Abstract
Humic substances (HS) have been defined as a potential plant biostimulant to improve crop yield in a sustainable and environmentally friendly way. Leonardite-suspension concentrate (SC) is a type of HS extracted from lignite that is currently employed to enhance various physiological aspects of plants. However, the different effects between both modes of SC application (root and foliar) are poorly understood, especially on photosynthesis performance. Therefore, this study aimed to investigate the influence of a leonardite-SC-based product (BLACKJAK®), on lettuce growth and photosynthesis efficiency, while comparing both methods of application. For this purpose, four root (R): R1 (0.20 mL/L), R2 (0.40 mL/L), R3 (0.60 mL/L), and R4 (0.80 mL/L), and four foliar: F1 (5.00 mL/L), F2 (7.50 mL/L), F3 (10.00 mL/L), and F4 (12.50 mL/L) BLACKJAK® doses were applied to lettuce plants. Related shoot and root growth parameters, photosynthetic efficiency, and sugar and starch content were assessed in lettuce plants. The results showed that BLACKJAK® improved shoot and root biomass, foliar area, and root length, especially at intermediate doses (R2, R3, F2, and F3), with R3 demonstrating the greatest growth increases. Similarly, the main photosynthetic parameters analyzed (net photosynthetic rate and Rubisco carboxylation efficiency), and the soluble sugars and starch content were improved by the same doses, with R3 showing the best photosynthetic performance. Hence, our study suggests that BLACKJAK® improves lettuce yield and photosynthetic efficiency, particularly with radicular application at R3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Feeding the world’s growing population has been and continues to be the main challenge for most agricultural researchers. It is estimated that the global population will increase by 2 billion people by 2050, reaching a total of 9.7 billion people. As a result, food production will have to increase by 60% over current production to feed the growing population (Muhie 2022; Simkin et al. 2019). Besides, climate change and the abiotic stress conditions (salinity, drought, high temperatures, etc.) are expected to considerably reduce crop yield. Furthermore, a quarter of greenhouse gas emissions come from agricultural practices which contributes significantly to climate change (Muhie 2022). In this way, chemical fertilizers used in an uncontrolled manner greatly promote the release of carbon dioxide (CO2) into the atmosphere, by altering physical and chemical soil properties such as pH, aeration, C/N ratio, affecting soil carbon dynamics (Chi et al. 2020; Cao et al. 2023). Therefore, it is imperative to discover an equilibrium between augmenting food production for feeding the population while reducing the overuse of chemical fertilizers.
Plant biostimulants have been described as a potential tool to increase crop yield in a sustainable and environmentally friendly way, while reducing the uncontrolled use of chemical fertilizers. A brief definition of plant biostimulant could be “any substance (organic or inorganic compound) or microorganism that when it is applied to plant, improve crop yield and quality, nutrient uptake and utilization, and abiotic stress tolerance.” According to this definition, the main categories of biostimulants are plant and seaweed extracts, humic substances, protein hydrolysates, inorganic compounds such as silica (Si), and beneficial bacteria, and fungi (du Jardin 2015).
Humification is the process of biodegradation of the soil organic matter (plants, animals, and microorganisms waste) that results in humic substances (HS) generation. Based on their physicochemical properties, HS are classified into three categories: (i) humin (insoluble in all pH conditions); (ii) humic acids (HA) (soluble in alkaline pH); and (iii) fulvic acids (FA) (soluble in alkaline and acidic conditions) (Yang et al. 2021). Different natural sources are used for HS extraction: composts and vermicomposts, volcanic soils, peat, and oxidation products of lignite as leonardite (du Jardin 2015; Wei et al. 2023). Leonardite is widely used by companies for commercial HS generation as suspension concentrate (SC), which are frequently employed by farmers and researchers (Conselvan et al. 2017). HS may be applied to plants by foliar spraying or to the soil solution (radicular or root application), and the most studies published that can be found in literature employ a single method of application.
Concerning to the beneficial effects of humic substances on plant growth, they can exert their influence at various levels, spanning from the soil to the leaves. Thus, HS improve the bioavailability of essential nutrients such as nitrogen, phosphorous, potassium, and others, as well as their uptake by roots and the nutrient use efficiency (Yuan et al. 2022; Zanin et al. 2019). Besides, different research studies have shown the positive influence of HS in primary and secondary metabolism, improving photosynthetic efficiency (Fan et al. 2014), tricarboxylic acid cycle (Nardi et al. 2007), nitrogen and sulfur assimilation (Jannin et al. 2012; Zanin et al. 2018), and phenolic metabolism (Savarese et al. 2022). In addition, HS may change phytohormonal profile and act as physiological signal emulating hormone functions (Chen et al. 2022). As a result, plant growth can be enhanced by HS application, increasing fresh and dry matter of shoots and roots. Therefore, HS play an important role in modern agriculture to increase crop production in a sustainable way with a reduced environmental impact compared to chemical fertilizers (Tiwari et al. 2023).
With respect to photosynthesis, improving the process of light conversion into biomass is synonymous with increased plant growth and productivity (Simkin et al. 2019). Some studies have shown that biomass production under glasshouse and field conditions may be increased by enhancing the photosynthesis process (Ding et al. 2016; Simkin et al. 2017). There are three main avenues for increase photosynthetic efficiency: (i) improving the stomatal conductance (g) and consequently the mesophyll intercellular CO2 (Ci) (ii) enhancing the carboxylation efficiency of Rubisco, and (iii) improving electron transport flux efficiency at photosynthetic complex level (Araus et al. 2021). To achieve these goals, most studies use techniques for the genetic manipulation of enzymes in the photosynthetic process. Alternatively, plant biostimulants such as HS have significance potential to enhance photosynthesis by reducing fluorescence dissipation. This reduction results in more light energy utilization for photosynthesis process (Canellas et al. 2013; Fan et al. 2014). Furthermore, HS may enhance Rubisco activity, leading to increased CO2 fixation and, consequently, a higher net photosynthetic rate (Chen et al. 2022). However, research comparing the effect on photosynthesis process between root and foliar application of HS extracted from different sources, such as leonardite, is scarce. Therefore, this study aims to evaluate the growth and photosynthesis performance of lettuce plants subjected to both root and foliar applications of HS. As the HS source, we used a leonardite-SC named BLACKJAK®, which has yet to be examined for its effects on photosynthesis performance of horticultural crops through different application strategies.
Materials and Methods
Plant Material and Growing Conditions
Lettuce plants (Lactuca sativa cv. Capitata) were used as plant material for this work. Seeds of these plants were germinated and grown in a tray with cells (size 3 × 3 × 10 cm) for 45 days. Afterward, lettuce seedlings were transferred to individual pots (13 cm upper diameter, 10 cm lower diameter, 12.5 cm high, and a volume of 2 L) filled with 3:1 mixture of vermiculite:perlite, and distributed in a culture chamber under controlled environmental conditions with temperature 25/15 °C (day/night), relative humidity 60–80%, and 16/8 h of photoperiod with a photosynthetic photon-flux density of 350 μmol m−1 s−1 (measured with a sensor SB quantum 190, LI-COR Inc., Lincoln, NE, USA). Throughout the experiment, lettuce plants were irrigated with a complete Hoagland nutritive solution (Hoagland and Arnon 1950), with small modifications for the correct growth of lettuce, composed of 4 mM KNO3, 1 mM KH2PO4, 1 mM NaH2PO4·2H2O, 3 mM Ca(NO3)2·4H2O, 2 mM MgSO4·7H2O, 5 µM Fe-chelate (Sequestrene; 138FeG100), 2 µM MnCl2·4H2O, 0.25 mM CuSO4·5H2O, 1 µM ZnSO4·7H2O, 10 µM HBO3, and 0.1 µM Na2MoO4·2H2O (pH 5.5–6). The nutrient solution was renewed every 3 days.
Humic Substances Application and Experimental Design
Treatments started 7 days after transplantation and maintained for 30 days. These treatments consisted of the application of HS using BLACKJAK®, a leonardite-SC-based product provided by the company Sofbey, S.A. (Mendrisio, Switzerland), composed of 30% of organic matter, with an acidic pH (4, 5). BLACKJAK® was applied to lettuce plants 3 times with 10-days intervals by both radicular and foliar application. Radicular (‘R’) HS were added to Hoagland nutritive solution at four different concentrations: 0.20 mL/L (R1), 0.40 mL/L (R2), 0.60 mL/L (R3), and 0.80 mL/L (R4). On the other hand, foliar (‘F’) application was carried out by spraying lettuce leaves with HS at four doses: 5.00 mL/L (F1), 7.50 mL/L (F2), 10.00 mL/L (F3), and 12.50 mL/L (F4). Those concentrations were selected based on commercial ranges and according to a previous screening using radicular and foliar doses on lettuce, where lower doses than those employed had no physiological effect, whereas higher doses resulted in toxicity. The foliar treatments were made 2 h after switching on the light of the growth chamber. Furthermore, a control treatment was conducted applying Hoagland nutritive solution without HS. The experimental design consisted of a complete randomized block with nine treatments, eight plants per treatment arranged in individual pots with the treatments randomly distributed in the culture chamber.
Sampling and Plant Growth Measurements
Plants of each treatment were sampled 30 days after the first HS application. The leaves and roots of all lettuce plants were sampled, washed using distilled water, dried on filter paper, and weighed for the fresh weight (FW) determination. These lettuce leaves of each treatment were frozen at − 45 °C for subsequent biochemical analyses. To determine the relative growth rate (RGR), leaves and roots were sampled before starting the HS application (initial time, Ti = 0 days) and weighed (initial fresh weight, FWi). At the end of the experiment (final time, Tf = 30 days), the FW of leaves and roots (final fresh weight, FWf) from each treatment was used to estimate the RGR, using the equation RGR = (ln FWf − ln FWi)/(Tf − Ti) (Navarro-León et al. 2019). On the other hand, a LI-COR optical reader, model LI-3000A (LI-COR Inc. Nebraska, USA), was employed to determine the leaf area and root surface area, while the root length was measured using a ruler.
Quantification of Photosynthetic Pigments
Chlorophylls (Chl a and Chl b) as well as carotenoids were extracted from 0.1 g of frozen lettuce leaves by adding 1 mL of methanol and then centrifugated at 5000×g for 5 min. Absorbance from the supernatant was measured at 3 different wavelengths: 653 nm, 666 nm, and 470 nm. From the absorbance values, photosynthetic pigments concentration was calculated using the equations described by Wellburn (1994).
Estimation of Chl a Fluorescence Parameters
Six plants from each treatment were adapted to dark for 30 min before Chl a fluorescence measure using a special leaf clip holder allocated in fully expanded leaves at midstem position. Chl a fluorescence kinetics was measured using the Handy PEA Chlorophyll Fluorimeter (Hansatech Ltd., King’s Lynn, Norfolk, UK) by the induction of red light (650 nm) with 3000 μmol photons m−2 s−1 light intensity. Before taking measurements in the experiment, the Handy PEA was calibrated by measuring a lettuce leaf. The JIP-test was used to analyze the Chl a fluorescence transient. The parameters employed in the present study to determine the effect of HS on photosynthetic activities were as follows: maximum quantum yield for primary (Fv/Fm, where Fv is variable fluorescence calculated as Fv = Fm − Fo, being Fm the maximum fluorescence and Fo the initial fluorescence), proportion of active reaction centers (RCs) (RC/ABS), performance index for energy conservation from photons absorbed by photosystem II (PSII) antenna to the reduction of QB (PIABS), the efficiency/probability with which a PSII trapped electron is transferred from QA to QB (ΨEo), maximum quantum yield of electron transport (ΦEo), and the number of times that QA is reduced from time 0 to time that Fm is reached (N) (Roháček 2002; Strasser et al. 2004).
Determination of Leaf Gas Exchange Measurements
Gas exchange parameters were recorded between 10.00 a.m and 02.00 p.m in fully expanded leaves at midstem position in six plants per treatment using a LI-6800 Portable Photosynthesis System infrared gas analyzer (IRGA: LI-COR Inc. Nebraska, USA), equipped with 6800-01A leaf chamber (6 cm2 aperture). Version 1.3.17 of the LI-COR software was used for data collection in the present study, and system warmup tests were run before measurements according to manufacturer’s recommendations. After successfully passing warmup tests, environmental conditions of leaf chamber were adapted to physiological demands of lettuce as described by some authors (Hidalgo-Santiago et al. 2021). In this way, all gas exchange parameters were measured at 350 μmol m−2 s−1 of photosynthetically active radiation (PAR), 400 µmol mol−1 CO2 concentration, leaf temperature at 30 °C, relative humidity at 70%, and chamber fan mixing speed at 10,000 rpm. For each plant, 9 measurements were taken, and the mean was expressed for the parameters analyzed: net photosynthetic rate (A), transpiration rate (E), intercellular CO2 (Ci), and stomatal conductance (gs) (Márquez et al. 2021; Saathoff and Welles 2021). The water use efficiency (WUE) was calculated as A/E, the carboxylation efficiency (CE) was estimated as A/Ci, and the stomatal limitation (Ls) was assayed as 1 − Ci/Ca (where Ca represents the ambient CO2 concentration) (Ma et al. 2019; do Rosário Rosa et al. 2021).
On the other hand, after each measurement of these parameters, a rapid A-Ci response curve (RACiR) was used to estimate the Rubisco maximum carboxylation rate (Vcmax) and the maximum rate of electron transport (Jmax). For the RACiR curve, the reference CO2 was adjusted so that increasing concentrations of CO2 from 10 to 510 µmol mol−1 were applied to the leaf through the chamber. As the applied CO2 concentration increased, the LI-6800 measured the net photosynthetic rate and the intercellular CO2 every 2 s for 7 min. To correct the RACiR curve data recorded in LI-6800 software, a RACiR curve was done with the chamber closed and without plant leaf before the first measure. To determine Vcmax and Jmax, the ‘plantecophys’ package in R described by Duursma (2015) was used by fitting the data with the traditional Farquhar et al. (1980) model.
Quantification of Soluble Sugars and Starch Concentration
The extraction and quantification of soluble sugars (sucrose, glucose, and fructose) and starch were assayed according to Dien et al. (2019) with small modifications. 0.1 g of frozen lettuce leaves were homogenized in 1 mL of ethanol 83%. After centrifugation (2800×g for 10 min), supernatant was used for soluble sugars determination, while the pellet was oven-dried at 40 °C for 48 h and the resulting dry residue was employed to quantify the starch content. 100 µL of supernatant was added into glass tubes of 50 mL capacity with 3 mL anthrone 0.1%. This mixture was incubated at 100 °C for 10 min. Afterward, soluble sugars were determined at 650 nm against a standard curve of glucose. On the other hand, 250 µL of distilled water, 250 µL of 4 M sodium acetate buffer (pH 4.5), and 250 µL of glucoamylase 0.5% were added to the dry residue for starch determination. After incubation at 40 °C for 48 h, the samples were filtered and 100 µL of supernatant were added into glass tubes of 50 mL capacity with 3 mL anthrone 0.1%. After 10 min of incubation at 100 °C, starch was quantified at 650 nm using a standard curve of glucose.
Statistical Procedures
The data were subjected to a simple ANOVA at 95% confidence, using the Statgraphics Centurion 16.1.03 software. Means were compared by Fisher’s least significant differences (LSD) and the significance levels were expressed as *p < 0.05, **p < 0.01, ***p < 0.001, or NS (not significant). For growth parameters, a total of eight replicates were employed, whereas six replicates were used for photosynthetic parameters and nine for biochemical analysis.
Results
Effect of HS on Lettuce Plants Growth
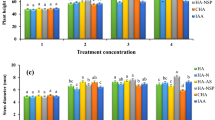
Leonardite-based product significantly increased biomass production in terms of shoot fresh weight with respect to the control at doses: R1 (16%), R2 (17%), R3 (23%), F2 (13%), and F3 (13%). In this way, the leaf area and leaf RGR were also increased by the same HS doses. The application of root-HS offered better results in terms of shoot growth than foliar HS, showing plants treated with R3 dose the highest values (Table 1; Fig. 1A, B). On the other hand, radicular HS applied at doses R2, R3, F2, F3, and F4 enhanced root fresh weight with an increase of 32%, 33%, 15%, 16%, and 21%, respectively, compared to control. Besides, root RGR was also improved by the same treatments. Therefore, as for shoot growth, radicular applications resulted in greatest increase in root biomass production and root RGR, with R3 presenting the highest values. Furthermore, R2, R4, and F3 significantly enhanced root length with respect to control plants, whereas no significant difference was observed for root surface area at any HS dose and form (Table 1; Fig. 2).
Photosynthetic Efficiency of Lettuce Plants Subjected to Radicular and Foliar HS
Foliar HS at dose F2 increased Chl a concentration, while R1 and F1 showed a significant reduction of Chl a compared to control plants. R4 enhanced Chl b, whereas F2 increased total chlorophylls concentration. Furthermore, all radicular doses decreased carotenoids concentration, whereas F3 enhanced it (Table 2).
With respect to leaf gas exchange parameters, A was generally increased by radicular and foliar humic substances compared to control plants, except for R1 and F4 doses. Plants subjected to radicular R3 HS presented the highest A. The foliar application of HS at doses F1, F2, and F3 enhanced E, Ci, and gs, while F4 significantly decreased E. Furthermore, plants treated with radicular HS R2 showed an increased in E and gs, while Ci was enhanced by R2, and R3. Concerning Ls, a significant decrease was observed in R2, F1, F2, and F3 treatments. The WUE was enhanced by R2, R3, R4, F3, and F4, showing plants of R3 treatment the highest value (Table 3). Respect to Vcmax and CE, all doses and forms of HS increased both parameters except R1 and F4, with the highest values presented in R3 dose (Fig. 3A, B). Besides, R3, R4, F1, and F2 significantly enhanced Jmax (Fig. 3C).
Regarding fluorescence parameters, no significant differences were recorded for Fv/Fm (Fig. 2A). Lettuce plants treated with R2, R3, and F4 showed higher values of RC/ABS (Fig. 2B). Concerning PIABS, it was also enhanced by HS application at R2, R3, F2, F3, and F4, with the highest value recorded by R3 dose (Fig. 4C). Besides, ΨEo and ΦEo were increased by F3 and F4 application (Fig. 4D, E), while a significant reduction of N was found in lettuce plants treated with R2 and R4 humic substances doses (Fig. 4F).
Effect of root and foliar application of humic substances on fluorescence parameters Fv/Fm (A), RC/ABS (B), PIABS (C), ΨEo (D), ΦEo. (E), and N (F). Values are expressed as means ± standard error (n = 6). Columns marked with the same letters were not significantly different based on the LSD test (p < 0.05)
Effect of HS on Soluble Sugars and Starch Concentration
The soluble sugars and starch concentration followed the same trend. Thus, radicular application of HS at doses R1, R2, and R4 as well as foliar doses F1, F2, and F3 significantly enhanced soluble sugars and starch concentrations, showing radicular doses the largest increases. However, R3 and F4 decreased soluble sugars, with the lowest value presented by plants treated with R3, whereas both doses did not affect starch concentration (Fig. 5A, B).
Discussion
Plant biostimulants have been defined as a potential tool to reduce the uncontrolled use of chemical fertilizers due to their low impact on the environment and their positive effects on plant growth and agricultural production (Li et al. 2022). A biostimulant product that has been studied for decades is humic substances, which constitute more than 60% of soil organic matter (Canellas et al. 2015). The irrigation or spraying with humic and/or fulvic acids increase shoot growth, as has been previously demonstrated by different authors. In this way, Maji et al. (2017) observed a significant increase in the fresh weight of shoots in Pisum sativum L. plants treated with humic acid-rich vermicompost mixed with soil. Likewise, the pretreatment of Oryza sativa L. with HA as well as the foliar application of HA to Physalis alkekengi L. plants also enhanced shoot growth (Huertas Tavares et al. 2019; Kazemi et al. 2023). For leafy vegetables that are consumed fresh, such as lettuce, the shoot fresh weight, the RGR, and the leaf area are reliable parameters of crop productivity (Tan et al. 2020). In the present study, we used BLACKJAK®, a product previously demonstrated to enhance crop yield under field conditions (Černý et al. 2018). However, under environmental controlled growth conditions, it has not been analyzed. Additionally, the comparative efficacy of its application methods (root versus foliar) remains unstudied. Therefore, the present study evaluated which application method is more effective in enhance lettuce growth. Thus, BLACKJAK® application increased all growth parameters at doses R1, R2, R3, F2, and F3 compared to control plants. As previously reported, the physiological effects of HS depend on different factors such as the mode and rate of application (Canellas et al. 2015). Thereby, our results suggest that intermediate radicular doses of the HS-based product used for this study could provide a greater shoot growth compared to foliar applications, being R3 the dose that offered better results.
Similarly, changes in root growth stimulated by HS are one of the most reported beneficial effects of this type of plant biostimulant (Canellas et al. 2015; Olaetxea et al. 2017; Rathor et al. 2023). Nunes et al. (2019) observed an enhanced in root biomass production in maize plants by adding HA to the culture. Similar results were obtained in wheat plants by radicular FA (Yao et al. 2019), in maize seedlings roots treated with HA (Zandonadi et al. 2019), in cucumber plants after foliar spraying with sedimentary HA (De Hita et al. 2020), as well as in cucumber and Arabidopsis plants after radicular HA application (Aranaz et al. 2023). In this experiment, root biomass and root RGR were improved by HS application at doses R2, R3, F2, F3, and F4 with the highest values presented by plants treated with radicular HS, especially at R3 dose. In addition, the positive effects of HA and FA on crop productivity are mainly due to the modifications in root architecture with the subsequent stimulation of nutrients and water uptake (Nunes et al. 2019). Qin and Leskovar (2020) observed that the application of a commercial lignite HS-based product, composed by 32% HA and 3% FA, increased root length and root surface area in four different species (pepper, tomato, watermelon, and lettuce). In the present work, only R2, R4, and F3 increased root length of lettuce plants, although HS application did not affect root surface area. As it is well known, the source from which humic substances are obtained, the type of humic substance applied, the proportion of HA and FA, the chemical composition, among other factors, may cause different physiological results between different HS (Canellas et al. 2015). This could explain that our HS-based product did not affect the root surface area in lettuce plants grown under control conditions.
Fahramand et al. (2014) suggested that the increase of root growth induced by HS is more pronounced than that in the shoot which may be verified with the results obtained in our experiment. The improvement of root growth by HS has been defined as “auxin-like effect” through the induction of root plasma membrane ATPase activity, with the subsequent apoplast acidification and increased root cell wall plasticity (de Azevedo et al. 2019; Monda et al. 2021; Rathor et al. 2023). This could explain the increased root growth observed in our study, both in terms of root biomass and length. Comparing both modes of application, radicular HS doses offered better results than foliar HS, which could be attributed to the direct contact of HS with the root during radicular applications, subsequently leading to the activation of the root plasma membrane ATPase (Olaetxea et al. 2017). In addition, previous studies have reported that HS increase nutrient bioavailability through the formation of HS-nutrient complexes and also enhance the activity of root nutrient transporters (García-Mina et al. 2004; Jindo et al. 2016; Tomasi et al. 2013). All of these factors contribute to improved plant growth. Consequently, direct contact of HS applied at root level may enhance water and nutrient uptake, resulting in increased shoot growth compared to foliar applications. Therefore, the root application of HS used in this experiment, particularly at R3, could be a better option than foliar spraying for lettuce growth.
The mechanisms of HS-induced growth enhancement have been studied with a focus on different aspects of plant physiology, particularly nutrient uptake and use efficiency (Canellas et al. 2015; Jindo et al. 2016) as well as induced changes in phytohormonal profile and the emulation of hormone functions (Chen et al. 2022; De Hita et al. 2020). In the present experiment, we focused on photosynthesis performance as a mechanism of action, aiming to compare between both modes of application effects on this essential process. The hypothesis that an increase in photosynthesis efficiency results in an increase in crop yield has been extensively studied and confirmed (Milenković et al. 2021). During the photosynthesis process, photosynthetic pigments such as Chl a and b, and carotenoids are responsible for light harvesting as well as photoprotection (Simkin et al. 2022). Although the enhancement in photosynthetic pigments concentration induced by humic substances has been extensively studied by different research authors (Bayat et al. 2021; Fan et al. 2014), no effect on pigments has also been observed by others under control conditions (Ali et al. 2015; Bijanzadeh et al. 2021; Hernandez et al. 2015). In our experiment, only F2 and F3 enhanced total chlorophylls and carotenoids concentration, respectively, while radicular HS decreased carotenoids. Hence, the results obtained suggest that only foliar spraying with HS positively affected pigments concentration at doses that enhanced plant biomass. Moreover, photosynthetic pigments concentration is not necessarily linked with plant growth (Trevisan et al. 2010), as can be observed in radicular-treated plants in our study.
The leaf gas exchange parameters such as A, gs, Ci, Vcmax, Jmax, and PSII activity offer us an approximation about leaf photosynthesis efficiency (Coursolle et al. 2019; Fan et al. 2014; Mumtaz et al. 2020). In our study, R2, R3, R4, F1, F2, and F3 enhanced A and Jmax by R3, R4, F1, and F2 treatments. Hence, the results obtained in our experiment suggest that the leonardite-SC-based product generally enhanced the photosynthesis performance of lettuce plants. If we compare both application methods, intermediate root doses, especially R3, offered better results in terms of photosynthesis efficiency compared to foliar applications. Furthermore, an increase in WUE was observed in plants treated with R2, R3, R4, F3, and F4, which suggest that these doses of leonardite-HS could be appropriate for future experiments to increase drought stress tolerance (Rabbani and Kazemi 2022). The positive influence of HS on photosynthesis has been reported by different authors. Thus, Azcona et al. (2011) observed an increase in net photosynthesis in pepper plants subjected to radicular HS derived from composted sludge, although they did not observe changes in chlorophylls content. Similar results were obtained by Haghighi et al. (2012), where HA application to nutrient solution significantly enhanced photosynthesis efficiency in lettuce plants. Likewise, foliar HA derived from sediments improved photosynthesis rate in chrysanthemum (Fan et al. 2014), while similar results were shown in canola plants treated with foliar HA extracted from vermicompost (Hemati et al. 2022), and in maize after foliar HA application (Wang et al. 2023).
One of the main mechanisms by which HS enhance photosynthesis performance is the improvement of Rubisco activity and its CE. In this way, Ertani et al. (2011) found that a commercial lignosulfonate-humate enhanced Rubisco activity and consequently the photosynthesis performance, as has been observed by recent studies in maize plants (Chen et al. 2022; Ertani et al. 2019). These results are in line with those obtained in our experiment, where HS doses that enhanced photosynthesis efficiency also improved Vcmax, which is synonymous with Rubisco activity (Coursolle et al. 2019) and CE. Therefore, an increase in Rubisco CE and activity that results in better photosynthesis performance is correlated with an enhance in biomass production (Milenković et al. 2021; Simkin et al. 2019). Likewise, in the present experiment, the doses that most enhanced the photosynthetic efficiency of lettuce plants also increased shoot biomass, except for F1 dose which did not affect shoot growth. In addition, consistent with biomass production results, applying HS to the nutrient solution, particularly at the R3 dose, could be more effective in enhancing photosynthesis efficiency compared to foliar HS applications. As previously described, an increase in plasma membrane ATPase activity is associated with improved nutrient uptake, which directly enhances photosynthesis performance (Zhang et al. 2021). Furthermore, as discussed earlier, direct contact of radicular HS with roots could lead to a greater increase in water and nutrient bioavailability, uptake, and utilization compared to foliar HS, as previously observed by Atero-Calvo et al. (2023). These positive effects could result in better stomatal gas exchange, thereby enhancing Rubisco activity, and WUE, which as described by Guo et al. (2019), Liu et al. (2013), and Wang et al. (2022), enhances net photosynthesis and plant growth.
Chlorophyll a fluorescence measurement is used to study the photochemical reactions of photosynthesis through different parameters (Navarro-León et al. 2023). Thus, RC/ABS represents how much energy is usable for photosynthesis, PIABS determines the plant vitality, while ΨEo and ΦEo indicate the electron transport efficiency (Navarro-León et al. 2018, 2023). Overall, the application of HS improved the PSII capability to capture light energy according to the results of fluorescence parameters, especially at doses R2, R3, F2, F3, and F4, which contributed to photosynthesis performance of lettuce plants. Fan et al. (2014) found that the foliar fertilization with HA enhanced the capability of PSII to use light energy with the consequent increase in A, which agrees with the results obtained in our study.
The CO2 fixation by Rubisco enzyme results in the generation of soluble sugars such as sucrose, glucose, and fructose, and starch, through the named Calvin-Benson cycle (Simkin et al. 2019). These carbohydrates are the final products of the photosynthesis process, and its generation and concentration is correlated with crop yield (Simkin et al. 2019). The increase in sucrose and starch in tobacco plants with highest photosynthetic CO2 assimilation rates, resulted in a 30% increase in biomass (Lefebvre et al. 2005), as was later found in tomato plants with increases in biomass, sucrose, and starch (Ding et al. 2016). Similarly, our results showed that the increase in photosynthesis efficiency in leonardite-HS treatments at doses R2, F2, and F3 enhanced soluble sugars and starch concentration, which resulted in enhanced shoot biomass production. Radicular-HS applied at dose R2 showed higher soluble sugars and starch concentration increment compared to foliar applications. Similar results were found by Ertani et al. (2011) in maize plants treated with two different lignosulfonate-humate. Nevertheless, the R3 dose reduced soluble sugars and had no effect on starch concentration. This result might be attributed to the soluble sugars being converted into complex organic molecules that facilitate plant growth, similar to the mechanism proposed by Rosa et al. (2009). Thus, Canellas et al. (2013) showed that total carbohydrate content and soluble sugars decreased (− 60%) in maize plants after HS application, which was accompanied by a 17% increase in grain production. These results indicated that the soluble sugars may be redirected toward supporting growth.
In conclusion, the leonardite-SC-based product (BLACKJAK®) used in the present study clearly enhanced plant growth and photosynthesis efficiency at most of the doses applied, especially at intermediate doses (R2, R3, F2, and F3). Therefore, our findings show the potential implications of BLACKJAK® in enhancing crop yields by improving photosynthesis performance, which is of great importance for practical applications in agriculture given the need to feed the world’s growing population. Furthermore, the most suitable application method for farmers to achieve higher yields would be through irrigation (radicular application). Particularly, R3 was the dose that produced the largest increases in shoot and root growth, as well as photosynthetic activity.
Abbreviations
- A :
-
Net photosynthetic rate
- CE :
-
Carboxylation efficiency
- Ci :
-
Intercellular CO2
- E :
-
Transpiration rate
- FA:
-
Fulvic acids
- gs :
-
Stomatal conductance
- Jmax :
-
Maximum rate of electron transport
- Ls :
-
Stomatal limitation
- HA:
-
Humic acids
- HS:
-
Humic substances
- PS:
-
Photosystem
- RGR:
-
Relative growth rate
- Vcmax :
-
Rubisco maximum carboxylation rate
- WUE:
-
Water use efficiency
References
Ali S, Aslam Bharwana S, Rizwan M et al (2015) Fulvic acid mediates chromium (Cr) tolerance in wheat (Triticum aestivum L.) through lowering of Cr uptake and improved antioxidant defense system. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-015-4271-7
Aranaz J, de Hita D, Olaetxea M et al (2023) The molecular conformation, but not disaggregation, of humic acid in water solution plays a crucial role in promoting plant development in the natural environment. Front Plant Sci 14:1–16. https://doi.org/10.3389/fpls.2023.1180688
Araus JL, Sanchez-Bragado R, Vicente R (2021) Improving crop yield and resilience through optimization of photosynthesis: panacea or pipe dream? J Exp Bot 72:3936–3955. https://doi.org/10.1093/jxb/erab097
Atero-Calvo S, Magro F, Masetti G et al (2023) Assaying the use of a leonardite-suspension concentrate-based product as a potential biostimulant to enhance growth, NPK use efficiency, and antioxidant capacity in Lactuca sativa L. Agronomy 14:64. https://doi.org/10.3390/agronomy14010064
Azcona I, Pascual I, Aguirreolea J et al (2011) Growth and development of pepper are affected by humic substances derived from composted sludge. J Plant Nutr Soil Sci 174:916–924. https://doi.org/10.1002/JPLN.201000264
Bayat H, Shafie F, Aminifard MH, Daghighi S (2021) Comparative effects of humic and fulvic acids as biostimulants on growth, antioxidant activity and nutrient content of yarrow (Achillea millefolium L.). Sci Hortic 279:109912. https://doi.org/10.1016/j.scienta.2021.109912
Bijanzadeh E, Emam Y, Pessarakli M (2021) Biochemical responses of water-stressed triticale (X Triticosecale wittmack) to humic acid and jasmonic acid. J Plant Nutr 44:252–269. https://doi.org/10.1080/01904167.2020.1806312
Canellas LP, Balmori DM, Médici LO et al (2013) A combination of humic substances and Herbaspirillum seropedicae inoculation enhances the growth of maize (Zea mays L.). Plant Soil 366:119–132. https://doi.org/10.1007/s11104-012-1382-5
Canellas LP, Olivares FL, Aguiar NO et al (2015) Humic and fulvic acids as biostimulants in horticulture. Sci Hortic 196:15–27. https://doi.org/10.1016/J.SCIENTA.2015.09.013
Cao TND, Mukhtar H, Le LT et al (2023) Roles of microalgae-based biofertilizer in sustainability of green agriculture and food-water-energy security nexus. Sci Total Environ 870:161927. https://doi.org/10.1016/J.SCITOTENV.2023.161927
Černý I, Pačuta V, Ernst D, Gažo J (2018) Formation of sugar beet yield and sugar content depending on year and foliar application of biologically active substances and fertilizers. LCaR 134:141–145
Chen Q, Qu Z, Ma G et al (2022) Humic acid modulates growth, photosynthesis, hormone and osmolytes system of maize under drought conditions. Agric Water Manag 263:107447. https://doi.org/10.1016/j.agwat.2021.107447
Chi Y, Yang P, Ren S et al (2020) Effects of fertilizer types and water quality on carbon dioxide emissions from soil in wheat-maize rotations. Sci Tot Environ 698:134010. https://doi.org/10.1016/j.scitotenv.2019.134010
Conselvan GB, Pizzeghello D, Francioso O et al (2017) Biostimulant activity of humic substances extracted from leonardites. Plant Soil 420:119–134. https://doi.org/10.1007/S11104-017-3373-Z/TABLES/8
Coursolle C, Otis Prud’homme G, Lamothe M, Isabel N (2019) Measuring rapid A-Ci curves in boreal conifers: black spruce and balsam fir. Front Plant Sci 10:460954. https://doi.org/10.3389/FPLS.2019.01276/BIBTEX
de Azevedo IG, Olivares FL, Ramos AC et al (2019) Humic acids and Herbaspirillum seropedicae change the extracellular H+ flux and gene expression in maize roots seedlings. Chem Biol Technol Agric 6:1–10. https://doi.org/10.1186/s40538-019-0149-0
de Rosário RV, Farias dos Santos AL, Alves da Silva A et al (2021) Increased soybean tolerance to water deficiency through biostimulant based on fulvic acids and Ascophyllum nodosum L. seaweed extract. Plant Physiol Biochem 158:228–243. https://doi.org/10.1016/j.plaphy.2020.11.008
De Hita D, Fuentes M, Fernández V et al (2020) Discriminating the short-term action of root and foliar application of humic acids on plant growth: emerging role of jasmonic acid. Front Plant Sci 11:493. https://doi.org/10.3389/FPLS.2020.00493/BIBTEX
Dien DC, Mochizuki T, Yamakawa T (2019) Effect of various drought stresses and subsequent recovery on proline, total soluble sugar and starch metabolisms in rice (Oryza sativa L.) varieties. Plant Prod Sci 22:530–545. https://doi.org/10.1080/1343943X.2019.1647787
Ding F, Wang M, Zhang S, Ai X (2016) Changes in SBPase activity influence photosynthetic capacity, growth, and tolerance to chilling stress in transgenic tomato plants. Sci Rep 6:1–14. https://doi.org/10.1038/srep32741
du Jardin P (2015) Plant biostimulants: definition, concept, main categories and regulation. Sci Hortic 196:3–14. https://doi.org/10.1016/j.scienta.2015.09.021
Duursma RA (2015) Plantecophys—an R package for analysing and modelling leaf gas exchange data. PLoS ONE 10:1–13. https://doi.org/10.1371/journal.pone.0143346
Ertani A, Francioso O, Tugnoli V et al (2011) Effect of commercial lignosulfonate-humate on Zea mays L. metabolism. J Agric Food Chem 59:11940–11948. https://doi.org/10.1021/jf202473e
Ertani A, Nardi S, Francioso O et al (2019) Metabolite-targeted analysis and physiological traits of Zea mays L. in response to application of a leonardite-humate and lignosulfonate-based products for their evaluation as potential biostimulants. Agronomy 9:445. https://doi.org/10.3390/agronomy9080445
Fahramand M, Moradi H, Noori M, Sobhkhizi A (2014) Influence of humic acid on increase yield of plants and soil properties. Int J Farm Allied Sci 3:339–341
Fan HM, Wang XW, Sun X et al (2014) Effects of humic acid derived from sediments on growth, photosynthesis and chloroplast ultrastructure in chrysanthemum. Sci Hortic 177:118–123. https://doi.org/10.1016/j.scienta.2014.05.010
Farquhar GD, Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90. https://doi.org/10.1007/BF00386231
Garcia-Mina JM, Antolin MC, Sanchez-Diaz M (2004) Metal-humic complexes and plant micronutrient uptake: a study based on different plant species cultivated in diverse soil types. Plant Soil 258:57–68. https://doi.org/10.1023/B:PLSO.0000016509.56780.40
Guo J, Jia Y, Chen H et al (2019) Growth, photosynthesis, and nutrient uptake in wheat are affected by differences in nitrogen levels and forms and potassium supply. Sci Rep 9:1248. https://doi.org/10.1038/s41598-018-37838-3
Haghighi M, Kafi M, Fang P (2012) Photosynthetic activity and N metabolism of lettuce as affected by humic acid. Int J Veg Sci 18:182–189. https://doi.org/10.1080/19315260.2011.605826
Hemati A, Alikhani HA, Babaei M et al (2022) Effects of foliar application of humic acid extracts and indole acetic acid on important growth indices of canola (Brassica napus L.). Sci Rep 12:20033. https://doi.org/10.1038/s41598-022-21997-5
Hernandez OL, Calderín A, Huelva R et al (2015) Humic substances from vermicompost enhance urban lettuce production. Agron Sustain Dev 35:225–232. https://doi.org/10.1007/s13593-014-0221-x
Hidalgo-Santiago L, Navarro-León E, López-Moreno FJ et al (2021) The application of the silicon-based biostimulant Codasil® offset water deficit of lettuce plants. Sci Hortic 285:110177. https://doi.org/10.1016/j.scienta.2021.110177
Hoagland DR, Arnon DI (1950) Preparing the nutrient solution. Water-Cult Method Grow Plants without Soil 347:29–31
Huertas Tavares OC, Santos LA, Lima de Araújo OJ et al (2019) Humic acid as a biotechnological alternative to increase N-NO3− or N–NH4+ uptake in rice plants. Biocatal Agric Biotechnol 20:101226. https://doi.org/10.1016/j.bcab.2019.101226
Jannin L, Arkoun M, Ourry A et al (2012) Microarray analysis of humic acid effects on Brassica napus growth: involvement of N, C and S metabolisms. Plant Soil 359:297–319. https://doi.org/10.1007/s11104-012-1191-x
Jindo K, Soares TS, Peres LEP et al (2016) Phosphorus speciation and high-affinity transporters are influenced by humic substances. J Plant Nutr Soil Sci 179:206–214. https://doi.org/10.1002/jpln.201500228
Kazemi S, Pirmoradi MR, Karimi H et al (2023) Effect of foliar application of humic acid and zinc sulfate on vegetative, physiological, and biochemical characteristics of Physalis alkekengi L. under soilless culture. J Soil Sci Plant Nutr 23:3845–3856. https://doi.org/10.1007/s42729-023-01305-4
Lefebvre S, Lawson T, Zakhleniuk OV et al (2005) Erratum: increased sedoheptulose-1,7-bisphosphatase activity in transgenic tobacco plants stimulates photosynthesis and growth from an early stage in development. Plant Physiol 138:1174. https://doi.org/10.1104/pp.104.900163
Li J, Van Gerrewey T, Geelen D (2022) A meta-analysis of biostimulant yield effectiveness in field trials. Front Plant Sci 13:1–13. https://doi.org/10.3389/fpls.2022.836702
Liu X, Fan Y, Long J et al (2013) Effects of soil water and nitrogen availability on photosynthesis and water use efficiency of Robinia pseudoacacia seedlings. J Environ Sci 25:585–595. https://doi.org/10.1016/S1001-0742(12)60081-3
Ma J, Janoušková M, Ye L et al (2019) Role of arbuscular mycorrhiza in alleviating the effect of cold on the photosynthesis of cucumber seedlings. Photosynthetica 57:86–95. https://doi.org/10.32615/ps.2019.001
Maji D, Misra P, Singh S, Kalra A (2017) Humic acid rich vermicompost promotes plant growth by improving microbial community structure of soil as well as root nodulation and mycorrhizal colonization in the roots of Pisum sativum. Appl Soil Ecol 110:97–108. https://doi.org/10.1016/j.apsoil.2016.10.008
Márquez DA, Stuart-Williams H, Farquhar GD (2021) An improved theory for calculating leaf gas exchange more precisely accounting for small fluxes. Nat Plants 7:317–326. https://doi.org/10.1038/s41477-021-00861-w
Milenković I, Borišev M, Zhou Y et al (2021) Photosynthesis enhancement in maize via nontoxic orange carbon dots. J Agric Food Chem 69:5446–5451. https://doi.org/10.1021/acs.jafc.1c01094
Monda H, McKenna AM, Fountain R, Lamar RT (2021) Bioactivity of humic acids extracted from shale ore: molecular characterization and structure-activity relationship with tomato plant yield under nutritional stress. Front Plant Sci 12:1–17. https://doi.org/10.3389/fpls.2021.660224
Muhie SH (2022) Optimization of photosynthesis for sustainable crop production. CABI Agric Biosci 3:1–8. https://doi.org/10.1186/s43170-022-00117-3
Mumtaz MA, Munir S, Liu G et al (2020) Altered brassinolide sensitivity1 transcriptionally inhibits chlorophyll synthesis and photosynthesis capacity in tomato. Plant Growth Regul 92:417–426. https://doi.org/10.1007/s10725-020-00650-z
Nardi S, Muscolo A, Vaccaro S et al (2007) Relationship between molecular characteristics of soil humic fractions and glycolytic pathway and krebs cycle in maize seedlings. Soil Biol Biochem 39:3138–3146. https://doi.org/10.1016/j.soilbio.2007.07.006
Navarro-León E, Ruiz JM, Graham N, Blasco B (2018) Physiological profile of CAX1a TILLING mutants of Brassica rapa exposed to different calcium doses. Plant Sci 272:164–172. https://doi.org/10.1016/j.plantsci.2018.04.019
Navarro-León E, Oviedo-Silva J, Ruiz JM, Blasco B (2019) Possible role of HMA4a TILLING mutants of Brassica rapa in cadmium phytoremediation programs. Ecotoxicol Environ Saf 180:88–94. https://doi.org/10.1016/j.ecoenv.2019.04.081
Navarro-León E, Grazioso A, Atero-Calvo S et al (2023) Evaluation of the alkalinity stress tolerance of three Brassica rapa CAX1 TILLING mutants. Plant Physiol Biochem 198:107712. https://doi.org/10.1016/j.plaphy.2023.107712
Olaetxea M, De Hita D, Andrés Garcia C et al (2017) Hypothetical framework integrating the main mechanisms involved in the promoting action of rhizospheric humic substances on plant root-and shoot-growth. Appl Soil Ecol 123:521–537. https://doi.org/10.1016/j.apsoil.2017.06.007
Oliveira Nunes R, Abrahão Domiciano G, Sousa Alves W et al (2019) Evaluation of the effects of humic acids on maize root architecture by label-free proteomics analysis. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-48509-2
Qin K, Leskovar DI (2020) Humic substances improve vegetable seedling quality and post-transplant yield performance under stress conditions. Agric 10:1–18. https://doi.org/10.3390/agriculture10070254
Rabbani M, Kazemi F (2022) Water need and water use efficiency of two plant species in soil-containing and soilless substrates under green roof conditions. J Environ Manag 302:113950. https://doi.org/10.1016/j.jenvman.2021.113950
Rathor P, Gorim LY, Thilakarathna MS (2023) Plant physiological and molecular responses triggered by humic based biostimulants—a way forward to sustainable agriculture. Plant Soil. https://doi.org/10.1007/s11104-023-06156-7
Roháček K (2002) Chlorophyll fluorescence parameters: the definitions, photosynthetic meaning, and mutual relationships. Photosynthetica 40:13–29. https://doi.org/10.1023/A:1020125719386
Rosa M, Prado C, Podazza G et al (2009) Soluble sugars: Metabolism, sensing and abiotic stress: a complex network in the life of plants. Plant Sign Behavior 4:388–393
Saathoff AJ, Welles J (2021) Gas exchange measurements in the unsteady state. Plant, Cell Environ 44:3509–3523. https://doi.org/10.1111/pce.14178
Savarese C, Cozzolino V, Verrillo M et al (2022) Combination of humic biostimulants with a microbial inoculum improves lettuce productivity, nutrient uptake, and primary and secondary metabolism. Plant Soil 481:285–314. https://doi.org/10.1007/s11104-022-05634-8
Simkin AJ, Lopez-Calcagno PE, Davey PA et al (2017) Simultaneous stimulation of sedoheptulose 1,7-bisphosphatase, fructose 1,6-bisphophate aldolase and the photorespiratory glycine decarboxylase-H protein increases CO2 assimilation, vegetative biomass and seed yield in Arabidopsis. Plant Biotechnol J 15:805–816. https://doi.org/10.1111/pbi.12676
Simkin AJ, López-Calcagno PE, Raines CA (2019) Feeding the world: improving photosynthetic efficiency for sustainable crop production. J Exp Bot 70:1119–1140. https://doi.org/10.1093/JXB/ERY445
Simkin AJ, Kapoor L, Doss CGP et al (2022) The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth Res 152:23–42. https://doi.org/10.1007/s11120-021-00892-6
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. Springer, Dordrecht, pp 321–362
Tan WK, Goenadie V, Lee HW et al (2020) Growth and glucosinolate profiles of a common Asian green leafy vegetable, Brassica rapa subsp. chinensis var. parachinensis (choy sum), under LED lighting. Sci Hortic 261:108922. https://doi.org/10.1016/j.scienta.2019.108922
Tiwari J, Ramanathan AL, Bauddh K, Korstad J (2023) Humic substances: structure, function and benefits for agroecosystems-a review. Pedosphere 33:237–249. https://doi.org/10.1016/j.pedsph.2022.07.008
Tomasi N, De Nobili M, Gottardi S et al (2013) Physiological and molecular characterization of Fe acquisition by tomato plants from natural Fe complexes. Biol Fertil Soils 49:187–200. https://doi.org/10.1007/s00374-012-0706-1
Trevisan S, Francioso O, Quaggiotti S, Nardi S (2010) Humic substances biological activity at the plant-soil interface. Plant Signal Behav 5:635–643. https://doi.org/10.4161/psb5611211
Wang C, Yue L, Cheng B et al (2022) Mechanisms of growth-promotion and Se-enrichment in Brassica chinensis L. by selenium nanomaterials: beneficial rhizosphere microorganisms, nutrient availability, and photosynthesis. Environ Sci: Nano 9:302–312. https://doi.org/10.1039/D1EN00740H
Wang Y, Lu Y, Wang L et al (2023) Analysis of the molecular composition of humic substances and their effects on physiological metabolism in maize based on untargeted metabolomics. Front Plant Sci 14:1–17. https://doi.org/10.3389/fpls.2023.1122621
Wei J, Tu C, Xia F et al (2023) Enhanced removal of arsenic and cadmium from contaminated soils using a soluble humic substance coupled with chemical reductant. Environ Res 220:115120. https://doi.org/10.1016/j.envres.2022.115120
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313. https://doi.org/10.1016/S0176-1617(11)81192-2
Yang F, Tang C, Antonietti M (2021) Natural and artificial humic substances to manage minerals, ions, water, and soil microorganisms. Chem Soc Rev 50:6221–6239. https://doi.org/10.1039/d0cs01363c
Yao Y, Wang C, Wang X et al (2019) Activation of fulvic acid-like in paper mill effluents using H2O2/TiO2 catalytic oxidation: characterization and salt stress bioassays. J Hazard Mater 378:2–9. https://doi.org/10.1016/j.jhazmat.2019.05.095
Yuan Y, Tang C, Jin Y et al (2022) 2022 Contribution of exogenous humic substances to phosphorus availability in soil-plant ecosystem: a review. Crit Rev Environ Sci Technol. https://doi.org/10.1080/10643389.2120317
Zandonadi DB, Matos CRR, Castro RN et al (2019) Alkamides: a new class of plant growth regulators linked to humic acid bioactivity. Chem Biol Technol Agric 6:1–12. https://doi.org/10.1186/s40538-019-0161-4
Zanin L, Tomasi N, Zamboni A et al (2018) Water-extractable humic substances speed up transcriptional response of maize roots to nitrate. Environ Exp Bot 147:167–178. https://doi.org/10.1016/j.envexpbot.2017.12.014
Zanin L, Tomasi N, Cesco S et al (2019) Humic substances contribute to plant iron nutrition acting as chelators and biostimulants. Front Plant Sci 10:675. https://doi.org/10.3389/FPLS.2019.00675/BIBTEX
Zhang M, Wang Y, Chen X et al (2021) Plasma membrane H+-ATPase overexpression increases rice yield via simultaneous enhancement of nutrient uptake and photosynthesis. Nat Commun 12:735. https://doi.org/10.1038/s41467-021-20964-4
Acknowledgements
This research was supported by the PAI program (Plan Andaluz de Investigación, Grupo de Investigación AGR282) and by a Grant from the FPU of the Ministerio de Educación y Ciencia awarded to S.A.C. grant number [FPU20/05049].
Funding
Funding for open access publishing: Universidad de Granada/CBUA.
Author information
Authors and Affiliations
Contributions
FM, GM, JJR, BB, and JMR conceived and designed research. SAC and ENL conducted experiments. FM and GM provided biostimulant. SAC and ENL analyzed data. SAC wrote the manuscript. FM, GM, JJR, BB, and JMR did a critical revision of the article. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Approval
The authors declare that manuscript reporting studies do not involve any human participants, human data, or human tissue.
Additional information
Handling Editor: Soumya Mukherjee.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Atero-Calvo, S., Magro, F., Masetti, G. et al. Comparative Effects of Root and Foliar Leonardite-Suspension Concentrate Application on Plant Growth and Photosynthetic Efficiency of Lettuce Plants (Lactuca sativa L.). J Plant Growth Regul (2024). https://doi.org/10.1007/s00344-024-11424-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00344-024-11424-6