Abstract
Purpose
To evaluate outcomes for men with biochemically recurrent prostate cancer who were selected for transponder-guided salvage radiotherapy (SRT) to the prostate bed alone by 68Ga-labelled prostate-specific membrane antigen positron emission tomography (68Ga-PSMA-PET).
Methods
This is a single-arm, prospective study of men with a prostate-specific antigen (PSA) level rising to 0.1–2.5 ng/mL following radical prostatectomy. Patients were staged with 68Ga-PSMA-PET and those with a negative finding, or a positive finding localised to the prostate bed, continued to SRT only to the prostate bed alone with real-time target-tracking using electromagnetic transponders. The primary endpoint was freedom from biochemical relapse (FFBR, PSA > 0.2 ng/mL from the post-radiotherapy nadir). Secondary endpoints were time to biochemical relapse, toxicity and patient-reported quality of life (QoL).
Results
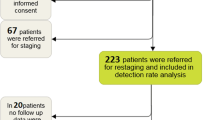
Ninety-two patients (median PSA of 0.18 ng/ml, IQR 0.12–0.36), were screened with 68Ga-PSMA-PET and metastatic disease was found in 20 (21.7%) patients. Sixty-nine of 72 non-metastatic patients elected to proceed with SRT. At the interim (3-year) analysis, 32 (46.4%) patients (95% CI 34.3–58.8%) were FFBR. The median time to biochemical relapse was 16.1 months. The rate of FFBR was 82.4% for ISUP grade-group 2 patients. Rates of grade 2 or higher gastrointestinal and genitourinary toxicity were 0% and 15.2%, respectively. General health and disease-specific QoL remained stable.
Conclusion
Pre-SRT 68Ga-PSMA-PET scans detect metastatic disease in a proportion of patients at low PSA levels but fail to improve FFBR. Transponder-guided SRT to the prostate bed alone is associated with a favourable toxicity profile and preserved QoL.
Trial registration number
ACTRN12615001183572, 03/11/2015, retrospectively registered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One in three men with prostate cancer (PCa) will experience recurrence within 10 years of radical prostatectomy (RP), initially presenting as a rising serum prostate-specific antigen (PSA) level [1]. The recommended treatment for these patients is salvage radiotherapy (SRT) to the prostate bed at the earliest sign of biochemical recurrence [2], ideally when PSA levels are less than 0.5 ng/mL [3].
However, conventional imaging modalities have poor sensitivity to metastatic disease at these PSA levels [3]. Therefore, clinicians must estimate the likelihood that a patient has locally recurrent, non-metastatic disease to reduce the risk of futile local salvage therapy. Elective pelvic nodal irradiation and/or androgen deprivation therapy (ADT) can be added to SRT for patients at higher risk of microscopic metastatic disease. While ADT may improve progression-free [4] and overall [5] survival, these benefits come at the expense of reduced quality of life (QoL) and increased mortality from other causes [5,6,7,8]. Therefore, men with recurrence locally in the prostate bed alone are at risk of overtreatment.
68Ga-labelled prostate-specific membrane antigen positron emission tomography (68Ga-PSMA-PET) is superior to conventional imaging for staging patients with newly diagnosed high-risk PCa [9], providing improved imaging accuracy and sensitivity for men who are candidates for salvage therapy [10, 11]. Compared to the findings of conventional imaging, 68Ga-PSMA-PET can change the treatment strategy in up to two-thirds of patients [12], leading to reduced use of ADT and the increased deployment of PET-directed local therapy [13].
Real-time target-tracking via implantable electromagnetic transponders is a further technological advancement which can optimise the delivery of SRT in the post-prostatectomy setting [14], however, long-term oncological and toxicity outcomes have not been previously reported. Movement of the prostate bed during SRT is not trivial, as it increases the risk of rectal toxicity and undertreatment of the target [15].
The purpose of this study was to prospectively evaluate clinical outcomes over 10 years for patients offered transponder-guided SRT to the prostate bed alone, without ADT, based on a negative 68Ga-PSMA-PET result or a positive finding in the prostate bed only. Herein are the interim (3-year) results, describing the rate of freedom from biochemical relapse (FFBR), toxicity and patient-reported health-related QoL.
Patients and methods
Study design, setting and patients
This was a prospective, single-centre, single-arm cohort study of men with biochemically recurrent PCa following RP. The study was approved by the Epworth Human Research Ethics Committee (675-15). All participants provided informed consent.
Eligible patients had previously undergone a RP for histologically confirmed, clinical stage T1–3 PCa, and subsequently presented with a rising PSA in the range of 0.1–2.5 ng/mL. Patients with metastatic disease outside the prostate bed, significant sarcomatoid, ductal or spindle-cell histology or ADT within 6 months prior to enrolment were excluded.
Outcomes
The primary endpoint was the rate of FFBR following SRT. Biochemical relapse was defined as the first increase in PSA greater than 0.2 ng/mL from the post-radiotherapy nadir, given that there was a second confirmatory reading [3]. Secondary endpoints were: (i) time from the end of SRT to biochemical relapse, (ii) acute (≤ 6 weeks) and late toxicity (> 6 weeks), defined by the Common Terminology Criteria for Adverse Events (version 4.03) and, (iii) changes in patient-reported general health and disease-specific QoL from baseline, assessed via the Short Form 12 Health Survey (SF-12, version 1) and Expanded Prostate Cancer Index Composite Short Form (EPIC-26), respectively.
Trial procedures
The study was conducted in two phases. First, eligible patients were screened with 68Ga-PSMA-PET, in addition to a standard-of-care contrast-enhanced CT scan of the chest, abdomen and pelvis. A low-dose whole-body CT scan was performed simultaneously to the PET scan and the imaging datasets fused to assist with image interpretation. All images were reviewed by an experienced nuclear medicine physician and equivocal findings were discussed by a multidisciplinary team.
Patients were included in the subsequent treatment phase of the trial if they had a negative 68Ga-PSMA-PET result or a positive finding limited to the prostate bed only. Eligible patients received SRT to the prostate bed alone to a total dose of 70 Gray (Gy) in 35 fractions (68Ga-PSMA-PET negative) or 74Gy in 37 fractions (68Ga-PSMA-PET positive in the prostate bed) [16]. Patients with a suitable body habitus (anterior–posterior separation between the anterior skin surface and greater trochanter notch less than 17 cm when lying supine) were implanted with three electromagnetic transponders in the prostate bed for real-time target-tracking using the Calypso® localisation system (Varian Medical Systems, Palo Alto, CA) during treatment. Transponders were implanted via a transperineal route under ultrasound guidance. Contraindications for transponder implantation were the presence of metal prosthetic implants in the pelvis and the use of anti-coagulant or anti-platelet therapy.
The clinical target volume (CTV) included the entire prostate bed, and for patients with relevant pathological features, the region of the seminal vesicle bed at risk of microscopic disease [17]. Magnetic resonance imaging was used to assist with target volume delineation when the planning CT scan was adversely affected by image artefact. For patients with Calypso® transponders, the planning target volume (PTV) was an isotropic 5-mm expansion of the CTV. For patients without transponders, the expansion was 5 mm posteriorly and 10 mm in all other directions from the CTV.
Treatment was delivered using intensity-modulated radiotherapy with daily corrections for isocentre position via dual orthogonal kV imaging and a weekly (at minimum) cone-beam CT scan. For patients with transponders, the Calypso® system gated treatment when transponders indicated that the target was greater than 3 mm from the planned position.
Follow-up
Follow-up was conducted 6 weeks after SRT, quarterly for the next 2 years, then half-yearly thereafter, until a patient reached the primary endpoint of biochemical relapse. Each follow-up point included PSA testing, clinical evaluation, toxicity assessment and administration of QoL questionnaires.
Statistical analysis
Data were analysed using the Stata statistical package (version 16, Stata Corporation, College Station, TX). Categorical data were summarised using frequencies and percentages while continuous/interval data were shown as means (with standard deviation) or medians with the interquartile range (IQR). Chi-squared test, t test and Wilcoxon rank-sum test were used to compare characteristics of non-metastatic and metastatic patients.
FFBR was reported as a percentage with the 95% confidence interval (CI). The relationship between biochemical relapse and patients’ clinical characteristics was examined using logistic regression, with results reported as odds ratios (OR) with 95% CI. Additionally, Cox proportional hazards model was used to assess if time to relapse varied by patient characteristics, with findings reported as hazard ratio (HR) and 95% CI. A proportional hazards test based on Schoenfeld residuals was also conducted to ensure the proportional hazards assumption was satisfied [18].
Change in QoL over time was examined using random effects models and results were reported as difference from baseline with the 95% CI. QoL data were also evaluated by a minimal clinically important difference (MCID) in scores compared to baseline. The MCID was set to one-third of a standard deviation at baseline for each specific sub-domain [19, 20].
A conservative critical alpha value of P < 0.01 was employed for both the primary and secondary endpoints.
Results
Participants
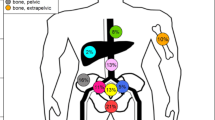
Ninety-two men were enrolled into the screening phase of the trial between July 2015 and January 2017. In 20 (21.7%) patients, there was a positive 68Ga-PSMA-PET/CT finding beyond the prostate bed. Baseline characteristics are presented in Table 1 and split into two groups: non-metastatic (negative 68Ga-PSMA-PET or positive 68Ga-PSMA-PET finding in the prostate bed only) and metastatic (a positive 68Ga-PSMA-PET finding outside the prostate bed). The majority (75%) of patients with non-metastatic PCa had received RP, while 13 of 20 (65%) of patients in the metastatic group had RP with lymph-node dissection (P = 0.001), however, this difference was attenuated by adjusting for ISUP grade-group, pathological T stage and pre-surgery PSA (adjusted P = 0.07).
Three patients in the non-metastatic group withdrew from the trial following screening, leaving a total of 69 men to proceeded to the treatment phase of the trial. Four patients had a local recurrence in the prostate bed. Treatment was delivered with real-time target-tracking for 61 (88.4%) patients. The median follow-up following SRT was 34.7 months (IQR 13.1–49.0 months).
Biochemical relapse
At the time of interim analysis, 32 (46.4%) patients (95% CI 34.3–58.8%) were FFBR, having a median follow-up of 49.0 months (IQR 43.6–49.9). Seventeen (24.6%) of these patients had an undetectable PSA reading. Of the 37 patients who failed biochemically, 21 had no radiographic evidence of metastatic disease, 3 had out-of-field seminal vesicle bed recurrence, 9 had locoregional nodal or bony metastases and 4 had distant metastases. Risk of relapse was significantly higher for ISUP grade groups 4–5 and 3 compared to grade group 2 (Table 2). No other variable was associated with relapse at P < 0.01.
The median time to biochemical relapse was 16.1 (IQR 6.9–25.5) months. Factors associated with a shorter time to relapse (Supplementary Table 1) were ISUP grade group 4–5 (HR 7.90, 95% CI 2.27–27.49, P = 0.001) or 3 (HR 5.44, 95% 1.66–17.80, P = 0.005), the presence of lymphovascular invasion (HR 2.34, 95% CI 1.23–4.44, P = 0.009) and pre-PSMA PSA (HR 2.65, 95% CI 1.35–5.18, P = 0.004).
Toxicity
Acute toxicity was assessed in 62 patients. The maximum acute genitourinary (GU) toxicity was grade 1 or grade 2 for 13 (21.0%) patients and 1 (1.6%) patient, respectively, with urinary incontinence being reported most frequently (n = 8). Seven (11.3%) patients had a maximum grade 1 GI toxicity, most commonly proctitis (n = 4). No grade 2 or higher acute GI toxicities were reported.
Sixty-six patients had late toxicity assessments. Three patients experienced biochemical progression prior to having a late toxicity assessment. There was one (1.5%) late grade 3 adverse event (urethral stricture) attributable to SRT. The maximum late GU toxicity was grade 1 and grade 2 in 30 (45.5%) and 10 (15.2%) patients, respectively, with urinary incontinence being reported most frequently (grade 1, n = 23; grade 2, n = 9). Twelve (18.2%) patients had a maximum grade 1 late GI toxicity. No grade 2 or higher late GI toxicities were recorded.
Quality of life
There were no clinically or statistically significant differences in physical component summary or mental component summary score from baseline up to 3 years post-treatment (Supplementary Fig. 1A). Disease-specific QoL for the cohort also remained within the MCID thresholds across all four sub-domains (Supplementary Fig. 1B).
Discussion
To our knowledge, this is the first prospective study of 68Ga-PSMA-PET-guided SRT to the prostate bed alone, without ADT, for men with biochemically recurrent PCa following RP. At the interim analysis, 46.4% of patients were FFBR. There were no grade 2 or higher GI toxicities and one treatment-related grade 3 urinary toxicity after a median follow-up of 34.7 months. QoL was preserved across all general health and disease-specific sub-domains. Risk factors for biochemical relapse or time to biochemical relapse were ISUP grade-group ≥ 3 and lymphovascular invasion, consistent with previous studies [21,22,23].
SRT to the prostate bed alone, with no ADT, has historically been associated with 3–5-year rates of biochemical progression-free survival (bPFS) in the order of 50% [24, 25]. The 5-year interim results of the recent NRG Oncology/RTOG 0534 SPPORT trial report higher rates of bPFS (71%), however, the threshold for biochemical failure was 2 ng/mL above the post-SRT [26] nadir compared to 0.2 ng/mL in the present study. In addition, over 50% of patients had pathological T2 stage disease [26] (versus 23% in the present study).
The lack of improvement in the rate of FFBR in the present study compared to historical data is likely to reflect the limitations of 68Ga-PSMA-PET in the early SRT setting. A meta-analysis of 14 studies using 68Ga-PSMA-PET re-staging for biochemical recurrence after prostatectomy found that the rate of positive scans was 46% and 33% when the pre-imaging PSA was 0.2–0.49 ng/mL and 0–0.19 ng/mL, respectively [27]. The median pre-PSMA PSA level of non-metastatic patients in this study was 0.17 ng/mL (IQR 0.12–0.26), making it likely that a proportion of patients had microscopic disease outside the prostate bed despite a negative PSMA-PET finding. Therefore, early salvage treatment strategies should still be guided primarily by known risk factors for metastatic disease and options including ADT and pelvic node irradiation should be considered by both the patient and clinician.
Contemporary studies of SRT which add ADT and/or extended pelvic radiotherapy report rates of 3–5-year bPFS ranging from 47 to 83% [8, 21, 26, 28]. In a study also utilising pre-SRT 68Ga-PSMA-PET screening, Emmett et al. [28], report 81% FFBR at 3 years for patients with negative or prostate bed confined disease on imaging, similarly using a threshold for biochemical failure of 0.2 ng/mL above the post-SRT nadir. However, nearly half of these patients also had radiotherapy to their pelvic nodes and 14% received ADT. The rate of FFBR for patients with ISUP grade-group 2 disease in the present study was 82.4% at a median follow-up of over 4 years, suggesting the possibility of long-term biochemical control outcomes equivalent to those treated more aggressively with ADT and/or elective pelvic node irradiation. In contrast, 73.4% of patients with ISUP grade-group 4–5 disease relapsed, suggesting an upfront role for ADT and pelvic node irradiation in the salvage setting, even with a negative 68Ga-PSMA-PET scan.
The potential extension of progression-free survival associated with ADT [4, 5], however, must be considered in the context of substantially more toxicity in sexual and hormonal domains which can outlast the duration of treatment by up to a year, while testosterone levels normalise [29]. Studies adding ADT to SRT report substantially increased rates of grade 2 or higher hot flushes, sweating and gynaecomastia [5, 8]. In the present study, sexual and hormonal QoL were preserved up to 3 years post-treatment. The trade-off between disease control and QoL detriment contributes to a lack of consensus regarding the use of ADT at the time of SRT [3, 30].
Finally, the rates of toxicity in this study compare favourably to contemporary trials using SRT alone. Rates of late grade 2 or higher GU and GI toxicity are reported to be 31–38% and 10–15%, respectively [5, 26] and grade 3 or worse GU toxicity is reported in up to 8% of patients [5, 8, 26]. The lower rates of late grade 2 or higher GU (17%) and GI (0%) toxicity in the present study are likely to reflect the use of intensity-modulated, transponder-guided radiotherapy with real-time target tracking.
This study has a number of limitations. Several of the sub-groups are small and may have limited power to detect significant differences in FFBR. The number of patients with a positive 68Ga-PSMA-PET in the prostate bed only was too small for a sub-group analysis. Second, there was no comparator arm in this study, preventing an assessment of outcomes following SRT alone against other treatment strategies. Finally, this interim analysis presents toxicity outcomes at a median follow-up of 34.7 months, which is too early to evaluate the true rate of late urinary sequelae. The final results of this study will be reported at 10 years post-treatment.
In conclusion, the interim results of this study show that 68Ga-PSMA-PET screening prior to SRT to the prostate bed alone fails to detect metastatic disease in the majority of patients who subsequently relapse. These findings are indicative of the limitations of 68Ga-PSMA-PET in the early salvage setting. However, there is a subset of patients (ISUP grade-group 2) with a rising PSA post-prostatectomy who are at a low risk of metastases and can avoid overtreatment while expecting durable bPFS and preserved QoL using Calypso® transponder-guided SRT.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
References
Roehl KA, Han M, Ramos CG, Antenor JV, Catalona WJ (2004) Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3478 consecutive patients: long-term results. J Urol 172:910–914. https://doi.org/10.1097/01.ju.0000134888.22332.bb
Valicenti RK, Thompson I, Albertsen P, Davis BJ, Goldenberg SL, Wolf JS, Sartor O, Klein E, Hahn C, Michalski J, Roach M, Faraday MM (2013) Adjuvant and salvage radiation therapy after prostatectomy: American Society for Radiation Oncology/American Urological Association guidelines. Int J Radiat Oncol Biol Phys 86:822–828. https://doi.org/10.1016/j.ijrobp.2013.05.029
Cornford P, Bellmunt J, Bolla M, Briers E, Santis MD, Gross T, Henry AM, Joniau S, Lam TB, Mason MD, van der Poel HG, van der Kwast TH, Rouvière O, Wiegel T, Mottet N (2017) EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol 71:630–642. https://doi.org/10.1016/j.eururo.2016.08.002
Carrie C, Magné N, Burban-Provost P, Sargos P, Latorzeff I, Lagrange J-L, Supiot S, Belkacemi Y, Peiffert D, Allouache N, Dubray BM, Servagi-Vernat S, Suchaud J-P, Crehange G, Guerif S, Brihoum M, Barbier N, Graff-Cailleaud P, Ruffion A, Dussart S, Ferlay C, Chabaud S (2019) Short-term androgen deprivation therapy combined with radiotherapy as salvage treatment after radical prostatectomy for prostate cancer (GETUG-AFU 16): a 112-month follow-up of a phase 3, randomised trial. Lancet Oncol 20:1740–1749. https://doi.org/10.1016/S1470-2045(19)30486-3
Shipley WU, Seiferheld W, Lukka HR, Major PP, Heney NM, Grignon DJ, Sartor O, Patel MP, Bahary J-P, Zietman AL, Pisansky TM, Zeitzer KL, Lawton CAF, Feng FY, Lovett RD, Balogh AG, Souhami L, Rosenthal SA, Kerlin KJ, Dignam JJ, Pugh SL, Sandler HM (2017) Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med 376:417–428. https://doi.org/10.1056/NEJMoa1607529
Spratt DE, Dess RT, Efstathiou JA, Zietman AL, Wallington DG, Jairath NK, Jackson WC, Den RB, Stish BJ, Morgan TM, Dignam JJ, Pisansky TM, Rosenthal SA, Michalski JM, Sartor O, Feng FY, Schipper M, Sandler HM, Sun Y, Shipley WU (2019) Two years of anti-androgen treatment increases other-cause mortality in men receiving early salvage radiotherapy: a secondary analysis of the NRG Oncology/RTOG 9601 randomized phase III trial. Int J Radiat Oncol Biol Phys 105:680. https://doi.org/10.1016/j.ijrobp.2019.08.029
Spratt DE, Dess RT, Zumsteg ZS, Lin DW, Tran PT, Morgan TM, Antonarakis ES, Nguyen PL, Ryan CJ, Sandler HM, Cooperberg MR, Posadas E, Feng FY (2018) A systematic review and framework for the use of hormone therapy with salvage radiation therapy for recurrent prostate cancer. Eur Urol 73:156–165. https://doi.org/10.1016/j.eururo.2017.06.027
Carrie C, Hasbini A, de Laroche G, Richaud P, Guerif S, Latorzeff I, Supiot S, Bosset M, Lagrange J-L, Beckendorf V, Lesaunier F, Dubray B, Wagner J-P, N’Guyen TD, Suchaud J-P, Créhange G, Barbier N, Habibian M, Ferlay C, Fourneret P, Ruffion A, Dussart S (2016) Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol 17:747–756. https://doi.org/10.1016/S1470-2045(16)00111-X
Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, Rutherford N, Martin JM, Frydenberg M, Shakher R, Wong L-M, Taubman K, Lee ST, Hsiao E, Roach P, Nottage M, Kirkwood I, Hayne D, Link E, Marusic P, Matera A, Herschtal A, Iravani A, Hicks RJ, Williams S, Murphy DG (2020) Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. The Lancet 395:1208–1216. https://doi.org/10.1016/S0140-6736(20)30314-7
Caroli P, Sandler I, Matteucci F, De Giorgi U, Uccelli L, Celli M, Foca F, Barone D, Romeo A, Sarnelli A, Paganelli G (2018) 68Ga-PSMA PET/CT in patients with recurrent prostate cancer after radical treatment: prospective results in 314 patients. Eur J Nucl Med Mol Imaging 45:2035–2044. https://doi.org/10.1007/s00259-018-4067-3
Bluemel C, Krebs M, Polat B, Linke F, Eiber M, Samnick S, Lapa C, Lassmann M, Riedmiller H, Czernin J, Rubello D, Bley T, Kropf S, Wester H-J, Buck AK, Herrmann K (2016) 68Ga-PSMA-PET/CT in patients with biochemical prostate cancer recurrence and negative 18F-Choline-PET/CT. Clin Nucl Med 41:515–521. https://doi.org/10.1097/RLU.0000000000001197
Bianchi L, Schiavina R, Borghesi M, Ceci F, Angiolini A, Chessa F, Droghetti M, Bertaccini A, Manferrari F, Marcelli E, Cochetti G, Porreca A, Castellucci P, Fanti S, Brunocilla E (2019) How does 68Ga-prostate-specific membrane antigen positron emission tomography/computed tomography impact the management of patients with prostate cancer recurrence after surgery? Int J Urol 26:804–811. https://doi.org/10.1111/iju.14012
Ekmekcioglu O, Busstra M, Klass ND, Verzijlbergen F (2019) Bridging the imaging gap: PSMA PET/CT has a high impact on treatment planning in prostate cancer patients with biochemical recurrence—a narrative review of literature. J Nucl Med 60:1394–1398. https://doi.org/10.2967/jnumed.118.222885
Canter D, Greenberg RE, Horwitz EM, Kutikov A, Li J, Long C, Buyyounouski M, Boorjian SA (2010) Implantation of electromagnetic transponders following radical prostatectomy for delivery of IMRT. Can J Urol 17:5365–5369
Klayton T, Price R, Buyyounouski MK, Sobczak M, Greenberg R, Li J, Keller L, Sopka D, Kutikov A, Horwitz EM (2012) Prostate bed motion during intensity-modulated radiotherapy treatment. Int J Radiat Oncol Biol Phys 84:130–136. https://doi.org/10.1016/j.ijrobp.2011.11.041
Shelan M, Odermatt S, Bojaxhiu B, Nguyen DP, Thalmann GN, Aebersold DM, Dal Pra A (2019) Disease control with delayed salvage radiotherapy for macroscopic local recurrence following radical prostatectomy. Front Oncol 9:12. https://doi.org/10.3389/fonc.2019.00012
Sidhom MA, Kneebone AB, Lehman M, Wiltshire KL, Millar JL, Mukherjee RK, Shakespeare TP, Tai K-H (2008) Post-prostatectomy radiation therapy: consensus guidelines of the Australian and New Zealand Radiation Oncology Genito-Urinary Group. Radiother Oncol 88:10–19. https://doi.org/10.1016/j.radonc.2008.05.006
Schoenfeld D (1982) Partial residuals for the proportional hazards regression model. Biometrika 69:239–241. https://doi.org/10.2307/2335876
Mazariego CG, Egger S, King MT, Juraskova I, Woo H, Berry M, Armstrong BK, Smith DP (2020) Fifteen year quality of life outcomes in men with localised prostate cancer: population based Australian prospective study. BMJ. https://doi.org/10.1136/bmj.m3503
Skolarus TA, Dunn RL, Sanda MG, Chang P, Greenfield TK, Litwin MS, Wei JT, PROSTQA Consortium (2015) Minimally important difference for the Expanded Prostate Cancer Index composite short form. Urology 85:101–105. https://doi.org/10.1016/j.urology.2014.08.044
Tendulkar RD, Agrawal S, Gao T, Efstathiou JA, Pisansky TM, Michalski JM, Koontz BF, Hamstra DA, Feng FY, Liauw SL, Abramowitz MC, Pollack A, Anscher MS, Moghanaki D, Den RB, Stephans KL, Zietman AL, Lee WR, Kattan MW, Stephenson AJ (2016) Contemporary update of a multi-institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. J Clin Oncol 34:3648–3654. https://doi.org/10.1200/JCO.2016.67.9647
Briganti A, Karnes RJ, Joniau S, Boorjian SA, Cozzarini C, Gandaglia G, Hinkelbein W, Haustermans K, Tombal B, Shariat S, Sun M, Karakiewicz PI, Montorsi F, Van Poppel H, Wiegel T (2014) Prediction of outcome following early salvage radiotherapy among patients with biochemical recurrence after radical prostatectomy. Eur Urol 66:479–486. https://doi.org/10.1016/j.eururo.2013.11.045
Jeong J-U, Nam T-K, Song J-Y, Yoon MS, Ahn S-J, Chung W-K, Cho IJ, Kim Y-H, Cho SH, Jung SI, Kwon DD (2019) Prognostic significance of lymphovascular invasion in patients with prostate cancer treated with postoperative radiotherapy. Radiat Oncol J 37:215–223. https://doi.org/10.3857/roj.2019.00332
Song C, Byun SJ, Kim YS, Ahn H, Byun S-S, Kim C-S, Lee SE, Kim J-S (2019) Elective pelvic irradiation in prostate cancer patients with biochemical failure following radical prostatectomy: a propensity score matching analysis. PLoS ONE. https://doi.org/10.1371/journal.pone.0215057
Ramey SJ, Agrawal S, Abramowitz MC, Moghanaki D, Pisansky TM, Efstathiou JA, Michalski JM, Spratt DE, Hearn JWD, Koontz BF, Liauw SL, Pollack A, Anscher MS, Den RB, Stephans KL, Zietman AL, Lee WR, Stephenson AJ, Tendulkar RD (2018) Multi-institutional evaluation of elective nodal irradiation and/or androgen deprivation therapy with postprostatectomy salvage radiotherapy for prostate cancer. Eur Urol 74:99–106. https://doi.org/10.1016/j.eururo.2017.10.009
Pollack A, Karrison TG, Balogh AG, Low D, Bruner DW, Wefel JS, Gomella LG, Vigneault E, Michalski JM, Angyalfi S, Lukka H, Faria SL, Rodrigues G, Beauchemin MC, Seaward SA, Allen AM, Monitto DC, Seiferheld W, Sandler HM (2018) Short term androgen deprivation therapy without or with pelvic lymph node treatment added to prostate bed only salvage radiotherapy: the NRG Oncology/RTOG 0534 SPPORT trial. Int J Radiat Oncol Biol Phys 102:1605. https://doi.org/10.1016/j.ijrobp.2018.08.052
Perera M, Papa N, Roberts M, Williams M, Udovicich C, Vela I, Christidis D, Bolton D, Hofman MS, Lawrentschuk N, Murphy DG (2020) Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer—updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol 77:403–417. https://doi.org/10.1016/j.eururo.2019.01.049
Emmett L, Tang R, Nandurkar R, Hruby G, Roach P, Watts JA, Cusick T, Kneebone A, Ho B, Chan L, van Leeuwen PJ, Scheltema MJ, Nguyen A, Yin C, Scott A, Tang C, McCarthy M, Fullard K, Roberts M, Francis R, Stricker P (2020) 3-Year freedom from progression after 68Ga-PSMA PET/CT–triaged management in men with biochemical recurrence after radical prostatectomy: results of a prospective multicenter trial. J Nucl Med 61:866–872. https://doi.org/10.2967/jnumed.119.235028
Spiegel DY, Hong JC, Oyekunle T, Waters L, Lee WR, Salama JK, Koontz BF (2019) A nomogram for testosterone recovery after combined androgen deprivation and radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 103:834–842. https://doi.org/10.1016/j.ijrobp.2018.11.007
Pisansky TM, Thompson IM, Valicenti RK, D’Amico AV, Selvarajah S (2019) Adjuvant and salvage radiation therapy after prostatectomy: ASTRO/AUA guideline amendment, executive summary 2018. Pract Radiat Oncol 9:208–213. https://doi.org/10.1016/j.prro.2019.04.008
Acknowledgements
This study was supported by funding from the EJ Whitten Foundation and the Epworth Medical Foundation.
Funding
This study was supported by funding from the EJ Whitten Foundation and the Epworth Medical Foundation.
Author information
Authors and Affiliations
Contributions
PB protocol/project development, patient management, data collection, analysis and interpretation of data, manuscript writing. AWS protocol/project development, patient management, data collection. KS protocol/project development, patient management, data collection, critical revision of manuscript. NL protocol/project development, patient management, data collection. DM protocol/project development, patient management, data collection. DGM protocol/project development, patient management, data collection, critical revision of manuscript. RR patient management, data collection. AC protocol/project development, patient management, data collection. DK patient management, data collection. HH patient management, data collection. JG patient management, data collection, critical revision of manuscript. PR protocol/project development, patient management, data collection. DG patient management, data collection. AL patient management, data collection. NC patient management, data collection. MF protocol/project development, patient management, data collection, critical revision of manuscript. LMLS data collection, analysis and interpretation of data, manuscript writing. SN Trial management, data collection. SMG analysis and interpretation of data, manuscript writing. DPM analysis and interpretation of data, manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
DGM has received honoraria for speaking and consulting activities from Astellas, Janssen, Bayer, Ferring, Ipsen and Astra Zeneca. The authors have no other conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
This study was approved by the Epworth Human Research Ethics Committee (675–15; Phase II trial harnessing PSMA-PET and Calypso® real-time tracking to precisely locate and treat recurrent prostate cancer (PINPOINT)). This study was conducted in line with the principles of the Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to participate
All patients gave their informed and written consent to participate in this trial.
Consent for publication
Consent for publication was provided by all patients within the trial informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
345_2021_3735_MOESM2_ESM.pdf
Supplementary Figure 1. Mean scores for general health (A) and disease specific (B) sub-domains. Quality of life remained stable over the course of three years for both general health (A) and disease specific (B) subdomains, as measured by SF-12 and EPIC-26 patient-reported quality of life instruments. Error bars reflect one standard deviation. Mean scores remained within minimal clinically important difference thresholds, signified by the pink shaded regions (+/− one third of a standard deviation of baseline scores, per sub-domain). In addition, there were no statistically significant differences in score compared to baseline on regression analysis. (PDF 48 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bowden, P., See, A.W., So, K. et al. 68Ga-PSMA-PET screening and transponder-guided salvage radiotherapy to the prostate bed alone for biochemical recurrence following prostatectomy: interim outcomes of a phase II trial. World J Urol 39, 4117–4125 (2021). https://doi.org/10.1007/s00345-021-03735-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-021-03735-0