Abstract
Introduction
Treatment advances in metastatic renal cell carcinoma (mRCC) have improved overall survival (OS) in mRCC patients over the last two decades. This single center retrospective analysis assesses if the purported survival benefits are also applicable in elderly mRCC patients.
Methods
401 patients with mRCC treated at Hannover Medical School from 01/2003–05/2016 were identified and evaluated by chart review. Treatment periods were defined as 01.01.2003–31.12.2009 (P1) and 01.01.2010–31.05.2016 (P2). Age groups were defined according to WHO classes (≤ 60 years: younger, > 60–75 years: elderly and > 75 years: old). Descriptive statistics, Kaplan–Meier analysis and logistic regression were performed.
Results
Median OS improved from 35.1 months in P1 to 59.1 months in P2. Sub-division into the respective age groups revealed median survival of 38.1 (95%-CI: 28.6–47.6) months in younger patients, 42.9 (95%-CI: 29.5–56.3) months among elderly patients and 27.3 (95%-CI: 12.8–41.8) months among old patients. Risk reduction for death between periods was most evident among old patients (young: HR 0.71 (95%-CI: 0.45–1.13, p = 0.2); elderly: HR 0.62 (95%-CI: 0.40–0.97, p = 0.04); old: HR 0.43 (95%-CI: 0.18–1.05, p = 0.06)). Age ≥ 75 years was an independent risk factor for death in P1 but not in P2.
Conclusion
Improved OS in the targeted treatment period was confirmed. Surprisingly elderly and old patients seem to profit the most form expansion of therapeutic armamentarium, within the TKI-dominated observation period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
400.000 patients have been diagnosed worldwide with renal cell carcinoma (RCC) in 2018 [1]. Currently the mean age of RCC diagnosis in Germany is 70 years, and patients often exhibit significant comorbidity or fragility [2].
Twenty years ago, mRCC treatment options were limited to interferon and IL-2 conferring a median overall survival (OS) of approximately 13 months [3]. Collectively, Sunitinib and Sorafenib changed the medical treatment landscape, introducing the decade of the VEGF-R-Tyrosine kinase inhibition (TKI) [4]. Checkpoint inhibitors (CPI) have also entered the fray, with nivolumab being approved and demonstrating for the first time an OS benefit with second line treatments [5, 6]. Developing strategies further, CPI-doublet and CPI/TKI combination therapies have entered clinic practice, moving towards a new post-TKI-Mono-therapy era [7, 8].
It has, however, been well-documented, that both combined or sequential use of the aforementioned agents is accompanied by a variety of adverse events. This may be particularly pertinent in elderly patients, who not only may be particularly susceptible to chronic toxicity but also traditionally underrepresented in the relevant clinical trials. It remains largely unknown if older patients benefit in terms of OS from these targeted treatments, or improved numbers of therapeutic options. Data in other forms of cancer suggest that treatment advances do not necessarily improve OS in older sub-populations [9].
Patients ≥ 65 years represent only 30–40% of study populations in most pivotal phase 3 trials [10]. This is of particular relevance in RCC, given that the median age of diagnosis is around 70 years [2]. Therefore, here we evaluate if elderly patients demonstrate similar benefits in OS following the introduction of these various novel treatments, mainly with an observation period wherein therapeutic landscape was mainly enriched by TKI.
Patients and methods
Study design and data acquisition
All mRCC patients treated at our center (Department of Hematology, Hemostasis, Oncology and Stem Cell Transplantation, Hannover Medical School, Hannover) between 1 January 2003 until 31 May 2016 were identified. Patient and tumor characteristics, along with treatment data were evaluated by chart review. Inclusion criteria were: Age > 18 years, date of diagnosis of mRCC, application of first line treatment with targeted therapy, documented adherence to respective treatment guidelines and local standards of care.
Data were anonymized prior to storage and assessmentwas done by physicians and data managers in accordance to the both recommendations of the local ethics committee (No: 3171-2016) as well as the declaration of Helsinki in its latest version. Last follow up was performed on 31.05.2016. Data were stored in a Microsoft Access database. After documentation plausibility control was performed.
Statistical analyses
Patients were divided into two time-dependent subgroups for comparison: those commencing treatment between 01.01.2003 and 31.12.2009 (period 1) and those starting between 01.01.2010 and 31.05.2016 (period 2). Period cut-offs were arbitrarily chosen to generate two samples of comparable size, but also reflecting to some extend the market authorization of the first TKI, considering a slow transformation of real world treatment patterns. Herein, sequential treatment applications, being affected by introduction of targeted therapies, disabled a certain approval date of targeted therapy as selection cut off. Furthermore “extended access programs” and clinical trials in our center as well as a heterogeneous implementation of new therapies in Germany with patients being referred to our clinic only after first line therapy failure create a major limitation and bias to define a reliable cut off by market authorization. Age subgroups were defined according to WHO as young (≤ 60 years), older (60.1–75 years) and old (> 75 years) at start of first systemic therapy [11].
Group comparisons were performed using Chi-square test, Fisher’s exact test, and t-test as applicable. OS was calculated from first diagnosis of metastatic disease (i.e. mRCC) until death or last follow up. Patients lost in follow up were censored at time of last documented follow up. Kaplan–Meier analysis was applied for overall population and subgroups and compared using the log-rank test. Univariate Cox regression models were performed to evaluate potential OS risk factors. Multivariable analysis was only applied for variables with statistical significance in univariate analysis. Missing values meet missing-completely-at-random criteria, meaning there is no significant bias on cox regression analysis [12]. A two-sided p-value below 0.05 was considered as statistically significant. SPSS 21.0 was used for statistical analysis (IBM, Armonk, New York). Documented parameters in the Access Data base were controlled prior to SPSS import.
Results
Patient and treatment characteristics
In total, 314 /401 patients with mRCC treated at our institution in the observation period fulfilled the predefined inclusion criteria (Supplement Fig. 1). Patients were not included due to missing date of mRCC diagnosis, missing date of therapy initiation and because they never received systemic therapy. Age distribution in patients not receiving systemic therapy was similar to those who received systemic therapy with a median OS of 46.2 months (95%-CI 3.3–89.1 months). Out of 314 patients who received systemic therapy, 173 patients (55%) were treated in period 1 and 141 (45%) in period 2. Median follow-up in period 1 was 33.8 (range (r): 1–181.6) months, compared to 27.3 (r: 0.6–179.5) months in period 2.
No significant differences in the majority of patient demographics between the two periods were observed (Table 1). However, patients in the second period tended to be older, although this didn’t achieve significance (p = 0.086). There were significantly higher rates of synchronous metastasis (42% vs. 29%, p = 0.015) and lung metastasis (59% vs. 48%, p = 0.043) in the earlier period, whereas performance status tended to be poorer in the more recent period (ECOG ≥ 1: 14 vs. 20 patients, p = 0.63). Conversely, these targeted period patients exhibited higher grade histology and received more complex medical strategies (grading: p = 0.001, therapeutic lines, p = 0.005) (Table 1). Inevitably, shifts in treatment strategies arose between periods, moving from cytokine based therapies towards TKI and mTOR inhibition were observed (Fig. 1). In first line therapy only 59.5% received TKI in period 1 compared to 85.8% in period 2, while cytokine-based therapies shrunk from 35.3% in period 1 to 2.8% in period 2 (Fig. 1).
Overall survival analysis
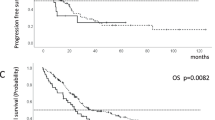
Within the entire cohort, median OS was 39.7 (95%-CI: 33.1–46.3) months within the complete observation period (Fig. 2A), being much improved in the more recent compared to the earlier period (59.1 [95%-CI: 36.1–82.2] vs. 35.1 [95%-CI: 28.3–41.9] months; Log-rank: p = 0.002, Fig. 2B).
Within the defined age groups, OS among younger, elderly, and old patient groups was 38.1 (95%-CI: 28.6–47.6), 42.9 (95%-CI: 29.5–56.3) and 27.3 (95%-CI: 12.8–41.8) months, respectively. No significant inter-group differences in OS were observed.
Comparing OS within age-groups between treatment periods, no improvements were apparent in young patients (OS 68.9 (95%-CI: 27.4–110.4) months vs. 35.1 (95%-CI: 28.2–42.0) months; Log-rank p = 0.149; Fig. 3A). Early deaths in period 2 are probably causing a non-significant difference in OS of young patients, while in particular ECOG and MSKCC are equally distributed in this subgroup. By contrast, significant improvements among elderly patients (59.1 (95%-CI: 22.9–95.3) months vs. 40.1 (95%-CI: 30.5–49.7) months, Log-Rank p = 0.034; Fig. 3B) were observed, with similar trends being evident among old patients (45.7 (95%-CI: 12.0–79.4) vs. 18.8 (95%-CI: 16.1–21.5) months; Log-rank p = 0.056; Fig. 3C). Interestingly, the greatest reduction in risk of death was observed in old patients in period 2 vs. period 1 (Hazard ratio [HR] 0.43, 95%-CI: 0.18–1.05, p = 0.06, log rank, Fig. 3C).
Risk factors for overall survival in dependence of different treatment periods
Several independent OS risk factors were identified. In the early period, multivariable analysis revealed age > 75 years (HR: 4.32, 95%-CI: 1.69–11.02, logistic regression: p = 0.002), non-ccRCC histology (HR: 2.79, 95%-CI: 1.48–5.27, logistic regression: p = 0.002), MSKCC (HR: 1.94, 95%-CI: 1.16–3.26, logistic regression: p = 0.012) and ≥ 2 sites of metastatic involvement as independent risk factors for OS (HR: 2.03, 95%-CI: 1.13–3.66, p = 0.018, logistic regression) (Table 2). In the more recent period, only ECOG ≥ 1 performance score was found to influence OS (HR 7.04, 95%-CI: 3.79–13.10, logistic regression: p < 0.001). Specifically, in Period 2 in contrast to period 1 age could not be identified as increasing risk in OS (Table 2).
Discussion
Medical treatment options for mRCC have changed dramatically in recent years, offering a variety of different effective treatments, enabling impressive improvements in OS [13]. The relevance of study outcomes in the typically older patients more commonly found in clinical practice has remained elusive, given their underrepresentation in clinical trials [10], especially given that in other forms of cancer it has been shown that elderly patients did not share reported OS benefits from such novel treatments [9, 14, 15].
This current retrospective analysis attempts to address this specific question, comparing survival outcomes dependent upon age at diagnosis of mRCC in different treatment periods. Herein, we focused on the landscape switch, resulting from introduction of mainly TKI and mTOR inhibitors, while the period of checkpoint inhibition (CPI) is hardly reflected due to the observation periods.
Within our entire cohort, substantial improvements in OS were apparent over time, improving to 59.1 months in period 2 compared to 35.1 months in period 1 (Log-rank p = 0.003). This improvement is particularly encouraging given that patients treated more recently exhibited higher tumor grades and poorer performance status. However, we observed lower rates of synchronous disease in period 2, which may have led to an overestimation of observed OS improvement. The implementation of CPI, due to the low frequency of administration (n = 13; within first to third line treatment) does not seem to introduce relevant bias. Acknowledging this improvement of OS the most relatable change within treatment pattern from period 1 to period 2 were a notable decline in utilization of cytokines in first line therapy and an increase in utilization of mTOR inhibition in second line therapy. None the less, as mentioned, our observation period allows no definite conclusion about CPI introduction to the therapeutic landscape.
Considering further age-related OS, over the entirety of the study no significant differences between groups were identified, with all age groups demonstrating improved OS in the targeted treatment period (young 35.1 vs. 68.9 months, elderly 40.1 vs. 59.1 months, old 18.8 vs. 45.7 months). In the young OS nearly doubled, but did not reach statistical significance probably due to early deaths that can unfortunately not be elucidated due to the retrospective character of this study. Surprisingly, there was a notable risk reduction for death in the targeted treatment period particularly in old patients (57%; young: 29%, elderly 38%). This would suggest that targeted treatment options, like TKI and mTOR-inhibitor might be particularly beneficial towards OS in older patients. Underlining this further, is the elimination of age as an independent risk factor for death in the more recent period. An explanation for this remains beyond the scope of this study, but it does at least support use of these novel treatments especially in old patients that traditionally did not fit in the “one size fits all” approach due to comorbidities or frailty [14, 15]. Considering other malignancies, evidence can be found that careful patient selection may improve outcomes, particularly for frail patients and that generalized application of aggressive treatments may not be universally beneficial, and may indeed be harmful [14, 15]. Although we do not have sufficient data regarding adverse events to provide meaningful analysis, issues around age-dependent toxicity profiling remain highly interesting. Comprehensive geriatric assessment/screening is not currently routinely performed, but should be considered in future, and might further improve benefits in the old [16].
Given the limited available data for treating older mRCC patients, our study adds relevant information for this common and somewhat vulnerable patient population [10]. Median age in pivotal mRCC trials centers around 59–62 years, while real world data suggests that more than half of mRCC patient are diagnosed at median 65 years [2, 15, 17]. A recent update on the efficacy of the Javelin-renal-101 trial showed no difference in median progress-free survival or OS in patients aged ≥ 75 vs 65–75 [18]. This supports the hypothesis, that decision-making regarding targeted treatments in mRCC patients should be independent of patient age. Although the majority of our cohort were not CPI treated, we would advocate against increasing age being an unfavorable predictor of survival, and it cannot be justified as argument for withholding either VEGFR or mTOR inhibitors as a minimum and potentially all novel treatment options. Previous studies examining mRCC outcomes in geriatric patients present an incomplete picture: Bellmunt et al. have previously concluded that elderly and old mRCC patients exhibit equivocal benefit from sunitinib with respect to PFS and OS [19]. Pooled analysis of phase 2 and 3 trials in mRCC encompassing 4736 patients examined the question if age represented an independent prognostic determinant of OS. Stratified according to three age groups—young (< 50 years), intermediate (50–70 years) and elderly (> 70 years)—no significant differences in OS were observed, returning at 20, 17.3 and 21 months, respectively [20]. However, in that analysis, patients above 70 years did experience more adverse events, irrespective of improvements in OS [20]. It has also been demonstrated that mRCC patients ineligible for clinical trials tend to experience inferior outcomes with a median OS of 12.5 months for ineligible vs. 28.4 months for eligible patients [21]. This might be explained by chronic and cumulative toxicity of targeted medical therapies reducing net therapeutic benefit, particularly in elderly subjects [10]. Our study stands in line with previous findings suggesting, that elderly and old mRCC patients might profit substantially from therapeutic advances in mRCC treatment.
Doubtless, our study is limited by its retrospective nature, selection bias and the small number of patients within age subgroups, especially old patients > 75 years. Selection bias favors a beneficial outcome in the elderly as no patients without treatment were included. In conjunction with its retrospective nature, a significant amount of information was missing - in particular the MSKCC - and may lead to a relevant bias, although our multivariable model counteracts this bias. Perhaps more pertinent, is that the analyzed periods fail to adequately depict the fundamental transition from cytokine based strategies into the VEGF-era, nor do they reflect the overwhelming rapid change of the current treatment armamentarium with little CPI therapies included. Nonetheless, our hypothesis that elderly should also participate and benefit from expanded and more potent therapies is robust and highly relevant for decision-making in current clinical practice. In well selected patients targeted therapy might lead to comparable outcomes regardless of age. Our findings contribute further to findings in previous publications and reemphasizes the need for further evaluation, ensuring that a relevant proportion of patients, namely the old and the elderly, are counselled appropriately.
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Robert Koch Institute (ed.) and the Association of Population-based Cancer Registries in Germany (2020) Cancer in Germany 2015/2016, p 158
Cohen HT, McGovern FJ (2005) Renal-cell carcinoma. N Engl J Med 353:2477–2490. https://doi.org/10.1056/NEJMra043172
Motzer RJ, Hutson TE, Tomczak P et al (2009) Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 27:3584–3590. https://doi.org/10.1200/JCO.2008.20.1293
Choueiri TK, Escudier B, Powles T et al (2015) Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1814–1823. https://doi.org/10.1056/NEJMoa1510016
Motzer RJ, Escudier B, McDermott DF et al (2015) Nivolumab versus everolimus in advanced renal cell carcinoma. N Engl J Med 373:1803–1813. https://doi.org/10.1056/NEJMoa1510665
Motzer RJ, Penkov K, Haanen J et al (2019) Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380:1103–1115. https://doi.org/10.1056/NEJMoa1816047
Rini BI, Plimack ER, Stus V et al (2019) Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380:1116–1127. https://doi.org/10.1056/NEJMoa1816714
Whitely R, Hannah P, Holmes F (1990) Survival in acute leukemia in elderly patients. J Am Geriatr Soc 38:527–530. https://doi.org/10.1111/j.1532-5415.1990.tb02402.x
Kanesvaran R, Saux OL, Motzer R et al (2018) Elderly patients with metastatic renal cell carcinoma: position paper from the International Society of Geriatric Oncology. Lancet Oncol 19:e317–e326. https://doi.org/10.1016/S1470-2045(18)30125-6
Burkhardt H, Gladisch R (2003) Pharmakotherapie des älteren Menschen aus klinischer Sicht. Internist 44:959–967. https://doi.org/10.1007/s00108-003-0941-5
Madley-Dowd P, Hughes R, Tilling K, Heron J (2019) The proportion of missing data should not be used to guide decisions on multiple imputation. J Clin Epidemiol 110:63–73. https://doi.org/10.1016/j.jclinepi.2019.02.016
Motzer RJ, Escudier B, McDermott DF et al (2020) Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. J Immunother Cancer 8:e000891. https://doi.org/10.1136/jitc-2020-000891
Tamirisa N, Lin H, Shen Y et al (2020) Association of chemotherapy with survival in elderly patients with multiple comorbidities and estrogen receptor-positive, node-positive breast cancer. JAMA Oncol 6:1548–1554. https://doi.org/10.1001/jamaoncol.2020.2388
Kantarjian H, Ravandi F, O’Brien S et al (2010) Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood 116:4422–4429. https://doi.org/10.1182/blood-2010-03-276485
Wildiers H, Heeren P, Puts M et al (2014) International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 32:2595–2603. https://doi.org/10.1200/JCO.2013.54.8347
National Cancer Institute (2021) Cancer stat facts: kidney and renal pelvis cancer. https://seer.cancer.gov/statfacts/html/kidrp.html. Accessed Sept 30, 2021
Tomita Y, Motzer RJ, Choueiri TK et al (2021) Efficacy and safety of avelumab plus axitinib (A + Ax) versus sunitinib (S) in elderly patients with advanced renal cell carcinoma (aRCC): extended follow-up results from JAVELIN Renal 101. JCO 39:301–301. https://doi.org/10.1200/JCO.2021.39.6_suppl.301
Bellmunt J, Négrier S, Escudier B et al (2009) The medical treatment of metastatic renal cell cancer in the elderly: position paper of a SIOG Taskforce. Crit Rev Oncol Hematol 69:64–72. https://doi.org/10.1016/j.critrevonc.2008.08.002
Panian J, Lin X, Simantov R et al (2020) The impact of age and gender on outcomes of patients with advanced renal cell carcinoma treated with targeted therapy. Clin Genitourin Cancer 18:e598–e609. https://doi.org/10.1016/j.clgc.2020.03.010
Heng DYC, Choueiri TK, Rini BI et al (2014) Outcomes of patients with metastatic renal cell carcinoma that do not meet eligibility criteria for clinical trials. Ann Oncol 25:149–154. https://doi.org/10.1093/annonc/mdt492
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
CS: manuscript writing, data analysis, and data management, HE: manuscript writing/editing, data analysis, and data management, VG: manuscript editing and protocol development, LR: data collection, MLT: data collection and Data management, CR: manuscript editing, AG: manuscript editing, IP: protocol/project development, manuscript writing/editing and data management.
Corresponding author
Ethics declarations
Conflict of interest
HE: Travel grants by Ipsen. VG: Honoraria from BMS, Novartis and Pfizer. Fees as an advisor and speaker from BMS, Novartis, Pfizer and Bayer. PI: Advisory and speaker fees from Novartis, GSK, Bayer, BMS, Merck Serono, MSD, EISAI, EUSA and Pfizer. All other authors have no conflicts of interest to declare.
Ethical standards
Data safety and GCP conformity: Data was stored and assessed by physicians and data managers in anonymized manner following the recommendations of the local ethics committee (Nr.: 3171-2016) and in concordance with the declaration of Helsinki in its latest version.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eggers, H., Schünemann, C., Grünwald, V. et al. Improving survival in metastatic renal cell carcinoma (mRCC) patients: do elderly patients benefit from expanded targeted therapeutic options?. World J Urol 40, 2489–2497 (2022). https://doi.org/10.1007/s00345-022-04110-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-022-04110-3