for whom occupational exposure to asbestos was possible (e.g. electricity workers or chimney sweeps [10, 14]), we used the criterion of a standardized mortality ratio (SMR) or standardized incidence ratio (SIR) for mesothelioma > 2 as marker of significant exposure, leading to the retention of the study for the meta-analysis. Descriptive studies, other systematic reviews or meta-analyses, community-based studies (either of cohort or case-control design), as well as conference proceedings, theses, and letters to the editor were excluded. Articles for which the full text was not available either online or by direct request to the journal in which they were published were also excluded. Two reviewers independently assessed the papers against the inclusion and exclusion criteria. Disagreement was solved by discussion.
Data extraction
For each mortality study we extracted the SMR for bladder cancer and its 95% confidence interval (CI); when these measures were not directly available from publications, but raw data were reported, we calculated them. Similarly, we abstracted, or calculated if not specified in the text, the SIR and its corresponding CI for cancer incidence studies. If 90% CI was reported, it was converted to 95% CI. Results of internal analysis (e.g., based on hazard ratios) were used for dose-response. In cases of multiple reports from the same cohort, the most comprehensive results (i.e. those based on the largest number of cases) were used.
The following study characteristics were also extracted where available: publication year, study design, country, cohort size (or number of cases and controls), number of person-years, duration of employment, duration of follow-up, minimum time of exposure, duration of exposure, type of outcome (incidence or mortality). Data were extracted independently by two reviewers and any disagreement was solved by a third reviewer.
Quality assessment
The methodological quality of the included studies was assessed through the National Institute of Health quality assessment tool for each study design [15]. The tools evaluate the presence of potential sources of bias, confounding factors, study power, and the strength of the association between the exposure and the outcome. The quality of the articles was rated as poor (score <9), fair (score = 9) and good (score >9). Quality assessment was performed by two independent reviewers, and results were discussed with the other authors until reaching consensus.
Statistical analysis
The main analysis included results for ever vs. never asbestos exposure. Results across studies were combined, separately for studies of bladder cancer incidence and mortality, using random-effect models meta-analyses [16] based on the log-transformation of the SMR/SIR and its standard error. Inter-study heterogeneity was evaluated with the I2 test [17]. Stratified meta-analyses were conducted to explore potential sources of heterogeneity, by sex (>90% males, >90% females and <90% for both), period of first employment of cohort members (1908–1940, 1941–1949 and 1950–1993), type of asbestos (amphiboles, chrysotile, mixed and unspecified type), geographic region (Europe, UK, North America, Australia and Asia), and quality score (poor, fair and good). Finally, we assessed the presence of publication bias by visual inspection of the funnel plot and applying the test proposed by Egger et al. [18]. A meta-regression was performed to assess the association between the duration of asbestos exposure and bladder cancer. For studies reporting multiple SMR or SIR for different durations of exposure, a single meta-regression was performed and the results were then meta-analyzed.
Results
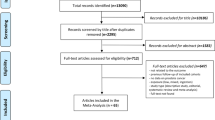
A total of 13,267 articles were retrieved from Medline, Scopus, and Embase databases. After reviewing the titles, 2948 articles were considered potentially relevant, and after duplicates were removed, 2379 articles remained. Of these, 1639 articles were discarded following a review of the abstract. The full texts of the remaining 740 articles were examined in detail and assessed against the inclusion and exclusion criteria; 643 did not meet the inclusion criteria as described, and the full text was not available for a further 38. A manual search of the reference lists of included articles did not reveal any additional pertinent studies. Thus, 59 articles [9,10,11, 14, 19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73] met the inclusion criteria for the systematic review (Online Information 2).
The number of workers in each cohort ranged from 88 [65] to 160,640 [41], the number of incidences of bladder cancer per cohort varied from four [45] to 1257 [14], and that of bladder cancer deaths from zero [65] to 310 [9]. Bladder cancer incidence was evaluated in 26 cohorts, bladder cancer mortality was evaluated in 38, and both outcomes were reported in four cohorts [23, 24, 29, 63, 64]. The total number of bladder cancer cases and deaths across all articles was 3596 (out of 525,585 subjects) and 1169 (out of 385,552 subjects), with a follow-up duration ranging from five [59] to 88 years [10]. Selected characteristics of the cohorts are reported in Table 1.
Meta-analysis
The results of the random-effects meta-analysis showed that bladder cancer incidence and mortality were not associated with occupational asbestos exposure. Results are shown in Figure 1a and b respectively (pooled SIR: 1.04, 95% CI: 0.95–1.13, P=0.000; pooled SMR: 1.06, 95% CI: 0.96–1.17, P=0.031). Both the pooled SIR and the pooled SMR showed significant heterogeneity (P<=0.001; I2=72.9%; P=0.031; I2=32.6%, respectively). One of the two cohorts analyzed in Tomioka et al. 2011 [65] could not be included in the meta-analysis because it reported no bladder cancer deaths (SMR: 0.00, 95%CI: 0.00–15.50).
a Forest plot of the pooled standardized incidence ratio and 95% confidence intervals of urinary bladder cancer incidence associated with occupational asbestos exposure, using random-effect models. b Forest plot of the pooled standardized mortality ratio and 95% confidence intervals of urinary bladder cancer mortality associated with occupational asbestos exposure, using random-effect models
Subgroup analysis stratified by first year of employment, industry, asbestos type, and geographic region showed that bladder cancer incidence increased significantly among workers employed between 1908 and 1940 (SIR: 1.15, 95% CI: 1.01–1.313) and bladder cancer mortality increased among women (SMR: 1.83, 95% CI: 1.22–2.75) and asbestos workers (SMR: 1.12, 95% CI: 1.06–1.30) (Table 2)
Meta-analysis of meta-regressions was possible for 5 studies. The RRs for each year of exposure were 0.97 (CI: 0.905–1.041) and 1.019 (CI: 0.997–1.041) for mortality and incidence respectively.
Funnel plots indicated no obvious outliers, and no evidence of publication bias was observed for either bladder cancer incidence or mortality. No small-study effect was found for mortality (p = 0.984) and incidence (p = 0.824) (Online Information 3).
Discussion
We investigated the relation between occupational asbestos exposure and risk of bladder cancer with a meta-analysis of the results obtained from a wide systematic review. Incidence of and mortality from bladder cancer were not significantly increased among asbestos-exposed workers (pooled SIR: 1.04, 95% CI: 0.95–1.13, P=0.000; pooled SMR: 1.06, 95% CI: 0.96–1.17, P=0.031). These results were in line with the largest studies done on asbestos workers [14, 20, 21, 29, 47, 64], and were confirmed in subgroup analyses stratified by type of asbestos fibers, country and quality assessment.
Also, from a biological point of view, there is no evidence of a reasonable mechanism to elucidate how respiratory asbestos fibers could reach the bladder. In the literature, there are no established physio-pathological pathways nor pathological evidence that could explain increased bladder cancer incidence or mortality among workers exposed to respiratory asbestos fibers.
When stratifying by year of first exposure, an increased risk of bladder cancer was found for workers employed between 1908 and 1940. This result should be interpreted with caution because it derives from multiple stratified analyses.
Our results showed that female workers had a higher bladder cancer mortality rate. This result was based on five studies comprising a total of 27 observed deaths. The pooled result was greatly influenced by the article by Ferrante et al. [9], and was not confirmed in the parallel analysis of cancer incidence among women. Ferrante et al. reported that their preliminary analyses suggested that the risk of bladder cancer was concentrated in the industrial sectors where asbestos exposure was associated with combustion fumes and other agents related to metalworking and painting. These industries had been classified in relation to carcinogenic risk, including bladder cancer [74, 75]. Furthermore, in four out of five studies no data on smoking habits, the most important environmental risk factor for bladder cancer, were considered. Cigarette smoking prevalence is high among women working in the construction industry and in construction and extraction occupations, as shown in a study done by Mazurek et al. in the United States [76]. Also, the higher bladder cancer mortality in the asbestos workers sub-cohort can probably be attributed to the increased smoking habit rates in this subgroup compared to the general population, as shown in a recent study by Frost et al. [77].
Regarding the major influence of tobacco smoke in the etiopathogenesis of bladder cancer, results from a recent meta-analysis [78] delineate a pooled relative risk of bladder cancer disease-specific mortality of 1.47 (95% CI: 1.24–1.75) for all smokers. In line with this result, a combined analysis of 11 case-control studies from Europe shows that the proportion of bladder cancer cases among women attributable to ever smoking was 0.30 [79].
Furthermore, for other organs there is no evidence of a difference between men and women in the development of asbestos-related diseases. This datum could suggest that the excess in bladder cancer risk for women only is not likely to represent a causal association.
This systematic review and meta-analysis suffers from some limitations. Information about smoking habits of workers, which is the main risk factor for bladder cancer and a potential confounder, is lacking from the studies we reviewed. Thus, the pooled SMR and SIR could be overestimated.
Another limitation is the lack of quantitative data on asbestos exposure and duration of the employment in most cohorts. To analyze the dose-response effect we considered the duration of the employment as a proxy for the dose, but only 12 out 60 studies provided this datum. However, the meta-regression resulted in an absence of a dose-response effect.
Conclusions
Our meta-analysis provides evidence that workers with occupational asbestos exposure have a bladder cancer incidence and mortality rate similar to the general population.
Excesses in bladder cancer risk in selected groups of workers, and in particular women, are not likely to represent causal associations; however, further studies are needed to evaluate whether female workers are more likely to develop bladder cancer when occupationally exposed to asbestos.
Due to the limited data on the duration of the exposure, it is not clear whether length of employment has a significant role in bladder cancer.
Data availability
The data that support the findings of this study are available from the corresponding author, MC, upon reasonable request.
References
Pira E, Donato F, Maida L, Discalzi G (2018) Exposure to asbestos: past, present and future. J Thorac Dis 10:S237–S245. https://doi.org/10.21037/jtd.2017.10.126
Seaton A (1995) Asbestos: past, present and future. Schweiz Med Wochenschr 125:453–457
U.S. Geological Survey (2021) Mineral commodity summaries 2021
Boffetta P, Donato F, Pira E et al (2019) Risk of mesothelioma after cessation of asbestos exposure: a systematic review and meta-regression. Int Arch Occup Environ Health 92:949–957. https://doi.org/10.1007/s00420-019-01433-4
Klebe S, Leigh J, Henderson DW, Nurminen M (2019) Asbestos, smoking and lung cancer: an update. Int J Environ Res Pub Health 17(1):258. https://doi.org/10.3390/ijerph17010258
Camargo MC, Stayner LT, Straif K et al (2011) Occupational exposure to asbestos and ovarian cancer: a meta-analysis. Environ Health Perspect 119:1211–1217. https://doi.org/10.1289/ehp.1003283
Hall AL, Kromhout H, Schüz J et al (2020) Laryngeal cancer risks in workers exposed to lung carcinogens: exposure-effect analyses using a quantitative job exposure matrix. Epidemiology 31:145–154. https://doi.org/10.1097/EDE.0000000000001120
Kwak K, Paek D, Zoh KE (2019) Exposure to asbestos and the risk of colorectal cancer mortality: a systematic review and meta-analysis. Occup Environ Med 76:861–871. https://doi.org/10.1136/oemed-2019-105735
Ferrante D, Chellini E, Merler E et al (2017) Italian pool of asbestos workers cohorts: mortality trends of asbestos-related neoplasms after long time since first exposure. Occup Environ Med 74:887–898. https://doi.org/10.1136/oemed-2016-104100
Hogstedt C, Jansson C, Hugosson M et al (2013) Cancer incidence in a cohort of Swedish chimney sweeps, 1958–2006. Am J Pub Health 103:1708–1714. https://doi.org/10.2105/AJPH.2012.300860
Richardson DB, Wing S, Keil A, Wolf S (2013) Mortality among workers at Oak Ridge National Laboratory. Am J Ind Med 56:725–732. https://doi.org/10.1002/ajim.22164
Molinini R, Paoletti L, Albrizio M et al (1992) Occupational exposure to asbestos and urinary bladder cancer. Environ Res 58:176–183. https://doi.org/10.1016/s0013-9351(05)80213-0
Moher D, Shamseer L, Clarke M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1. https://doi.org/10.1186/2046-4053-4-1
Sorahan TM (2019) Cancer incidence in UK electricity generation and transmission workers, 1973–2015. Occup Med (Chic Ill) 69:342–351. https://doi.org/10.1093/occmed/kqz082
NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
DerSimonian R, Laird N (2015) Meta-analysis in clinical trials revisited. Contemp Clin Trials 45:139–145. https://doi.org/10.1016/j.cct.2015.09.002
Higgins JPT, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558. https://doi.org/10.1002/sim.1186
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634. https://doi.org/10.1136/bmj.315.7109.629
Acheson ED, Gardner MJ, Winter PD, Bennett C (1984) Cancer in a factory using amosite asbestos. Int J Epidemiol 13:3–10. https://doi.org/10.1093/ije/13.1.3
Allen EM, Alexander BH, MacLehose RF et al (2015) Cancer incidence among Minnesota taconite mining industry workers. Ann Epidemiol 25:811–815. https://doi.org/10.1016/j.annepidem.2015.08.003
Andersson E, Westberg H, Bryngelsson I-L et al (2013) Cancer incidence among Swedish pulp and paper mill workers: a cohort study of sulphate and sulphite mills. Int Arch Occup Environ Health 86:529–540. https://doi.org/10.1007/s00420-012-0785-1
Band PR, Le ND, Fang R et al (2001) Cohort cancer incidence among pulp and paper mill workers in British Columbia. Scand J Work Environ Health 27:113–119. https://doi.org/10.5271/sjweh.597
Barbiero F, Zanin T, Pisa FE et al (2018) Cancer incidence in a cohort of asbestos-exposed workers undergoing health surveillance. Int Arch Occup Environ Health 91:831–841. https://doi.org/10.1007/s00420-018-1326-3
Barbiero F, Zanin T, Pisa FE et al (2018) Mortality in a cohort of asbestos-exposed workers undergoing health surveillance. Med Lav 109:83–86. https://doi.org/10.23749/mdl.v109i2.5865
Berry G, Newhouse ML, Wagner JC (2000) Mortality from all cancers of asbestos factory workers in east London 1933–80. Occup Environ Med 57:782–785. https://doi.org/10.1136/oem.57.11.782
Bertolotti M, Ferrante D, Mirabelli D et al (2008) Mortality in the cohort of the asbestos cement workers in the Eternit plant in Casale Monferrato (Italy). Epidemiol Prev 32:218–28
Bonneterre V, Mathern G, Pelen O et al (2012) Cancer incidence in a chlorochemical plant in Isère, France: an occupational cohort study, 1979–2002. Am J Ind Med 55:756–767. https://doi.org/10.1002/ajim.22069
Clemmesen J, Hjalgrim-Jensen S (1981) Cancer incidence among 5686 asbestos-cement workers followed from 1943 through 1976. Ecotoxicol Environ Saf 5:15–23. https://doi.org/10.1016/0147-6513(81)90042-7
Daniels RD, Kubale TL, Yiin JH et al (2014) Mortality and cancer incidence in a pooled cohort of US firefighters from San Francisco, Chicago and Philadelphia (1950–2009). Occup Environ Med 71:388–397. https://doi.org/10.1136/oemed-2013-101662
Danielsen TE, Langård S, Andersen A, Knudsen O (1993) Incidence of cancer among welders of mild steel and other shipyard workers. Br J Ind Med 50:1097–1103. https://doi.org/10.1136/oem.50.12.1097
Enterline PE, Hartley J, Henderson V (1987) Asbestos and cancer: a cohort followed up to death. Br J Ind Med 44:396–401. https://doi.org/10.1136/oem.44.6.396
Fazzo L, Cernigliaro A, De Santis M et al (2020) Occupational cohort study of asbestos-cement workers in a contaminated site in Sicily (Italy). Epidemiol Prev 44:137–144. https://doi.org/10.19191/EP20.2-3.P137.036
Finkelstein MM, Verma DK (2004) A cohort study of mortality among Ontario pipe trades workers. Occup Environ Med 61:736–742. https://doi.org/10.1136/oem.2003.011916
Habib RR, Abdallah SM, Law M, Kaldor J (2005) Mortality rates among nuclear industry workers at Lucas Heights Science and Technology Centre. Aust N Z J Pub Health 29:229–237. https://doi.org/10.1111/j.1467-842x.2005.tb00760.x
Hughes JM, Weill H, Hammad YY (1987) Mortality of workers employed in two asbestos cement manufacturing plants. Br J Ind Med 44:161–174. https://doi.org/10.1136/oem.44.3.161
Johansen C, Olsen JH (1998) Risk of cancer among Danish utility workers–a nationwide cohort study. Am J Epidemiol 147:548–555. https://doi.org/10.1093/oxfordjournals.aje.a009486
Koskinen K, Pukkala E, Reijula K, Karjalainen A (2003) Incidence of cancer among the participants of the Finnish Asbestos Screening Campaign. Scand J Work Environ Health 29:64–70. https://doi.org/10.5271/sjweh.706
Koutros S, Lubin JH, Graubard BI et al (2019) Extended mortality follow-up of a Cohort of 25,460 workers exposed to Acrylonitrile. Am J Epidemiol 188:1484–1492. https://doi.org/10.1093/aje/kwz086
Langseth H, Andersen A (2000) Cancer incidence among male pulp and paper workers in Norway. Scand J Work Environ Health 26:99–105. https://doi.org/10.5271/sjweh.518
Levin JL, Rouk A, Shepherd S et al (2016) Tyler asbestos workers: A mortality update in a cohort exposed to amosite. J Toxicol Environ Health B Crit Rev 19:190–200. https://doi.org/10.1080/10937404.2016.1195319
Lin CK, Chang YY, Wang JD, Lee LJH (2015) Increased standardised incidence ratio of malignant pleural mesothelioma in taiwanese asbestos workers: A 29-Year Retrospective Cohort Study. Biomed Res Int 2015:678598. https://doi.org/10.1155/2015/678598
McDonald JC, Liddell FD, Dufresne A, McDonald AD (1993) The 1891–1920 birth cohort of Quebec chrysotile miners and millers: mortality 1976–88. Br J Ind Med 50:1073–1081. https://doi.org/10.1136/oem.50.12.1073
Menegozzo S, Comba P, Ferrante D et al (2011) Mortality study in an asbestos cement factory in Naples, Italy. Annal Ist Super Sanita 47:296–304. https://doi.org/10.4415/ANN_11_03_10
Merlo DF, Bruzzone M, Bruzzi P et al (2018) Mortality among workers exposed to asbestos at the shipyard of Genoa, Italy: a 55 years follow-up. Environ Health 17:94. https://doi.org/10.1186/s12940-018-0439-1
Meurman LO, Pukkala E, Hakama M (1994) Incidence of cancer among anthophyllite asbestos miners in Finland. Occup Environ Med 51:421–425. https://doi.org/10.1136/oem.51.6.421
Michaels D, Zoloth S (1988) Asbestos disease in sheet metal workers: proportional mortality update. Am J Ind Med 13:731–734. https://doi.org/10.1002/ajim.4700130612
Nichols L, Sorahan T (2005) Mortality of UK electricity generation and transmission workers, 1973–2002. Occup Med (Lond) 55:541–548. https://doi.org/10.1093/occmed/kqi157
Nokso-Koivisto P, Pukkala E (1994) Past exposure to asbestos and combustion products and incidence of cancer among Finnish locomotive drivers. Occup Environ Med 51:330–334. https://doi.org/10.1136/oem.51.5.330
Parducci DA, Puccetti M, Bianchi Martini L et al (2005) Mortality among workers in a cigarette factory in Lucca (Tuscany). Epidemiol Prev 29:271–7
Pesch B, Taeger D, Johnen G et al (2010) Cancer mortality in a surveillance cohort of German males formerly exposed to asbestos. Int J Hyg Environ Health 213:44–51. https://doi.org/10.1016/j.ijheh.2009.09.001
Pira E, Turbiglio M, Maroni M et al (1999) Mortality among workers in the geothermal power plants at Larderello, Italy. Am J Ind Med 35:536–539. https://doi.org/10.1002/(sici)1097-0274(199905)35:5%3c536::aid-ajim12%3e3.0.co;2-g
Pira E, Romano C, Violante FS et al (2016) Updated mortality study of a cohort of asbestos textile workers. Cancer Med 5:2623–2628. https://doi.org/10.1002/cam4.824
Pira E, Romano C, Donato F et al (2017) Mortality from cancer and other causes among Italian chrysotile asbestos miners. Occup Environ Med 74:558–563. https://doi.org/10.1136/oemed-2016-103673
Raffn E, Lynge E, Juel K, Korsgaard B (1989) Incidence of cancer and mortality among employees in the asbestos cement industry in Denmark. Br J Ind Med 46:90–96. https://doi.org/10.1136/oem.46.2.90
Rafnsson V, Sulem P (2003) Cancer incidence among marine engineers, a population-based study (Iceland). Cancer Causes Control 14:29–35. https://doi.org/10.1023/a:1022505308892
Rapiti E, Turi E, Forastiere F et al (1992) A mortality cohort study of seamen in Italy. Am J Ind Med 21:863–872. https://doi.org/10.1002/ajim.4700210609
Richardson DB, Wing S, Wolf S (2007) Mortality among workers at the Savannah River Site. Am J Ind Med 50:881–891. https://doi.org/10.1002/ajim.20511
Rønneberg A, Haldorsen T, Romundstad P, Andersen A (1999) Occupational exposure and cancer incidence among workers from an aluminum smelter in western Norway. Scand J Work Environ Health 25:207–214. https://doi.org/10.5271/sjweh.425
Sandén A, Järvholm B (1987) Cancer morbidity in Swedish shipyard workers 1978–1983. Int Arch Occup Environ Health 59:455–462. https://doi.org/10.1007/BF00377839
Schnatter AR, Wojcik NC, Jorgensen G (2019) Mortality Update of a Cohort of Canadian Petroleum Workers. J Occup Environ Med 61:225–238. https://doi.org/10.1097/JOM.0000000000001523
Selikoff IJ, Seidman H (1991) Asbestos-associated deaths among insulation workers in the United States and Canada, 1967–1987. Ann N Y Acad Sci 643:1–14. https://doi.org/10.1111/j.1749-6632.1991.tb24439.x
Seidman H, Selikoff IJ, Gelb SK (1986) Mortality experience of amosite asbestos factory workers: dose-response relationships 5 to 40 years after onset of short-term work exposure. Am J Ind Med 10:479–514. https://doi.org/10.1002/ajim.4700100506
Simonato L, Fletcher AC, Andersen A et al (1991) A historical prospective study of European stainless steel, mild steel, and shipyard welders. Br J Ind Med 48:145–154. https://doi.org/10.1136/oem.48.3.145
Sorahan T (2007) Mortality of UK oil refinery and petroleum distribution workers, 1951–2003. Occup Med (Lond) 57:177–185. https://doi.org/10.1093/occmed/kql168
Tomioka K, Natori Y, Kumagai S, Kurumatani N (2011) An updated historical cohort mortality study of workers exposed to asbestos in a refitting shipyard, 1947–2007. Int Arch Occup Environ Health 84:959–967. https://doi.org/10.1007/s00420-011-0655-2
Tsai SP, Ahmed FS, Wendt JK et al (2007) A 56-year mortality follow-up of Texas petroleum refinery and chemical employees, 1948–2003. J Occup Environ Med 49:557–567. https://doi.org/10.1097/JOM.0b013e318057777c
Tulchinsky TH, Ginsberg GM, Iscovich J et al (1999) Cancer in ex-asbestos cement workers in Israel, 1953–1992. Am J Ind Med 35:1–8. https://doi.org/10.1002/(sici)1097-0274(199901)35:1%3c1::aid-ajim1%3e3.0.co;2-5
Ulvestad B, Kjaerheim K, Martinsen JI et al (2002) Cancer incidence among workers in the asbestos-cement producing industry in Norway. Scand J Work Environ Health 28:411–417. https://doi.org/10.5271/sjweh.693
Ulvestad B, Kjaerheim K, Martinsen JI et al (2004) Cancer incidence among members of the Norwegian trade union of insulation workers. J Occup Environ Med 46:84–89. https://doi.org/10.1097/01.jom.0000105981.46987.42
Van den Borre L, Deboosere P (2015) Enduring health effects of asbestos use in Belgian industries: a record-linked cohort study of cause-specific mortality (2001–2009). BMJ Open 5:e007384. https://doi.org/10.1136/bmjopen-2014-007384
Wang X, Lin S, Yu I et al (2013) Cause-specific mortality in a Chinese chrysotile textile worker cohort. Cancer Sci 104:245–249. https://doi.org/10.1111/cas.12060
Wilczyńska U, Szymczak W, Szeszenia-Dabrowska N (2005) Mortality from malignant neoplasms among workers of an asbestos processing plant in Poland: results of prolonged observation. Int J Occup Med Environ Health 18:313–326
Wu W-T, Lin Y-J, Shiue H-S et al (2014) Cancer incidence of Taiwanese shipbreaking workers who have been potentially exposed to asbestos. Environ Res 132:370–378. https://doi.org/10.1016/j.envres.2014.04.026
IARC (International Agency for Research on Cancer) (1984) Monographs on the evaluation of carcinogenic risks to humans, Volume 34, Polynuclear aromatic compounds, Part 3: Industrial exposures in aluminum production, goal gasification, coke production, and iron and steel founding. Lyon
IARC (International Agency for Research on Cancer) (1989) Monographs on the Evaluation of the Carcinogenic Risks to Human. Occupational exposures in paint manufacture and painting. Lyon
Mazurek JM, England LJ (2016) Cigarette smoking among working women of reproductive Age-United States, 2009–2013. Nicotine Tob Res 18:894–899. https://doi.org/10.1093/ntr/ntv292
Frost G, Darnton A, Harding A-H (2011) The effect of smoking on the risk of lung cancer mortality for asbestos workers in Great Britain (1971–2005). Ann Occup Hyg 55:239–247. https://doi.org/10.1093/annhyg/meq089
Cumberbatch MG, Rota M, Catto JWF, La Vecchia C (2016) The role of tobacco smoke in bladder and kidney carcinogenesis: a comparison of exposures and meta-analysis of incidence and mortality risks. Eur Urol 70:458–466. https://doi.org/10.1016/j.eururo.2015.06.042
Brennan P, Bogillot O, Greiser E et al (2001) The contribution of cigarette smoking to bladder cancer in women (pooled European data). Cancer Causes Control 12:411–417. https://doi.org/10.1023/a:1011214222810
Funding
Open access funding provided by Università degli Studi di Torino within the CRUI-CARE Agreement. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation was performed by NF, CZ, and AG. Data collection and analysis were performed by AG, MC, and PB. The first draft of the manuscript was written by NF, AG, MC, and PB. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Paolo Boffetta was involved in litigation on asbestos exposure. Enrico Pira has acted as Court-appointed expert witness and as consultant to parties (judge, prosecutor, and defendant attorney) in asbestos litigations. All other authors reported no conflict of interest.
Ethical approval
Ethical approval was not needed because data were extracted from primary published studies in which informed consent was obtained by investigators.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Franco, N., Godono, A., Clari, M. et al. Occupational asbestos exposure and urinary bladder cancer: a systematic review and meta-analysis. World J Urol 41, 1005–1015 (2023). https://doi.org/10.1007/s00345-023-04327-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-023-04327-w