Abstract
Purpose
To develop a model to predict recurrence for patients with intermediate-risk (IR) non-muscle-invasive bladder cancer (NMIBC) treated with intravesical chemotherapy which can be challenging because of the heterogeneous characteristics of these patients.
Methods
Data from three Dutch trials were combined. Patients treated with intravesical chemotherapy with characteristics according to the IR definition of the EAU guideline 2013 were included. Uni- and multivariable Cox regression with selection methods were used to identify predictors of recurrence at 1, 2, and 5 years. An easy-readable table for recurrence probabilities was developed. An external validation was done using data from Spanish patients.
Results
A total of 724 patients were available for analyses, of which 305 were primary patients. Recurrences occurred in 413 patients (57 %). History of recurrences, history of intravesical treatment, grade 2, multiple tumors, and adjuvant treatment with epirubicin were relevant predictors for recurrence-free survival with hazard ratios of 1.48, 1.38, 1.22, 1.56, and 1.27, respectively. A table for recurrence probabilities was developed using these five predictors. Based on the probability of recurrence, three risk groups were identified. Patients in each of the separate risk groups should be scheduled for less or more aggressive treatment. The model showed sufficient discrimination and good predictive accuracy. External validation showed good validity.
Conclusion
In our model, we identified five relevant predictors for recurrence-free survival in IR-NMIBC patients treated with intravesical chemotherapy. These recurrence predictors allow the urologists to stratify patients in risk groups for recurrence that could help in deciding for an individualized treatment approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bladder cancer remains a common problem in the Western world [1]. Approximately 75–85 % of bladder cancer presents as non-muscle-invasive bladder cancer (NMIBC); the remaining patients have muscle-invasive disease (MIBC) [2].
Treatment of NMIBC consists of complete transurethral resection of the bladder tumor (TURBT), followed by a single immediate postoperative instillation (POI) with chemotherapy. Further treatment depends on patients’ and tumor characteristics. In the guidelines of the European Association of Urology (EAU), patients are divided into three risk groups [2] (Supplementary Table [ST] 1). This stratification is similar to that provided by the International Bladder Cancer Group (IBCG) [3] and is partially based on the well-known risk tables developed by the European Organization for Research and Treatment of Cancer (EORTC) [4].
In general, treatment advises for low-risk and high-risk groups are clearly stated in the guidelines, but treatment advises for intermediate-risk (IR) patients are less clear. This is an important lack of information as the IR group covers a large number of patients with heterogeneous characteristics, making selection of appropriate therapy challenging. Therefore, we identified predictors of recurrence and developed a prediction model for recurrence probabilities for IR-NMIBC patients treated with intravesical chemotherapy.
Methods
Data of three prospective Dutch studies [5–7] were available for analyses, providing us with individual data of 2006 patients. Treatment and follow-up have been described in detail before [5–7] and can be found in ST2.
For the development of the prediction model, in this study, we included only patients with Ta G1/2 urothelial carcinoma without carcinoma in situ (CIS) and without the combination ‘multiple & recurrent & diameter >3 cm.’ This is consistent with the definition of IR group according to the EAU guideline [2]. All included patients received intravesical chemotherapy (either mitomycin C or epirubicin).
Primary outcome measurement was time to first recurrence (recurrence-free survival; RFS): time from randomization to the date of the first bladder recurrence.
Statistical methods
First, baseline demographics of the selected Dutch patients are presented (n = 724).
Univariable and multivariable Cox regression with selection procedures and likelihood analyses were used for selecting independent variables for RFS. Smoking and tumor diameter were removed from analyses based on the number of missing data and the hazard ratios (HRs). For the final model, the adjusted HRs are presented, including the 1, 2, and 5 years probabilities for recurrence. To assess the model’s accuracy (discrimination), Harrell’s bias corrected concordance index (c-index) was calculated at 1, 2, and 5 years and models were refitted 200 times with bootstrap resampling techniques.
Three risk groups were constructed based on the risk profiles of the final model: <P33, P33–P66, and >P66. In addition, sensitivity, specificity, negative, and positive predictive value (NPV, PPV) were calculated for the minor risk group at 2 years, as most recurrences occurred within 2 years.
External validation
Prospectively collected, independent, individual patient data provided by Fundacio Puigvert, Barcelona, Spain, were used to study the final prediction model (n = 137). However, this cohort included only data of primary patients. For the comparison, we used a subcohort of primary patients from the Dutch cohort (n = 305). The prediction model was applied to the data of these cohorts.
Statistical analyses were done with SPSS 20.0.0 for Windows (SPSS Inc., Chicago, Il, USA), in SAS 9.2 for Windows (SAS Institute Inc., Cary, NC, USA) and in R 2.2 for Windows.
Results
Demographics of Dutch cohort
Data of 724 Dutch patients met our inclusion criteria. The baseline demographics are presented in Table 1. The median follow-up was 29.6 months (range 2–239 months). A total of 413 patients (57 %) experienced a recurrence. As expected, only few patients progressed to MIBC (16 patients; 2.2 %), and therefore, we did not take progression into account as an outcome measurement.
Recurrence
In Tables 2 and 3, we show the crude and adjusted HR with 95 % confidence interval (CI) of the clinicopathological characteristics using uni- and multivariable Cox regression. The following five variables were included in the final model: history of previous recurrences, history of intravesical treatment, tumor grade 2, multiple tumors, and adjuvant treatment with epirubicin, with HRs of 1.48, 1.38, 1.22, 1.56, and 1.27, respectively. As can be seen in Table 3, the HR, 95 % CI and p value of tumor grade and adjuvant treatment are 1.22 (95 % CI 0.99–1.51; p value 0.061) and 1.27 (95 % CI 1.00–1.62; p value 0.048), respectively. Although the statistical significance of these two variables is around 0.05, the five-variable model outperformed the three-variable model, i.e., the model without tumor grade and adjuvant treatment (likelihood ratio test, Chi-square = 17.0, p = 0.0002).
Prediction model
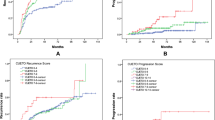
The recurrence probabilities of the final Cox regression model at 1, 2, and 5 years are presented in Table 4. The c-index for this RFS model was 0.60, 0.62, and 0.63 at year 1, 2, and 5, respectively. Three risk groups were constructed based on the risk profiles of the final model. The Kaplan–Meier curves of minor, moderate, and major risk based on the risk profiles of the final model are shown in Fig. 1. The model can distinguish clearly between recurrence outcomes, e.g., a patient with multiple G2 recurrences without previous treatment who received adjuvant treatment with epirubicin has, according to Table 4, 67 % chance of being recurrence free at 12 months, which is associated with major risk in Fig. 1. We calculated sensitivity, specificity, PPV, and NPV for minor risk patients at 2 years (ST3). PPV is 68.4 % and NPV is 65.2 %.
Demographics of Spanish cohort and external validation
For the external validation, a cohort of 137 Spanish patients was used (treatment and demographics can be found in ST2 and Table 1, respectively). It needs to be stressed that in the Spanish cohort, only ten patients had grade 1. Therefore, only the HR of the number of tumors was updated using the data of Dutch subcohort and the Spanish cohort. The HR of the number of tumors of 1.65 (95 % CI 1.28–2.00) in the combined cohorts (Dutch subcohort + Spanish cohort) was comparable to the development (Dutch) cohort: 1.56 (95 % CI 1.20–2.01). The associated Kaplan–Meier curves are shown in Fig. 2.
Discussion
We present a study comparing recurrence outcome and treatment options for the heterogeneous spectrum of IR patients, and we propose a prediction model on recurrence probabilities with external validation. We found five relevant predictors for RFS: a history of recurrences, history of previous treatment, tumor grade 2, multiple tumors, and adjuvant treatment with epirubicin, with HRs of 1.48, 1.38, 1.22, 1.56, and 1.27, respectively. There is a huge difference between 1 and 5 years outcome, and between having none or all of the independent predictors (Table 4). We defined the IR group into three subgroups (minor, moderate, and major risk) in a way that each risk group needs to be considered for a less or more aggressive adjuvant treatment schedule or treatment type. The recurrence probabilities as predicted in Table 4 can be related to a risk group in Fig. 1.
The EORTC have developed risk tables based on a group of 2596 patients [4]. However, the EORTC risk tables have several limitations: 22 % of patients received no intravesical treatment at all; only 171 patients (7 %) received treatment with bacillus Calmette–Guerin (BCG) and none received BCG maintenance. Therefore, the EORTC risk tables could be interpreted as probabilities of the untreated natural history of the disease, especially for progression. Another well-known prediction model is the scoring model of Club Urologico Español de Tratamiento Oncologico (CUETO) [8]. Data of 1062 patients, all treated with BCG, were used to identify risk factors for recurrence and progression after BCG treatment. Several other prediction models have been developed for NMIBC [9–15], but none of them included solely patients who, according to the guidelines, should have been and in fact were treated with intravesical chemotherapy.
Recently, Kamat et al. [16] developed an algorithm specifically for IR patients based on the consensus of the IBCG. They consider tumor size, tumor multiplicity, timing and frequency of recurrences, and previous treatment to be key factors. Based on these key factors, they divide IR patients in three groups: low-risk patients, ‘true’ IR patients, and high-risk patients. Our analyses and model support these recommendations; only tumor size, which is also a significant predictor in the EORTC risk model [4], is of no influence in our model, and tumor grade is not considered to be a key factor by the IBCG.
Concerning tumor size, Kamat et al. [16] do mention that the well-known cutoff of 3 cm might be no longer relevant as the number of patients with large tumors is very low, which we could confirm (in the Dutch cohort only 6 %). Within the IBCG, it was suggested to further study a new cutoff of 1 cm. When analyzing this cutoff in our cohort, no statistical significant influence on recurrence outcome was seen (p = 0.480), but this might be due to the high number of missing data, which is a limitation of our study.
We found that tumor diameter had many missing data and was no statistically significant predictor for RFS in the complete cohort. This is clearly different from other prediction models including the EORTC risk model [4], but our group of patients all received adjuvant intravesical chemotherapy and are therefore not comparable with, e.g., the EORTC risk model patients.
The term ‘low grade’ is based on the WHO 2004 grading system, which was not yet available during the inclusion period of the three Dutch studies. Therefore, we considered G1 and G2 tumors to be low grade, but G2 tumors are a mixture of low-grade and high-grade tumors. According to Chen et al. [17], approximately 80 % of the G2 tumors are low grade. Thus, in this study, we could have misclassified 75 patients, and consequently, these patients could be treated insufficiently with subsequently more recurrences.
The c-index of our model was 0.60, 0.62 and 0.63 at year 1, 2, and 5, respectively. This is comparable to the c-index for recurrence probabilities of the EORTC risk Tables (0.66 both at 1 and 5 years) and that of the CUETO scoring model (0.64 both at 1 and 5 years) [4, 8]. However, the clinical relevance of the c-index is doubtful and there is no consensus how high the c-index should be to make a model clinically relevant.
For a more practical approach, based on their risk factors, we divided the patients in three subgroups: minor, moderate and major risk. As can be seen in Fig. 1, this subdivision is clearly related to recurrence outcome, and thus, the major risk group could be considered for more aggressive treatment and the minor risk group for less aggressive treatment. The relevance of this subdivision is also reflected in the predictive accuracy of our model (ST3). For treatment options, the NPV and PPV are more important than sensitivity and specificity, as this is associated with under- and overtreatment. Compared to the EORTC and CUETO, our model is clearly better in preventing overtreatment in minor risk patients as PPV is much higher (68 % versus 21 and 24 %), but NPV is somewhat lower (65 versus 94 and 92 %) which, however, is less of a problem in minor risk patients [18]. Additionally, the external validation shows very good overlap in HR. However, as the Spanish cohort only included primary patients, it is in fact a partial external validation. Nevertheless, as agreement between the Dutch subcohort and the Spanish cohort is high, one could hypothesize that these results could be extrapolated to the total model. An external validation with primary and recurrent patients is needed to confirm our results.
Limitations of this study are the long inclusion period, the missing data, and the differences with the current standard of treatment including the quality of TURBT due to, e.g., the introduction of re-TURBT, the introduction of fluorescence cystoscopy, and the lack of immediate POIs in most patients (only 23 % of patients received immediate POI). On the other hand, fluorescence cystoscopy is most useful in CIS, but these patients were excluded in our analyses. In the Dutch cohort, no re-TURBT was done, but this is not always necessary in IR patients. Another limitation is the variability in adjuvant treatment, including the dose, the concentration of chemotherapy used, and the treatment schedule which might have influenced the outcome. Yet, the median number of instillations received was 10, and only 2.1 % of patients received less than six instillations. Furthermore, both European and American guidelines do not recommend specific chemotherapy schedules [2, 19].
Conclusion
We developed a risk table for IR-NMIBC patients treated with intravesical chemotherapy including five relevant predictors of RFS: history of recurrences, history of intravesical treatment, grade 2, multiple tumors and adjuvant treatment with epirubicin. These individual predictors were used to subdivide IR patients into three risk groups, which is related to recurrence outcome. With this subcategorization, the urologist together with the patient can choose for an individualized treatment approach.
Abbreviations
- BCG:
-
Bacillus Calmette–Guerin
- CI:
-
Confidence interval
- CIS:
-
Carcinoma in situ
- CUETO:
-
Club Urologico Espanol de Tratamiento Oncologica
- C-index:
-
Harrell’s bias corrected concordance index
- EAU:
-
European association of urology
- EORTC:
-
European Organization for Research and Treatment of Cancer
- HR:
-
Hazard ratio
- IBCG:
-
International bladder cancer group
- IR:
-
Intermediate risk
- MIBC:
-
Muscle-invasive bladder cancer
- NMIBC:
-
Non-muscle-invasive bladder cancer
- NPV:
-
Negative predictive value
- POI:
-
Postoperative instillation
- PPV:
-
Positive predictive value
- RFS:
-
Recurrence-free survival
- ST:
-
Supplementary table
- TURBT:
-
Transurethral resection of bladder tumor
- WHO:
-
World Health Organization
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Comperat E, Sylvester RJ, Kaasinen E, Bohle A, Palou Redorta J, Roupret M (2013) EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol 64(4):639–653. doi:10.1016/j.eururo.2013.06.003
Brausi M, Witjes JA, Lamm D, Persad R, Palou J, Colombel M, Buckley R, Soloway M, Akaza H, Bohle A (2011) A review of current guidelines and best practice recommendations for the management of nonmuscle invasive bladder cancer by the International Bladder Cancer Group. J Urol 186(6):2158–2167. doi:10.1016/j.juro.2011.07.076
Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW, Kurth K (2006) Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 49(3):466–477
Vegt PD, Witjes JA, Witjes WP, Doesburg WH, Debruyne FM, van der Meijden AP (1995) A randomized study of intravesical mitomycin C, bacillus Calmette-Guerin Tice and bacillus Calmette-Guerin RIVM treatment in pTa-pT1 papillary carcinoma and carcinoma in situ of the bladder. J Urol 153(3 Pt 2):929–933
Hendricksen K, Witjes WP, Idema JG, Kums JJ, van Vierssen Trip OB, de Bruin MJ, Vergunst H, Caris CT, Janzing-Pastors MH, Witjes JA (2008) Comparison of three schedules of intravesical epirubicin in patients with non-muscle-invasive bladder cancer. Eur Urol 53(5):984–991
Lammers RJ, Witjes WP, Janzing-Pastors MH, Caris CT, Witjes JA (2012) Intracutaneous and intravesical immunotherapy with keyhole limpet hemocyanin compared with intravesical mitomycin in patients with non-muscle-invasive bladder cancer: results from a prospective randomized phase III trial. J Clin Oncol. doi:10.1200/jco.2011.39.2936
Fernandez-Gomez J, Madero R, Solsona E, Unda M, Martinez-Pineiro L, Gonzalez M, Portillo J, Ojea A, Pertusa C, Rodriguez-Molina J, Camacho JE, Rabadan M, Astobieta A, Montesinos M, Isorna S, Muntanola P, Gimeno A, Blas M, Martinez-Pineiro JA (2009) Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette-Guerin: the CUETO scoring model. J Urol 182(5):2195–2203
Kiemeney LA, Witjes JA, Heijbroek RP, Verbeek AL, Debruyne FM (1993) Predictability of recurrent and progressive disease in individual patients with primary superficial bladder cancer. J Urol 150(1):60–64
Millan-Rodriguez F, Chechile-Toniolo G, Salvador-Bayarri J, Palou J, Algaba F, Vicente-Rodriguez J (2000) Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. J Urol 164(3 Pt 1):680–684
Shariat SF, Zippe C, Ludecke G, Boman H, Sanchez-Carbayo M, Casella R, Mian C, Friedrich MG, Eissa S, Akaza H, Sawczuk I, Serretta V, Huland H, Hedelin H, Rupesh R, Miyanaga N, Sagalowsky AI, Wians F Jr, Roehrborn CG, Lotan Y, Perrotte P, Benayoun S, Marberger MJ, Karakiewicz PI (2005) Nomograms including nuclear matrix protein 22 for prediction of disease recurrence and progression in patients with Ta, T1 or CIS transitional cell carcinoma of the bladder. J Urol 173(5):1518–1525. doi:10.1097/01.ju.0000154696.48217.75
Hong SJ, Cho KS, Han M, Rhew HY, Kim CS, Ryu SB, Sul CK, Chung MK, Park TC, Kim HJ (2008) Nomograms for prediction of disease recurrence in patients with primary Ta, T1 transitional cell carcinoma of the bladder. J Korean Med Sci 23(3):428–433. doi:10.3346/jkms.2008.23.3.428
Yamada T, Tsuchiya K, Kato S, Kamei S, Taniguchi M, Takeuchi T, Yamamoto N, Ehara H, Deguchi T (2010) A pretreatment nomogram predicting recurrence- and progression-free survival for nonmuscle invasive bladder cancer in Japanese patients. Int J Clin Oncol 15(3):271–279. doi:10.1007/s10147-010-0049-6
Pan CC, Chang YH, Chen KK, Yu HJ, Sun CH, Ho DM (2010) Constructing prognostic model incorporating the 2004 WHO/ISUP classification for patients with non-muscle-invasive urothelial tumours of the urinary bladder. J Clin Pathol 63(10):910–915. doi:10.1136/jcp.2010.079764
Shapur N, Pode D, Katz R, Shapiro A, Yutkin V, Pizov G, Appelbaum L, Zorn KC, Duvdevani M, Landau EH, Gofrit ON (2011) Predicting the risk of high-grade bladder cancer using noninvasive data. Urol Int 87(3):319–324. doi:10.1159/000328635
Kamat AM, Witjes JA, Brausi M, Soloway M, Lamm D, Persad R, Buckley R, Bohle A, Colombel M, Palou J (2014) Defining and treating the spectrum of intermediate risk nonmuscle invasive bladder cancer. J Urol. doi:10.1016/j.juro.2014.02.2573
Chen Z, Ding W, Xu K, Tan J, Sun C, Gou Y, Tong S, Xia G, Fang Z, Ding Q (2012) The 1973 WHO classification is more suitable than the 2004 WHO classification for predicting prognosis in non-muscle-invasive bladder cancer. PLoS ONE 7(10):e47199. doi:10.1371/journal.pone.0047199
Sylvester RJ (2012) The confusing world of prognostic models and their clinical use. Nijmegen, the Netherlands
Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD, Skinner EC, Wolf JS Jr, Schellhammer PF (2007) Guideline for the management of non muscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol 178(6):2314–2330
Acknowledgments
The 39 hospitals that participated in the original three studies are as follows: Onze Lieve Vrouwe Gasthuis, Amsterdam; Academic Medical Center, Amsterdam; Hospital Center Apeldoorn, Apeldoorn; Rijnstate Hospital, Arnhem; Wilhelmina Hospital, Assen; Lievensberg Hospital, Bergen op Zoom; Ignatius Hospital, Breda; Medical Center Haagland, Den Haag; Rode Kruis Hospital, Den Haag; St. Deventer Hospital, Deventer; Slingeland Hospital, Doetinchem; Gelderse Vallei, Ede; Catharina Hospital, Eindhoven; Medisch Spectrum Twente, Enschede; Martini Hospital, Groningen; St. Jansdal Hospital, Harderwijk; Jeroen Bosch Hospital, ’s-Hertogenbosch; Hospital Bethesda, Hoogeveen; Medical Center Leeuwarden Noorderbreedte, Leeuwarden; Diaconessen Hospital, Leiden; Leids University Medical Center, Leiden; Rijnland Hospital, Leiderdorp; Zuiderzee Hospital, Lelystad; Diaconessen Hospital, Meppel; Radboud University Nijmegen Medical Center, Nijmegen; Canisius Wilhelmina Hospital, Nijmegen; Pasteur Hospital, Oosterhout; St. Franciscus, Roermond; St. Laurentius, Roermond; St. Franciscus Hospital, Roosendaal; Academic Hospital Rotterdam, Rotterdam; Zuiderzee Hospital, Rotterdam; Elisabeth Hospital, Tilburg; Tweesteden Hospital, Tilburg; Academic Hospital Utrecht, Utrecht; Viecuri, Venlo; Hospital Walcheren, Vlissingen; Streek Hospital Queen Beatrix, Winterswijk; Isala, Zwolle. Furthermore, we would like to thank Christien T.M. Caris and Maria H.D. Janzing-Pastors from CuraTrial SMO & Research, Arnhem, the Netherlands, for collecting the data of the Dutch three studies. Finally, we would like to thank Dr. Sergio Skrobot for collecting the data in Fundacio Puigvert.
Ethical standard
The original studies of Hendricksen et al. [6] and Lammers et al. [7] have been approved by the appropriate ethics committees and have therefore been performed in concordance with the ethical standards laid down in the 1964 Declaration of Helsinki. The original study of Vegt et al. [5] was conducted before the current ethical standards. However, all patients gave their informed consent prior to inclusion in the study.
Conflict of interest
Prof J. A. Witjes has received a lecture honorarium from Medical Enterprise Ltd in the past and is an advisor for Medical Enterprise Ltd, Sanofi Pasteur, Telormedix, and Theracoat. The other authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lammers, R.J.M., Hendriks, J.C.M., Rodriguez Faba, O.R. et al. Prediction model for recurrence probabilities after intravesical chemotherapy in patients with intermediate-risk non-muscle-invasive bladder cancer, including external validation. World J Urol 34, 173–180 (2016). https://doi.org/10.1007/s00345-015-1598-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-015-1598-0