Abstract
Objective
To systematically evaluate evidence on prognostic factors for tumor recurrence in clinical stage I nonseminoma patients other than lymphovascular invasion (LVI).
Methods
We performed a systematic literature search in the biomedical databases Medline (via Ovid) and Cochrane Central Register of Controlled Trials (search period January 2010 to February 2021) for full text publications in English and German language, reporting on retro- or prospectively assessed prognostic factors for tumor recurrence in patients with stage I nonseminomatous germ cell tumors.
Results
Our literature search yielded eleven studies reporting on 20 potential prognostic factors. Results are based on cohort studies of mostly moderate to low quality. Five out of eight studies found a significant association of embryonal carcinoma (EC) in the primary tumor with relapse. Among the different risk definitions of embryonal carcinoma (presence, predominance, pure), presence of EC alone seems to be sufficient for prognostification. Interesting results were found for rete testis invasion, predominant yolk sac tumor, T-stage and history of cryptorchidism, but the sparse data situation does not justify their clinical use.
Conclusions
No additional factors that meet the prognostic value of LVI, especially when determined by immunohistochemistry, could be identified through our systematic search. The presence of EC might serve as a second, subordinate prognostic factor for clinical use as the data situation is less abundant than the one of LVI. Further efforts are necessary to optimize the use of these two prognostic factors and to evaluate and validate further potential factors with promising preliminary data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Testicular germ cell cancer is the most common malignancy in young men and its initial treatment consists of inguinal orchiectomy. Pathohistologic examination of the affected testis reveals nonseminoma in about 40% of the cases [1]. Upon radiographic assessment about 60% of the diagnosed nonseminomas exhibit no tumor-suspicious abnormalities and are, therefore, classified as clinical stage I [1]. Data from a meta-analysis including 7113 patients revealed that despite the absence of pathologic findings at the initial staging, about 18.6–41.3% of stage I nonseminoma patients managed by surveillance develop tumor recurrence during follow-up.
Established adjuvant treatment options to reduce the risk of relapse are adjuvant chemotherapy with one cycle of bleomycin, etoposide and cisplatin (BEP) or nerve-sparing modified retroperitoneal lymphadenectomy (nsRPLND) [3].
Adjuvant chemotherapy with 1 × BEP is highly effective in this situation as it results in a significant reduction of the relapse rate to ≤ 3% for 1 × BEP [4]. Possible drawbacks of 1xBEP are short-term complications as neutropenic fever, thromboembolism and reduced fertility as well as long-term toxicities with a possible decrease in relative survival manifesting after decades [5].
Another option, which is recommended only in very selected cases, is nsRPLND, which achieves an almost complete local control without the application of adjuvant chemotherapy in pathohistological stage I and IIA disease, but it is hampered by a 10.2% rate of metastasis developing outside the retroperitoneum [6].
The third option for nonseminoma stage I patients is Surveillance, which is associated with a high cure rate of > 99% [7]. However, about 15–45% of patients relapse during Surveillance with the need of an induction chemotherapy with at least 3 cycles of BEP [8].
In summary, all three approaches are characterized by an approximately 50–85% risk for overtreatment (for 1 × BEP or nsRPLND) or 15–50% risk for undertreatment (for Surveillance), leading to unnecessary adjuvant treatments in the former and to a higher burden of chemotherapy in the latter case.
To meet this issue, individualizing initial treatment for nonseminoma stage I patients by risk-adapted treatment approaches has been intensively studied during the last decades. A number of clinical trials has, therefore, focused on the identification of prognostic factors to stratify patients according to their relapse risk. The best validated prognostic factor in this context is lymphovascular invasion (LVI), i.e. infiltration of tumor cells into lymphatic or vascular structures within the primary [2, 9]. The application of LVI allows to form two risk cohorts, segregating the overall 25–30% relapse risk of nonseminoma stage I patients to high-risk group (LVI +) with a ~ 47.5% relapse risk and a low-risk group (LVI-) with a ~ 16.9% relapse risk [2]. LVI status can be implemented into a treatment algorithm that consists of 1 × BEP for the LVI + group and Surveillance for the LVI- group. This results in a significant advantage according to the necessary treatment burden to achieve cure in a larger collective of patients. Application of this risk-adapted approach results on average in 0.41 less cycles BEP per patient in the LVI + group and of 0.61 less cycles BEP per patient in the LVI- group, when compared with the respective alternative treatment option [10].
Consequent use of this broadly accepted risk-adapted approach is still far from an ideal state for tailored therapy in this young patient group. Using this approach still results in a about 50% risk of overtreatment for LVI + patients and in an ~ 15% risk of undertreatment for LVI- patients. Therefore, further effort is necessary to identify additional prognostic factors that might outperform or supplement LVI in the prognostication of relapse in non-semimoma stage I patients.
The aim of the present work is to review recent evidence the current knowledge on possible additional prognostic factors for non-semimoma stage I, which could help to optimize the decision on adjuvant treatment for these patients.
Methods
This work is based on a former systematic literature search that was conducted for the elaboration of the first German clinical practice guideline [11, 12]. In this context, several systematic literature searches were conducted. We present here the results combined with an update search on this topic.
Systematic literature search
We performed a systematic literature review in accordance to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [13]. Our search was limited to full text publications in English and German language published in the biomedical databases Medline (via Ovid) and Cochrane Central Register of Controlled Trials (search period January 2010 to February 2021). We considered randomized clinical trials and prospective and retrospective observational comparative studies. Case reports, case series, editorials, comments and conference abstracts were excluded. An additional search for unpublished data and ongoing studies was conducted in clinical trial registers (clinicaltrials.gov/ and www.who.int/ictrp/). We have contacted the study coordinators in case of missing information for studies identified in the trial registries. We also hand-searched the reference lists of included studies to determine additional, potentially relevant studies.
Studies were considered if they included patients (≥ 18 years) with initial stage I nonseminomatous germ cell tumors that were managed either by surveillance or by adjuvant therapies such as nsRPLND or 1–2 cycles of BEP chemotherapy. Studies had to report on prognostic markers for tumor recurrence, either as single marker or a combination of markers. As vascular invasion (VI) is an established prognostic marker for patients with stage I nonseminomatous germ cell tumors, our search focused on prognostic markers other than VI. Studies on seminomas only or other than stage I disease stages were excluded. Studies published between 2010 and 2021 in English or German language were considered.
Literature screening, data extraction and quality assessment
One review author screened the titles and abstracts and afterwards the full texts of the retrieved references and determined the relevance for inclusion. For included studies, one author extracted relevant data in evidence tables. The study quality was appraised by one author using the QUIPS tool for the risk of bias assessment in prognostic studies [14] and the level of evidence was rated according to the Oxford criteria [15]. In any case of uncertainty, another review author was involved in each of the above-mentioned steps and a consensus was reached by discussion.
Results
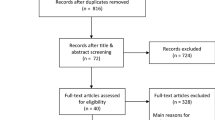
The literature search identified in total 2829 records (Supplemental Fig. 1). Eleven prognostic cohort studies, reporting a total of 2829 nonseminomatous germ cell tumor stage I (NSGCT I) patients, met our inclusion criteria (Supplemental Table 1). Study sample sizes ranged from 27 to 1226 NSGCT I patients. Further details on the study characteristics are summarized in Supplemental Table 1.
A total of 20 different pathohistological, clinical and molecular factors were reported (Table 1, Table 2, Table 3). Most of the factors (n = 7) were basic histopathologic characteristics of the primary tumor, including histological subtypes, features of differential pathology, tumor size, T-stage or rete testis invasion (RTI). Another three factors were also assessed on primary tumor material using immunohistochemistry or gene analysis. Out of ten clinical factors, four were of demographic (medical history), three were of serological (blood sample results) and another three were of radiologic nature.
The quality of the included studies can be depicted from Supplemental Table 1. Risk of bias ranged from low (5 studies) and moderate (4 studies) to high (2 studies), partly lowering the confidence in the presented results for the latter one. Studies with high risk of bias presented with insufficient information on the definition of prognostic factor or outcome, no reporting of dropouts during the study period or missing adjustment of potential confounders.
Despite many histopathological, clinical or molecular factors were identified by our literature search, most of the data on each of the factors rely on only one or two mostly small retrospective studies. The presence of embryonal carcinoma forms an exemption from this, as it was investigated by a total of eight studies included in our review. In the following section the most relevant prognostic factors retrieved by our search will be discussed more in detail.
Discussion
Reflecting on the precision of LVI assessment
LVI is the best validated prognostic factor for relapse in nonseminoma stage I patients, although its far away from an optimal prognosticator, as its sensitivity and specifity do not exceed 75% [2, 9]. However, significant disagreement between peripheral, general pathologies and pathology departments with more expertise in testicular germ cell tumor assessment and a high rate of interobserver variability using conventional hematoxylin & eosin (H&E) staining was reported [16, 17]. A recent study from the Netherlands, that was retrieved from our search, investigated the value of CD31, FVIII, and D2–40 immunohistochemistry for LVI assessment with 52 nonseminoma stage I patients undergoing surveillance [18]. Compared to H&E, the use of immunohistological markers resulted in improved sensitivity (71.0 vs. 61.3%) on the cost of decreased specificity (71.4 vs. 85.7%) and with identical accuracy (71.2 vs. 71.2%) (Table 1). This was clinically relevant, as after a median follow-up of 66 months three of the eight more cases detected by IHC staining developed relapse and the two cases in which LVI was reclassified to be absent on IHC did not develop relapse. Another interesting finding of this study was, that consideration of the origin of LVI positivity, differentiating between lymph vessel infiltration only (L1, V0), blood vessel infiltration only (L0, V1) and presence of both (L1, V1) had a high impact on the prediction of relapse [18]. The authors found, that all eight patients with “double-vascular invasion” (L1, V1) experienced disease recurrence. This interesting finding of improved prediction with consideration of “double-vascular invasion” has not yet been validated in a larger series.
Features of the primary tumor as prognostic factors
The presence of embryonal carcinoma (EC) is associated to LVI, as it is the most frequent tumor component that can be found within the blood or lymph vessels upon pathohistological examination [18]. Independent from the capacity to invade vessels, the role of EC tissue in the primary tumor for prognostification of disease relapse was investigated by eight studies retrieved from our literature search [8, 19,20,21,22,23,24,25](Table 2). Five of these studies found a significant correlation of EC with disease relapse [8, 20, 23,24,25]. Interestingly, the presence of EC alone was more reliantly associated with relapse than the presence of predominant EC (i.e. > 50% EC) in this analysis, as this was true for two out of four studies for the former and for none out of four studies for the latter definition. The largest study of our literature search by Daugaard et al. only differentiated absence or presence of EC without quantitative pathology assessment using a definite cutoff, for example, 50% [8]. This study revealed an around three fold higher risk for relapse for patients with the evidence of EC in their primary tumor [hazard ratio 2.73 (95% confidence interval (95% CI) 1.94–3.85)] [8]. The predictive value was even higher, when presence of EC was combined with LVI and RTI, reaching a hazard ratio of 5.65 (95% CI 3.74–8.53) with a 5-year relapse rate (5-year RR) of 50%. Of note, LVI alone had a surprisingly low predictive value with a hazard ratio of 1.57 (95% CI 1.22–2.02) and a 5 year RR of 18% in this large cohort, possibly affected by the known difficulties in reliable LVI assessment [18].
It is known, that LVI is positively correlated with rising EC proportions and inversely correlated with higher amounts of teratoma in the pathological specimen [26]. As the presence of EC and the presence of LVI seem to be interconnected, this might raise the question, if LVI alone might be sufficient for the prediction of relapse. In all of the five positive studies the presence of EC was prognostic for relapse independent from LVI upon multivariate analysis. This largely rules out a plain association of the presence of EC with the established prognostic factor LVI and underlines the independent value of EC in this context.
Different tumor components of nonseminomas as well as their quantitative evaluation was not only investigated for EC, but also for other tumor differentiations. The most comprehensive analysis on tumor histologies was conducted by a Chinese study, comprising 78 stage I nonseminomas [22]. Interestingly, in contrast to teratoma or seminoma, predominant yolk sac tumor (pYST) was strongly associated with relapse in their cohort. Ten out of 26 patients with pYST had a relapse during follow-up, whereas this was true only for 8 out of 52 patients without pYST, resulting in a considerable hazard ratio of 3.54 (95% CI 1.08–11.63) in multivariate analysis. This encouraging result is conflicting with data from another study included in our analysis, where the presence (not the predominance) of YST showed no correlation with relapse [24]. To what extent the percentage of YST might play a role in this context remains speculative, as the authors of the latter study did not perform quantitative pathology and we found no further data on this.
For rete testis invasion, a prognostic factor, which was extensively studied for stage I seminoma, our literature search yielded only one study for stage I nonseminoma [8, 27]. The large Danish cohort study found a weak but significant association of rete testis invasion with tumor recurrence with a hazard ratio of 1.30 (95% CI 1.02–1.69) [8].
For TNM T-stage, which is a composite of local tumor extension to neighbouring macroscopic structures (e.g., tunica albuginea or epididymis) as well as microscopic infiltration of blood or lymph vessels (corresponding to LVI), data from three studies were included in our report [22,23,24]. Two studies with a relatively small sample size found no association of T-stage with relapse [22, 24]. In contrast, a large series of 322 nonseminoma stage I patients revealed a strong correlation of a ≥ 2 T-stage with relapse after staging RPLND with an odds ratio of 4.4 (95% CI 1.7–11.7) p = 0.003 [23]. This result supports the EAU guideline recommendation, where a ≥ 2 T-stage and not the presence of LVI alone is used as a decision tool for or against adjuvant chemotherapy with 1 × BEP [3].
Size of the primary tumor, continuous or with a cutoff of 4 cm, which is the best described and well accepted prognostic factor for recurrence in stage I seminoma [27], is for nonseminoma patients only studied by Li et al. with data based on a circumscript sample of 78 patients only [22]. They found a non-significant trend towards a higher risk of recurrence for larger tumors with an odds ratio of 2.055 (95% CI 0.700–6.030). In a former large series from 2007, that comprised 779 patients, primary tumor size was also not predictive of occult metastatic disease (p = 0.167) [28]. This is supported by a recently published systematic review, in which tumor size was not predictive for recurrence in 5 out of 6 studies [2].
Serum tumor markers as prognostic factors
Serum tumor markers occupy a special position for the management of TGCT patients. Elevated pre-orchiectomy tumor markers support the suspicion of a TGCT, constantly or rising post-orchiectomy markers suggest metastatic disease even in the absence of suspect findings on cross-sectional imaging. Hypothetically, high pre-orchiectomy marker levels might be able to predict recurrence in stage I nonseminoma patients, although they initially decline to normal after surgery (Table 3). Two studies, reporting on preoperative HCG, AFP and LDH levels were identified by our search [22, 24]. None of the mentioned serum tumor markers were associated with tumor recurrence in their analyses, whereby the number of included patients was limited.
MiR-371a-3p (M371), a promising novel tumor marker, which showed high correlation with the presence of a germ cell tumor, was recently studied in a cohort of 151 stage I TGCT patients (101 seminomas, 50 nonseminomas) undergoing surveillance [29, 30]. Contrary to the expectations, this study found no association of post-orchiectomy M371 levels with relapse for seminoma patients (OR 1.3, 95% CI 0.87–2.02) as well as for nonseminoma patients (OR 0.45, 95% CI 0.21–1.00) [29]. If methodological differences, for example, the timepoints of postoperative M371 determinations, between the present study and original report of Dieckmann et al. might have played a role, is under debate. Two prospective studies, that should answer this question and help to elucidate the role of M371 as a predictive marker for occult or early metastasis, are currently recruiting (NCT04435756; GTCSG M371 follow-up trial).
Patient characteristics as prognostic factor
A prospective study from China comprising 89 patients had its focus on patient characteristics apart from histological features [19]. First, age as a continuous variable was reported to have no association with disease relapse, in line with another study that used a cutoff of 30 years [24]. Whereas laterality had no predictive value, history of cryptorchidism was strongly correlated with relapse [19]. In the surveillance group (n = 38), patients with history of cryptorchidism had a higher relapse rate than those without cryptorchidism (50 vs. 13.3%, p = 0.02). Cryptorchidism is part of the testicular dysgenesis syndrome (TDS), which also comprises hypospadia and semen parameters disorders. Selvi et al. found a strong correlation of TDS with the development of metastasis for TGCTs [31]. These results on cryptorchidism and TDS have to be interpreted with care, as the results are based on a small patient cohort and the characteristics of cryptorchidism as ipsi- or contralaterality and treatment history were not defined.
Obesity at the time of diagnosis has been associated with better outcomes in some metastatic cancers, such as renal-cell cancer [32]. The study of McGregor et al. focused on the association of obesity with outcomes in TGCT patients [33]. A high body mass index, dichotomized at 25 kg/m2, was not correlated with relapse in stage I nonseminoma patients (hazard ratio 0.78 (95% CI 0.34–1.81)).
Imaging features as prognostic factor
Howard et al. investigated the prognostic role of retroperitoneal lymph nodes smaller than 10 mm at the initial staging computed tomography [21]. The radiological parameters greatest short-axis node diameter (OR 1.18 (95% IC 0.88–1.59)) and nodal volume (OR 0.78 (95% CI 0.21–2.96)) were not correlated with tumor recurrence. Interestingly, craniocaudal node length was predictive for relapse in a significant but not clinically meaningful dimension (OR 1.15 (1.01–1.31)). A more sophisticated imaging to interpret data from computed tomographies beyond size measurements is radiomics. Radiomics uses data from the internal texture of lymph nodes, which help to train a diagnostic software-based algorithm for discriminating benign from malignant tissue. This technique has so far been only applied to lymph nodes larger than 10 mm (clinical stage ≥ IIA) for the assessment of post-chemotherapeutic residual masses and still requires testing for subclinical enlarged lymph nodes in stage I patients [34, 35].
Implications for further research
Future studies might concentrate towards an improved use and assessment of the existing prognostic factor LVI. Assessment of LVI should by now rely on an additional immunohistochemistry assessment with specific antibodies, such as CD31, FVIII, and D2–40 [18]. This improves the reliability of LVI assessment without a relevant increase in time or cost. Furthermore, a more differentiated view on LVI, which takes into account the invasive tumor component (EC or others) as well as the affected vessels (L1V0, L0V1, L1V1) is a promising approach to pull out more from LVI as to date.
Among the plethora of predictive factors presented in this work, history of cryptorchidism sticks out, as it had a very strong correlation with relapse in stage I nonseminoma patients [19]. As this was only investigated in one small study with a low event rate (n = 8), confirmative analyzes are necessary to further evaluate this interesting finding.
Summary
No additional prognostic factors that reach a similar prognostic value compared to LVI could be identified through our search. The presence of EC, a well described prognostic factor from previous reports, has some additional predictive value, although smaller than that one of LVI (see Fig. 1). In line with a recent meta-analysis, the trials included in our work were not able to define a cutoff for a necessary percentage of EC within the primary tumor. The information on the presence or absence of EC can be integrated into the decision-making on the necessity of adjuvant treatment in stage I nonseminoma, which is still mainly driven by a reliably assessed LVI status.
References
Fosså SD, Cvancarova M, Chen L, Allan AL, Oldenburg J, Peterson DR, Travis LB (2011) Adverse prognostic factors for testicular cancer-specific survival: a population-based study of 27,948 patients. J Clin Oncol 29(8):963–970. https://doi.org/10.1200/JCO.2010.32.3204 (Epub 2011 Feb 7)
Blok JM, Pluim I, Daugaard G, Wagner T, Jozwiak K, Wilthagen EA, Looijenga LHJ, Meijer RP, Bosch J, Horenblas S (2020) Lymphovascular invasion and presence of embryonal carcinoma as risk factors for occult metastatic disease in clinical stage I nonseminomatous germ cell tumor: a systematic review and meta-analysis. BJU Int 125(3):355–368. https://doi.org/10.1111/bju.14967
Laguna MP, Albers P, Algaba F, et al (2020) Guidelines on Testicular Cancer. In: EAU Guidelines. EAU Guidlines Office, Arnhem, The Netherlands.
Tandstad T, Stahl O, Hakansson U, Dahl O, Haugnes HS, Klepp OH, Langberg CW, Laurell A, Oldenburg J, Solberg A, Soderstrom K, Cavallin-Stahl E, Stierner U, Wahlquist R, Wall N, Cohn-Cedermark G (2014) One course of adjuvant BEP in clinical stage I nonseminoma mature and expanded results from the SWENOTECA group. Ann Oncol 25(11):2167–72. https://doi.org/10.1093/annonc/mdu375
Kvammen O, Myklebust TA, Solberg A, Moller B, Klepp OH, Fossa SD, Tandstad T (2019) Causes of inferior relative survival after testicular germ cell tumor diagnosed 1953–2015: a population-based prospective cohort study. PLoS ONE 14(12):e0225942. https://doi.org/10.1371/journal.pone.0225942
Hermans BP, Sweeney CJ, Foster RS, Einhorn LE, Donohue JP (2000) Risk of systemic metastases in clinical stage I nonseminoma germ cell testis tumor managed by retroperitoneal lymph node dissection. J Urol 163(6):1721–1724
Kollmannsberger C, Tandstad T, Bedard PL, Cohn-Cedermark G, Chung PW, Jewett MA, Powles T, Warde PR, Daneshmand S, Protheroe A, Tyldesley S, Black PC, Chi K, So AI, Moore MJ, Nichols CR (2015) Patterns of relapse in patients with clinical stage I testicular cancer managed with active surveillance. J Clin Oncol 33(1):51–57. https://doi.org/10.1200/JCO.2014.56.2116
Daugaard G, Gundgaard MG, Mortensen MS, Agerbaek M, Holm NV, Rorth M, von der Maase H, Christensen IJ, Lauritsen J (2014) Surveillance for stage I nonseminoma testicular cancer: outcomes and long-term follow-up in a population-based cohort. J Clin Oncol 32(34):3817–3823. https://doi.org/10.1200/JCO.2013.53.5831
Albers P, Siener R, Kliesch S, Weissbach L, Krege S, Sparwasser C, Schulze H, Heidenreich A, de Riese W, Loy V, Bierhoff E, Wittekind C, Fimmers R, Hartmann M, German Testicular Cancer Study Group (2003) Risk factors for relapse in clinical stage I nonseminomatous testicular germ cell tumors: results of the German Testicular Cancer Study Group Trial. J Clin Oncol. 21(8):1505–12. https://doi.org/10.1200/JCO.2003.07.169
Oldenburg J, Aparicio J, Beyer J, Cohn-Cedermark G, Cullen M, Gilligan T, De Giorgi U, De Santis M, de Wit R, Fosså SD, Germà-Lluch JR, Gillessen S, Haugnes HS, Honecker F, Horwich A, Lorch A, Ondruš D, Rosti G, Stephenson AJ, Tandstad T (2015) Personalizing, not patronizing: the case for patient autonomy by unbiased presentation of management options in stage I testicular cancer. Ann Oncol 26(5):833–838. https://doi.org/10.1093/annonc/mdu514
Kliesch S, Schmidt S, Wilborn D, Aigner C, Albrecht W, Bedke J, Beintker M, Beyersdorff D, Bokemeyer C, Busch J, Classen J, de Wit M, Dieckmann KP, Diemer T, Dieing A, Gockel M, Göckel-Beining B, Hakenberg OW, Heidenreich A, Heinzelbecker J, Herkommer K, Hermanns T, Kaufmann S, Kornmann M, Kotzerke J, Krege S, Kristiansen G, Lorch A, Müller AC, Oechsle K, Ohloff T, Oing C, Otto U, Pfister D, Pichler R, Recken H, Rick O, Rudolph Y, Ruf C, Schirren J, Schmelz H, Schmidberger H, Schrader M, Schweyer S, Seeling S, Souchon R, Winter C, Wittekind C, Zengerling F, Zermann DH, Zillmann R, Albers P (2021) Management of germ cell tumors of the testis in adult patients. German clinical practice guideline part I: epidemiology, classification, diagnosis, prognosis, fertility preservation, and treatment recommendations for localized stages. Urol Int 105(3–4):169–80. https://doi.org/10.1159/000510407
Kliesch S, Schmidt S, Wilborn D, Aigner C, Albrecht W, Bedke J, Beintker M, Beyersdorff D, Bokemeyer C, Busch J, Classen J, de Wit M, Dieckmann KP, Diemer T, Dieing A, Gockel M, Göckel-Beining B, Hakenberg OW, Heidenreich A, Heinzelbecker J, Herkommer K, Hermanns T, Kaufmann S, Kornmann M, Kotzerke J, Krege S, Kristiansen G, Lorch A, Müller AC, Oechsle K, Ohloff T, Oing C, Otto U, Pfister D, Pichler R, Recken H, Rick O, Rudolph Y, Ruf C, Schirren J, Schmelz H, Schmidberger H, Schrader M, Schweyer S, Seeling S, Souchon R, Winter C, Wittekind C, Zengerling F, Zermann DH, Zillmann R, Albers P (2021) Management of germ cell tumors of the testes in adult patients: German clinical practice guideline, part II - recommendations for the treatment of advanced, recurrent, and refractory disease and extra gonadal and sex cord/stromal tumors and for the management of follow-up, toxicity, quality of life, palliative care, and supportive therapy. Urol Int 105(3–4):181–191. https://doi.org/10.1159/000511245
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C (2013) Assessing bias in studies of prognostic factors. Ann Intern Med 158(4):280–286. https://doi.org/10.7326/0003-4819-158-4-201302190-00009
Oxford Centre for Evidence-Based Medicine (2009). Levels of Evidence (March 2009). https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/.
Sharma P, Dhillon J, Agarwal G, Zargar-Shoshtari K, Sexton WJ (2015) Disparities in interpretation of primary testicular germ cell tumor pathology. Am J Clin Pathol 144(2):289–294. https://doi.org/10.1309/AJCPJTX8R6CVWSRW
Harari SE, Sassoon DJ, Priemer DS, Jacob JM, Eble JN, Caliò A, Grignon DJ, Idrees M, Albany C, Masterson TA, Hanna NH, Foster RS, Ulbright TM, Einhorn LH, Cheng L (2017) Testicular cancer: the usage of central review for pathology diagnosis of orchiectomy specimens. Urol Oncol 35(10):605.e9-605.e16. https://doi.org/10.1016/j.urolonc.2017.05.018
Lobo J, Stoop H, Gillis AJM, Looijenga LHJ, Oosterhuis W (2019) Interobserver agreement in vascular invasion scoring and the added value of immunohistochemistry for vascular markers to predict disease relapse in stage I testicular nonseminomas. Am J Surg Pathol 43(12):1711–1719. https://doi.org/10.1097/pas.0000000000001352
Dong P, Liu ZW, Li XD, Li YH, Yao K, Wu S, Qin ZK, Han H, Zhou FJ (2013) Risk factors for relapse in patients with clinical stage I testicular nonseminomatous germ cell tumors. Med Oncol 30(1):494. https://doi.org/10.1007/s12032-013-0494-y
Gilbert DC, Al-Saadi R, Thway K, Chandler I, Berney D, Gabe R, Stenning SP, Sweet J, Huddart R, Shipley JM (2016) Defining a new prognostic index for stage I nonseminomatous germ cell tumors using CXCL12 expression and proportion of embryonal carcinoma. Clin Cancer Res 22(5):1265–1273. https://doi.org/10.1158/1078-0432.Ccr-15-1186
Howard SA, Gray KP, O’Donnell EK, Fennessy FM, Beard CJ, Sweeney CJ (2014) Craniocaudal retroperitoneal node length as a risk factor for relapse from clinical stage I testicular germ cell tumor. AJR Am J Roentgenol 203(4):W415–W420. https://doi.org/10.2214/ajr.13.11615
Li X, Guo S, Wu Z, Dong P, Li Y, Zhang Z, Yao K, Han H, Qin Z, Zhou F, Liu Z (2015) Surveillance for patients with clinical stage I nonseminomatous testicular germ cell tumors. World J Urol 33(9):1351–1357. https://doi.org/10.1007/s00345-014-1454-7
Nicolai N, Miceli R, Necchi A, Biasoni D, Catanzaro M, Milani A, Piva L, Pizzocaro G, Stagni S, Torelli T, Salvioni R (2010) Retroperitoneal lymph node dissection with no adjuvant chemotherapy in clinical stage I nonseminomatous germ cell tumors: long-term outcome and analysis of risk factors of recurrence. Eur Urol 58(6):912–918. https://doi.org/10.1016/j.eururo.2010.08.032
Shinoda Y, Matsui Y, Fujimoto H (2018) Outcomes of active surveillance of clinical stage I non-seminomatous germ cell tumors: sub-analysis of the multi-institutional nationwide case series of the Japanese urological association. Jpn J Clin Oncol 48(6):565–569. https://doi.org/10.1093/jjco/hyy051
Sturgeon JF, Moore MJ, Kakiashvili DM, Duran I, Anson-Cartwright LC, Berthold DR, Warde PR, Gospodarowicz MK, Alison RE, Liu J, Ma C, Pond GR, Jewett MA (2011) Non-risk-adapted surveillance in clinical stage I nonseminomatous germ cell tumors: the Princess Margaret Hospital’s experience. Eur Urol 59(4):556–562. https://doi.org/10.1016/j.eururo.2010.12.010
French BL, Zynger DL (2016) Do histopathologic variables affect the reporting of lymphovascular invasion in testicular germ cell tumors? Am J Clin Pathol 145(3):341–349. https://doi.org/10.1093/ajcp/aqv091 (Epub 2016 Feb 24)
Zengerling F, Kunath F, Jensen K, Ruf C, Schmidt S, Spek A (2018) Prognostic factors for tumor recurrence in patients with clinical stage I seminoma undergoing surveillance-a systematic review. Urol Oncol 36(10):448–458. https://doi.org/10.1016/j.urolonc.2017.06.047
Beck SD, Foster RS, Bihrle R, Donohue JP (2007) Significance of primary tumor size and preorchiectomy serum tumor marker level in predicting pathologic stage at retroperitoneal lymph node dissection in clinical Stage A nonseminomatous germ cell tumors. Urology 69(3):557–559. https://doi.org/10.1016/j.urology.2006.12.011
Dieckmann KP, Radtke A, Spiekermann M, Balks T, Matthies C, Becker P, Ruf C, Oing C, Oechsle K, Bokemeyer C, Hammel J, Melchior S, Wosniok W, Belge G (2017) Serum levels of microRNA miR-371a-3p: a sensitive and specific new biomarker for germ cell tumors. Eur Urol 71(2):213–220. https://doi.org/10.1016/j.eururo.2016.07.029
Lobo J, Leao R, Gillis AJM, van den Berg A, Anson-Cartwright L, Atenafu EG, Kuhathaas K, Chung P, Hansen A, Bedard PL, Jewett MAS, Warde P, O’Malley M, Sweet J, Looijenga LHJ, Hamilton RJ (2021) Utility of serum miR-371a-3p in predicting relapse on surveillance in patients with clinical stage I testicular germ cell cancer. Eur Urol Oncol 4(3):483–491. https://doi.org/10.1016/j.euo.2020.11.004
Selvi I, Ozturk E, Yikilmaz TN, Sarikaya S, Basar H (2020) Effects of testicular dysgenesis syndrome components on testicular germ cell tumor prognosis and oncological outcomes. Int Braz J Urol 46(5):725–740. https://doi.org/10.1590/s1677-5538.Ibju.2019.0387
Kim LH, Doan P, He Y, Lau HM, Pleass H, Patel MI (2021) A systematic review and meta-analysis of the significance of body mass index on kidney cancer outcomes. J Urol 205(2):346–355. https://doi.org/10.1097/JU.0000000000001377 (Epub 2020 Sep 18)
McGregor BA, Miller RE, O’Donnell E, Albiges LK, Sweeney CJ, Markt SC (2019) Body mass index and outcomes in germ-cell tumors. Clin Genitourin Cancer 17(4):283–290. https://doi.org/10.1016/j.clgc.2019.04.012
Baessler B, Nestler T, Pinto Dos Santos D, Paffenholz P, Zeuch V, Pfister D, Maintz D, Heidenreich A (2020) Radiomics allows for detection of benign and malignant histopathology in patients with metastatic testicular germ cell tumors prior to post-chemotherapy retroperitoneal lymph node dissection. Eur Radiol 30(4):2334–45. https://doi.org/10.1007/s00330-019-06495-z
Lewin J, Dufort P, Halankar J, O’Malley M, Jewett MAS, Hamilton RJ, Gupta A, Lorenzo A, Traubici J, Nayan M, Leão R, Warde P, Chung P, Anson Cartwright L, Sweet J, Hansen AR, Metser U, Bedard PL (2018) Applying radiomics to predict pathology of postchemotherapy retroperitoneal nodal masses in germ cell tumors. JCO Clin Cancer Inform 2:1–12. https://doi.org/10.1200/cci.18.00004
Funding
Open Access funding enabled and organized by Projekt DEAL. This work is based on a clinical guideline program, which was funded by the German Cancer Aid Foundation (DKH) (Reference No. 70112789).
Author information
Authors and Affiliations
Contributions
FZ: protocol/project development, data collection, data analysis, manuscript writing. DB: protocol/project development, manuscript editing. JB: protocol/project development, manuscript editing. JH: protocol/project development, manuscript editing. DP: protocol/project development. CR: protocol/project development. CW: protocol/project development. PA: protocol/project development, manuscript editing. SK: protocol/project development, manuscript editing. SS: protocol/project development, data collection, data analysis, manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
All other authors have no conflicts of interest to declare that are relevant to the content of this article.
Research involving human participants and/or animals
This systematic review involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zengerling, F., Beyersdorff, D., Busch, J. et al. Prognostic factors in patients with clinical stage I nonseminoma—beyond lymphovascular invasion: a systematic review. World J Urol 40, 2879–2887 (2022). https://doi.org/10.1007/s00345-022-04063-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-022-04063-7