Abstract
Under field conditions high temperatures are usually associated to high light intensity for periods of time that are getting longer because of global warming caused by climate change. These adverse conditions lead to significant reductions in yield and fruit quality in crops of great economic relevance such as citrus. In this work, the effect of high temperatures and high light intensity occurring alone or simultaneously has been studied in Carrizo citrange plants, a citrus genotype widely used as a rootstock, and the mitigating effect of kaolin (KL) evaluated. The combined stress conditions affected the plants in a unique manner at both, physiological and hormonal regulation levels, different to the effects of each individual stress. KL alleviated the deleterious effect of combined stress in different ways: (i) reducing leaf damage and abscission, (ii) improving physiological and gas exchange parameters, (iii) decreasing foliar proline content, (iv) increasing chlorophyll content, (v) preventing carotenoid degradation, and (vi) modulating levels of hormones and their precursors involved in plant responses to stress [abscisic acid (ABA), salicylic acid (SA), cinnamic acid (CA), indole-3-acetic acid (IAA), jasmonic acid (JA) and 12-oxophytodienoic acid (OPDA)].
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Citrus is one of the most widely grown fruit crop worldwide. Citrus production and exports have grown steadily over the past three decades, being world citrus production around 150,000 million of tones in 2019 (FAO 2020). Climate change has a significant impact on citrus growth, yield and fruit quality, playing drought, salinity, temperature, and high light intensity an essential role in determining the quantity and quality of the fruit. Although the effects of individually applied abiotic stress conditions on citrus plants have been widely studied (reviewed in Pérez-Clemente et al. 2013; Vives-Peris et al. 2017), it has been only recently that efforts to study responses of this crop to different stress situations occurring simultaneously have appeared (Zandalinas et al. 2016b, 2017a, 2017b, 2018a; Balfagón et al. 2019a, 2021, 2022a).
Citrus are considered subtropical plants. Although citrus grow between 10 and 35 °C, vegetative growth starts at 12.8 °C, while, the optimum growth is between 25 and 30 °C. This temperature range is considered the optimum for photosynthesis and dry matter production, which increase trees vigor and productivity (Abobatta 2021). It has been reported that, if leaf temperature hits 36 °C, trees start feeling mild heat stress (Guha 2022). On the other hand, recent studies conducted to study the physiological and hormonal responses of citrus to a combination of drought and high temperatures determined that 40 °C cause a mild stress in this crop (Zandalinas et al. 2017b).
Photosynthetic activity is highly susceptible to stresses caused by high temperatures. It has been reported that the activity of photosystem II (PSII) declines rapidly when the leaf is exposed to high light intensity (Ahammed et al. 2018). This phenomenon is called photoinhibition and it is responsible for a slow and reversible reduction of photosynthetic efficiency that leads to a partial loss of capacity to convert radiant energy into chemical energy (in the form of sugars). If leaf exposure to excessive radiation is maintained, it leads to photooxidation, a secondary phenomenon occurring after photoinhibition has progressed as a function of light intensity and exposure time, and causes the destruction of photosynthesizing pigments, manifesting as a discoloration of these pigments, and it may also cause cell death (Murata el al. 2007; Goh et al. 2012). However, pigment bleaching only takes place once a certain degree of photoinhibition has already occurred (reviewed in Blanke 2000; Takahashi et al. 2002). Under field conditions, adverse environmental situations do not occur independently but very often take place simultaneously. High temperatures are usually associated with periods of drought and, in many cases, combined with other stress factors such as high light intensity, nutrient deficiencies, or increased concentrations of heavy metals (Mittler 2006; Zandalinas et al. 2018b). In the case of high irradiation and high temperatures occurring simultaneously, studies described up to date are contradictory. While early studies, carried out decades ago, showed a protective effect of high irradiation on the photosynthetic performance of plants subjected to heat stress (HS; Schreiber and Berry 1977), more recent studies showed the opposite effect: strong irradiation in plants exposed to heat stress caused a decrease in the rate of CO2 assimilation (reviewed in Takahashi et al. 2002). According to this, an increase in the activity of the xanthophyll cycle in plants exposed to heat has been described as a protective mechanism from heat damage (Dongsansuk et al. 2013; Buchner et al. 2015). However, this possible protective effect should be confirmed, and new mechanisms involved in the plant response found.
Citrus responses to combined stress situations have been characterized at different levels. In response to combined water stress and heat, changes in plant physiology (Zandalinas et al. 2016b), hormonal levels (Balfagón et al. 2019b) and plant secondary metabolism (Zandalinas et al. 2017b) have been shown. However, up to date, there is no available information on how stress caused by high temperatures and high light intensity occurring simultaneously affects to this crop. Nonetheless, periods of drought in citrus growing areas are very frequent and usually coincident with the absence of clouds that causes that the noon radiation oversaturates the photosynthetic system. Forecasts indicate that both total and ultraviolet radiations to which the crops are exposed will continue increasing in the near future. Therefore, it seems crucial understanding how plants respond to this combination of stresses in order to establish strategies that mitigate the damage caused in citrus trees.
Plants have developed interconnected regulatory pathways that allow them to respond and adapt to a changing environment (Zhang et al. 2022). To counteract the effects of stress, plants accumulate different compounds, which also acts as a scavenger of free radicals (Kavi Kishor and Sreenivasulu 2014). However, plant responses to stress are mainly mediated by phytohormones. Abscisic acid (ABA) is a key signal when plants are exposed to drought or high salinity, regulating transpiration and stomatal closure, synthesis of compatible osmolytes and the expression of genes involved in stress-signaling response (Zandalinas et al. 2016a). Indole-3-acetic acid (IAA) is involved in plant acclimation to salt stress conditions (Wani et al. 2016). Recent studies point out the important role of salicylic acid (SA), traditionally associated to plant responses to biotic stresses, in abiotic stress signaling and tolerance (Miura and Tada 2014; Balfagón et al. 2019b). The role of jasmonic acid (JA) in plant acclimation to combined stress conditions involving high light and heat stress has also been described (Balfagón et al. 2019a). Similarly, it has been demonstrated the involvement of cinnamic acid (CA), a precursor of SA, in plant photoprotection against high light irradiance (Weremczuk-Jeżyna et al. 2021).
In recent years, numerous strategies have been implemented to mitigate the deleterious impact of abiotic stress on yields in many crops. Examples of success in citrus are the use of tolerant rootstocks and cultivars (Balfagón et al. 2022b); inoculation of beneficial bacteria such as Pseudomonas putida and Novosphingobium sp. (Vives-Peris et al. 2018) or the application of reflective material, as kaolin (KL), initially used to function in pest control (Braham et al. 2007).
Foliar sprays with inert reflective materials (such as KL) have been used in greenhouse and orchard systems to mitigate the negative effects of high irradiance and extreme temperature. They achieve this by reducing canopy temperature, minimizing water stress, and reflecting solar radiation from the leaf surface (reviewed in Brito et al. 2019). KL is a mineral clay that easily dissolves in water and their foliar application produced a protective particle film (Glenn 2012). In the literature, the use of KL at 5% has been described in citrus orchards (Lo Verde et al. 2011). Additionally, KL concentrations between 2 and 4% have been recommended for protecting fruits against sunburn (Ennab et al. 2017). Its beneficial effect has been reported on several crops such as olive trees (Brito et al. 2019), nut and kernel (Gharaghani et al. 2018), apple (Glenn 2010) and grapevines (Dinis et al. 2016), resulting in improvements in physiological responses such as photosynthetic and transpiration rates. However, information regarding KL applications in citrus is scarce.
Therefore, the aim of the present work is to elucidate physiological and hormonal responses of Carrizo citrange plants subjected to heat stress (HS) or high light stress (HL), and the combined occurrence of these stressors. Additionally, we aim to evaluate the effectiveness of KL application as a possible strategy to mitigate the harmful effect of these adverse conditions.
Materials and Methods
Plant Material
One-year-old Carrizo citrange (Poncirus trifoliata L. Raf. × Citrus sinensis L. Osb.) plants were purchased from a commercial nursery (Beniplant S.L., Peñíscola, Spain), and transplanted to 0.80 L plastic pots filled up with perlite as substrate. Plants were watered three times a week with a half-strength Hoagland solution (Arbona et al. 2009). Culture conditions in the greenhouse were natural photoperiod and temperatures of 25 ± 3.0 °C and 18 ± 2.0 °C (day/night, respectively). Plants were allowed to acclimate for 2 weeks in growth chambers at 25 °C, a photoperiod of 16 h light/8 h dark (from 8:00 to 24:00 h), 100 µmol m−2 s−1 of light intensity and 80% of relative moisture, as described in Balfagon et al. (2022c).
Stress Treatment
Leaves were sprayed with KL 1% (w/v) in distilled water 2 days before the experiment. Although it has been reported the application of KL at higher concentrations in mature citrus trees growing in the field (Ennab et al. 2017; Lo Verde et al. 2011), in our study, 1-year-old seedlings were used as plant material. A KL concentration of 1% was selected as mitigating stress treatment based on previous assays conducted to determine the minimum KL content that guarantees the formation of a uniform film covering the leaf surface (data not shown). Pulverization was made twice on the same day for ensuring the complete KL adhesion uniformity. For carrying out the experiment, eight experimental groups were established: on one hand, four groups consisted of KL-sprayed plants; on the other hand, four groups included non-KL treated plants (Fig. 1). Each one of the two main groups (KL treated and non-KL treated plants) included the control group (CT) and plants subjected to different stress treatments: heat stress (HS), high light stress (HL) and their combination (HS + HL). Heat stress was imposed maintaining plants at 40 °C/24 h. High light stress was applied by increasing light intensity to 1000 µmol m−2 s−1 during 8 h/day (from 12:00 to 20:00 h) and HS + HL was performed by simultaneously exposing plants to high temperature and high light stress treatments. Control plants (CT) were maintained at 100 µmol m−2 s−1 light intensity at 25 °C. The stress conditions were maintained during 5 days. The experiment was replicated three times with ten plants per group and replicate. The analytical determinations described below were performed with three replicates from each biological sample.
Visual Leaf Damage
For analyzing the effect of different stresses on plant phenotype and the effectivity of KL treatment as a stress damage palliative, leaf conditions were observed, and percentages of damaged and abscised leaves were calculated in each experimental group. Any leaf with chlorotic or necrotic spots was considered damaged.
Physiological Parameters
Quantum yield (ɸPSII) was analyzed with a portable fluorometer (Fluor Pen FP-MAX 100, Photon System Instruments, Czech Republic). These measurements were obtained on three leaves in five plant replicates per treatment (n = 15).
Leaf photosynthesis rate (A), transpiration rate (E) and stomatal conductance (gs) were measured with a LI-6800 Portable Photosynthesis System (Lincoln, USA). Supplemental light was provided by a PAR lamp at 100 or 1000 µmol m−2 s−1 photon flux density, CO2 reference was set in 400 ppm, and air flow was set at 150 µmol mol−1 as fully described in Zandalinas et al. (2016b) and Balfagón et al. (2021). After instrument stabilization, five measurements were taken per leaf in three plants from each stress treatment, being n = 15.
Chlorophyll and Carotenoids Content Analysis
The measurements of the chlorophyll and carotenoids content were performed following the procedure described by Wellburn (1994). Briefly, 20 mg frozen leaf tissue was incubated at 37 °C for 15 h in 2 mL DMSO. After the incubation, the absorbance at different wavelength (665 nm, 649 nm and 480 nm) was measured. The chlorophyll and carotenoid content were calculated as follows:
The results were expressed as µg chlorophyll per gram of fresh weight. Data are the average of three biological replicates in each experimental group.
Proline Analysis
The procedure used for proline analysis was described by (Bates and Waldren 1973). 100 mg frozen leaf tissue was extracted in 5 mL of 3% sulfosalicylic acid by sonication for 30 min. After centrifugation at 4000 × g during 20 min at 4 °C, 1 mL of the supernatant was mixed with 1 mL of glacial acetic acid and 1 mL of ninhydrin reagent in a 1:1:1 (v:v:v) ratio, and incubated in a water bath at 100 °C for 1 h to start the reaction. Then, the samples were centrifuged at 2000 × g for 5 min at 4 °C. Absorbance was measured at 520 nm and proline content was quantified by interpolation in a proline standard curve made with a commercial standard of this amino acid. Data are the average of three biological replicates in each experimental group.
Phytohormone Analysis
Analysis of leaf content of the phytohormones ABA; SA and its precursor CA; IAA; JA and its precursor OPDA was performed as described in Durgbanshi et al. (2005). 100 mg of fresh leaf tissue was extracted in 2 mL of ultrapure water with a mill ball equipment (MillMix 20, Domel Železniki, Slovenija), adding 25 ng of [2H6]-ABA, [13C6]-SA, dehydrojasmonic acid (DHJA), [2H6]-CA and 2.5 ng [2H5]-IAA as internal standards. After a centrifugation at 4000 × g and 4 °C for 10 min, supernatants were recovered and pH-adjusted to 2.8–3.2 with 80% acetic acid. The water extract was partitioned twice against 2 mL of diethyl ether (Fisher Scientific, Hampton, NH, USA) and the organic layer recovered and dried under vacuum in a centrifuge concentrator (Speed Vac, Jouan, Saint Herblain Cedex, France). Once dried, the pellet was resuspended in 0.5 mL of 10:90 methanol:H2O solution by a gentle sonication. The resulting solution was filtered through 0.22 µm polytetrafluoroethylene membrane syringe filtres (Albert S.A., Barcelona, Spain) and directly injected into an ultraperformance LC system (Acquity SDS; Waters Corp., Milford, MA, USA). Chromatographic separations were carried out on a reversed-phase C18 column (Gravity, 50 × 2.1 mm, 1.8-µm particle size; Macherey–Nagel GmbH, Durem, Germany) using methanol:H2O (both supplemented with 0.1% acetic acid) gradient at a flow rate of 300 µL min−1. The phytohormones were quantified with a TQS triple quadrupole mass spectrometer (Xevo TQ-S, Waters Corp., Milford, MA, USA) through an orthogonal Z-spray electrospray ion source. Standard curves were prepared injecting the commercial standards in the UPLC-MS system and used for quantifying sample hormone concentrations. Results were processed using MassLynx v4.1 software. Data are the average of three biological replicates in each experimental group.
Statistical Analysis
Statistical analysis was performed by one-way ANOVA followed by Duncan post hoc test with Statistical Package for the Social Sciences Statistics v.21 software (IBM SPSS, USA). Different letters indicate statistical significance at P < 0.05 among experimental groups.
Results
Leaf Damage and Abscission
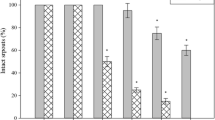
The effect of KL application on plant performance was estimated in terms of leaf damage (Fig. 2). In Fig. 3 the percentage of damaged and abscised leaves in plants under different culture conditions is shown. There were no statistical differences in the percentage of leaf damage among control plants (treated or not with KL), plants subjected to HS and those subjected to an individual stress and treated with KL (HS + KL and HL + KL). A significant percentage of leaves exhibited damage symptoms in plants subjected to HL, HS + HL and HS + HL + KL, in comparison to control plants. As also indicated in Fig. 3, during the experimental period, leaf abscission was only recorded in plants subjected to HS + HL conditions. KL prevented this abscission (HS + HL + KL) and reduced significantly leaf damage in these plants.
Representative images of Carrizo citrange plants. Non-treated leaf (A) Kaolin (KL)-treated leaf (B), control plants (C) plants subjected to heat and high light combined stress (D), and KL-treated plants subjected to heat and high light combined stress (E). The scale bar included in each figure represents a length of 1 cm
Percentage of damaged and abscised leaves, in Carrizo citrange plants subjected to different stress treatments. Data are mean values (n = 3) ± standard errors. Different letters denote statistical significance at P < 0.05, among treatments. KL KL-treated plants, HS heat stress, HS + KL KL-treated plants subjected to heat stress, HL high light stress, HL + KL KL-treated plants subjected to high light, HS + HL heat and high light combined stress, HS + HL + KL KL-treated plants subjected to heat and high light combined stress
Physiological and Gas Exchange Parameters
The quantum efficiency of PSII (ɸ PSII, Fig. 4A) significantly decreased under HL and HS + HL conditions, with values 17.60% and 20.90% lower than control plants, respectively. Under these stress conditions, KL application had a positive effect, and ɸPSII values in plants subjected to HL + KL and HS + HL + KL were similar to those in CT plants. KL application did not affect this parameter in CT plants and in those under HS (ɸ PSII values in these groups of plants ranged between 0.76 and 0.74). Leaf photosynthesis rate (A, Fig. 4B), E (Fig. 4C) and gs (Fig. 4D) were measured, finding that each stress condition affected these parameters in a different way. Under HS, there were significant increases in E (2.14-fold change) and gs (2.32-fold change) while similar A values with respect to CT were recorded. Under HL, A and gs values were similar to CT. Values of A, E and gs were also similar to CT when plants were subjected to HS + HL. KL application significantly affected A in plants cultured under all stress conditions (HS + KL, HL + KL and HS + HL + KL, Fig. 4B). Transpiration significantly increased after KL application under all environmental conditions (Fig. 4C). When KL was applied to CT plants, a 1.57-fold increase was recorded. Similarly, E values were 1.30-, 1.70- and 1.66-fold higher in plants under HS + KL; HL + KL and HS + HL + KL, respectively when compared to plants under the same conditions but not treated with KL. Results obtained when measuring gs were similar to those of transpiration (Fig. 4D). Thus, in plants under HS + KL, gs values were 1.38-fold higher than in plants under HS and, in plants under HS + HL + KL, gs values were 1.74-times fold higher than in plants under HS + HL.
Physiological parameters in Carrizo citrange plants subjected to different treatments. Quantum efficiency (ɸPSII) (A). Gas exchange parameters: leaf photosynthesis rate, A (B), transpiration, E (C), and stomatal conductance, gs (D). Data are mean values (n = 3) ± standard errors. Different letters denote statistical significance at P < 0.05 among treatments. CT control plants, HS heat stress, HS + KL KL-treated plants subjected to heat stress, HL high light stress, HL + KL KL-treated plants subjected to high light, HS + HL heat and high light stress combination, HS + HL + KL KL-treated plants subjected to heat and high light combined stress
Chlorophyll and Carotenoid Contents
Levels of chlorophylls and carotenoids did not change under HL (Fig. 5A, B, D) either when this stress condition was applied individually or combined with HS, compared to CT situations. However, the ratio Chl /Carotenoids (Fig. 5F) significantly decreased in plants subjected to these treatments (HL and HS + HL). Nevertheless, in plants under HS, chlorophyll B (Chl B) content (Fig. 5B) increased about 1.54-fold in comparison to CT plants. On the contrary, chlorophyll A (Chl A) content (Fig. 5A) decreased a 21.92% in plants under HS. The ratio Chl A/B (Fig. 5C) also decreased a 40.65% in plants under HS in comparation to CT plants. Furthermore, under HS, carotenoid content (Fig. 5D) was 26.66% lower than under CT conditions. KL application induced an increase in Chl B content (1.47-fold change) in CT + KL regarding to CT. On the contrary, the ratio of Chl A/B in CT plants treated with KL decreased by 28.53% compared to CT untreated plants. Nonetheless, KL application did not affect on chlorophyll and carotenoid contents in plants under HL or in plants under the two stress situations applied simultaneously (Fig. 5).
Chlorophyll and carotenoid contents in leaves of Carrizo citrange plants subjected to different treatments. Chlorophyll A content (A), chlorophyll B content (B), Chlorophyll A/B ratio (C), carotenoids content (D), chlorophyll total content (E) and Chlorophyll/carotenoids ratio (F). Data are mean values (n = 3) ± standard errors. Different letters denote statistical significance among treatments at P < 0.05. CT control plants, HS heat stress, HS + KL KL-treated plants subjected to heat stress, HL high light stress, HL + KL KL-treated plants subjected to high light, HS + HL heat and high light combined stress, HS + HL + KL KL-treated plants subjected to heat and high light combined stress
Proline Concentration
Leaf proline levels did not vary when plants were subjected to HS with regard to CT. On the contrary, proline concentration slightly increased in plants under HL and much notably in plants under combined stress (Fig. 6). In CT plants and in those under HL or HS, KL application did not modify proline levels. However, KL treatment reduced proline concentration by 18.16% in plants under HS + HL.
Proline concentration in leaves of Carrizo citrange plants subjected to different treatments. Data are mean values (n = 3) ± standard errors. Different letters denote statistical significance at P < 0.05 among treatments. CT control plants, HS heat stress, HS + KL KL-treated plants subjected to heat stress, HL high light stress, HL + KL KL-treated plants subjected to high light, HS + HL heat and high light combined stress, HS + HL + KL KL-treated plants subjected to heat and high light combined stress
Leaf Phytohormone Contents
Different stress conditions (HS, HL, HS + HL with or without KL application) affected leaf phytohormone content in different ways depending on the analyzed phytohormone. Leaf SA concentration (Fig. 7A) did not significantly vary among treatments, regardless of the stress conditions or KL application. Leaf CA content increased in plants under HL and HS + HL, with values 1.63- and 1.43-fold higher than CT, respectively (Fig. 7B). KL application induced a decrease in CA content under HS + HL, reaching levels similar to CT plants. ABA content decreased in plants under HS and HS + HL (27.96% and 24.76%, respectively) compared to CT (Fig. 7C). HL induced an increase in ABA content (1.95-fold higher than in CT plants). KL application did not affect ABA levels under control conditions or when a single stress condition was applied. However, in plants under HS + HL + KL, there was an increase in ABA content with respect to plants under HS + HL, showing values similar to CT. Plants under HS recorded similar IAA levels than CT (Fig. 7D), auxin concentration strongly increased in plants under HL and HS + HL (2.75- and 2.09-fold higher than CT plants, respectively). KL induced a decrease in IAA leaf content under HL; however, there were no changes in IAA content after KL treatment in HS + HL + KL. Focusing on JA (Fig. 7E), plants under HL showed increased JA values (6.19-fold higher than CT). However, in plants under HL + KL, JA concentration was similar to CT. No differences in JA content, regarding to CT, were recorded in plants neither under HS nor HS + HL with or without KL treatment. The content of OPDA (a JA precursor), increased under HL, exhibiting values 1.47-fold higher than CT (Fig. 7F). KL application did not significatively modify OPDA concentration under any stress assayed.
Phytohormone contents in leaves of Carrizo citrange plants subjected to different treatments. salicylic acid (SA) content (A), cinnamic acid (CA) content (B), abscisic acid (ABA) content (C), IAA content (D), acid (JA) content (E), and 12-oxophytodienoic acid (OPDA) content (F); data are mean values (n = 3) ± standard errors. Different letters denote statistical significance at P < 0.05 among treatments. CT control plants, HS heat stress, HS + KL KL-treated plants subjected to heat stress, HL high light stress, HL + KL KL-treated plants subjected to high light, HS + HL heat and high light combined stress, HS + HL + KL KL-treated plants subjected to heat and high light combined stress
Discussion
The simultaneous incidence of different abiotic stresses on plants in the last decades has been exacerbated as a result of climate change, and it is responsible for large economic losses, negatively affecting plant yield and productivity (reviewed in Zandalinas et al. 2018b, 2022; Pascual et al. 2022). Although many efforts have been recently made to decipher plant responses to combined abiotic factors (Mittler 2006; Zandalinas et al. 2016b, 2017a, 2017b; Balfagón et al. 2019a, 2022a), there is a lack of information on how HL incidence and HS occurring simultaneously affect citrus plants at physiological and biochemical levels. To enhance plant performance under adverse environmental conditions, various strategies, including the application of KL have been recently used in citrus (Jifon and Syvertsen 2003; Gullo et al. 2020), grapevine (Bernardo et al. 2021), hazelnut tree (Cabo et al. 2020) and olive (Cirillo et al. 2021). Our results indicate that Carrizo citrange plants exposed to simultaneous conditions of HL and HS experienced damages, at physiological and biochemical levels, that do not correspond with the addition of the effects of both stress conditions applied individually. Furthermore, the application of KL sprayed onto the leaves improved plant performance under stress combination conditions.
No significant leaf damage was observed when plants were exposed for 5 days to HS, contrary HL and the combined stresses affected negatively leaf status. The most deleterious effects (important foliar damage and abscission) were observed in plants subjected to HS + HL (Fig. 3). This finding is consistent with the study by Balfagón et al. (2019a) which reported that these two adverse conditions applied simultaneously had a higher negative impact on Arabidopsis plants than each of the different stress components applied individually. KL application had a beneficial effect on plants under HL and HS + HL when considering this parameter (Fig. 3). It is important to point out that, as described in this work, early physiological and biochemical changes take place in stressed plants before visual symptoms are evident. This is relevant from a practical perspective as early detection of stress could allow applying preventive treatments.
Although it is widely accepted that the PSII reaction center is a sensitive component within the plant photosynthetic apparatus to most of abiotic stresses (reviewed in Blanke 2000), in citrus, HS did not affect ɸPSII whereas HL induced a significant reduction of values recorded for this parameter (Fig. 4A), in concordance with data in tomato plants (Gerganova et al. 2016). This decrease on ɸPSII could be explained considering that plants reduce the number of open PSII centers to restrict the utilization of excitation energy (highly increased under HL conditions) and the rate of electron transport which has been proposed as one of the mechanisms to cope with unfavorable conditions (Scheibe et al. 2005). Therefore, ɸPSII was negatively affected in plants exposed to HL, regardless the temperature conditions.
Stress factors have varying impacts on gas exchange parameters (Fig. 4); whereas E and gs values increased in plants under HS, no changes in these parameters were recorded in plants subjected to HL. As indicated above, the effect of the combined stresses on plants is unique and cannot be inferred from the sum of the two individual stresses. Thus, for the different parameters evaluated, the effect of individual stress condition prevails in a random way. For ɸPSII and A, the effect of HL clearly prevailed when both stresses were applied simultaneously but for E and gs an intermediate situation was observed in plants under combined stress. This indicates that HS was determinant in inducing a stomatal aperture to increase transpiration and cool the leaf surface (Zandalinas et al. 2016a), restricting the effect of HL (that induced a stomatal closure as described in Devireddy et al. (2018).
KL is a white mineral, chemically inert, non-abrasive, non-toxic and easily dispersible in water, used in agriculture as a stress mitigating treatment. When sprayed on the leaf surface, water evaporates leaving behind a protective particle film that alters the microclimate around leaves. This treatment increases water saving and improves crop performance, alleviating the severity of adverse environmental conditions (reviewed in Brito et al. 2019). Our results (Fig. 4) indicate that KL application led to an improvement in ɸPSII under HL and HS + HL, restoring this parameter to CT values in plants under HL + KL and HS + HL + KL. Moreover, under combined stress conditions KL application induced an increase in gs and E, probably provided by the reduced leaf-to-air vapor pressure deficit, which can decrease the driving force for transpiration (Zhang et al. 2017). This is in concordance with (Gharaghani et al. 2018), who observed that in nut and kernel trees subjected to heat and high light combination A, E and gs values increased after KL application.
It has been reported that in plants exposed to high temperature there is a reduction of chlorophyll A, which is related either to an impaired chlorophyll synthesis, due to an inhibition of various biosynthesis enzymes and/or to an accelerated chlorophyll degradation (Gerganova et al. 2016). Our results corroborate an important reduction in this pigment content when plants were cultured under HS (Fig. 5A). However, KL treatment enhanced chlorophyll A content under HS, revealing its protective role on chlorophyll degradation. Similar results have been described in grapevine exposed to high temperature when KL was sprayed on the leaf surface (Bernardo et al. 2021). On the contrary, chlorophyll B content increased in plants subjected to this adverse condition. In Zafar et al. (2017), a significant variation in chlorophyll B content among rice lines exposed to high temperatures, with important increases in the heat tolerant genotypes was reported. This may explain of the observed increase in chlorophyll B content in Carrizo citrange plants, which has been recently described as a heat-tolerant genotype (Zandalinas et al. 2018a). In contrast, it has been observed that under HL, plants tend to reduce Chl B levels whereas plants grown under low light intensity accumulate this molecule (Biswal et al. 2012). In this work, it was observed that control plants treated with KL exhibited increased content of chlorophyll B. This can be consequence of the limitation of light intensity arriving to the leaf surface after the application of the reflective compound. HS induced a significant decrease in carotenoid content which was not observed under HL or combined stress conditions (Fig. 5D) where similar values to those of control plants were recorded. Carotenoids play a crucial role in scavenging reactive oxygen species, protecting photosynthetic pigments and unsaturated fatty acids from being damaged (Haque et al. 2021). Considering the accumulation pattern of these metabolites, we observed that plants under HS + HL performed better than those subjected only to HS. Some other studies have reported beneficial effects as a result of the interaction of two stresses applied simultaneously (reviewed in Suzuki et al. 2014). On the contrary, HL did not negatively affect chlorophyll leaf concentration (Fig. 5). This observation evidences that 5 days of HL causes photoinhibition but not photooxidation as no destruction of photosynthesizing pigments was recorded.
It has been widely observed that proline content increases when plants are exposed to different abiotic stress conditions (reviewed in Carraro and Di Iorio 2022). Increased proline contents have been reported in different citrus genotypes under drought stress (Hussain et al. 2018) or under combined drought and heat stress (Zandalinas et al. 2017a). Results described in this work indicate that KL reduced the proline content under HS + HL (Fig. 6), highlighting once again the beneficial role of KL treatment in situations of combined abiotic stress. This finding is in line with the role of proline as a reliable stress-intensity marker (Zandalinas et al. 2018b).
The involvement of phytohormones is crucial in plants’ ability to acclimate to abiotic stress conditions (Zandalinas et al. 2016a; Balfagón et al. 2019a; Hasan et al. 2023 and reviewed in Ahammed et al. 2021). The analyzed phytohormones exhibited different accumulation patterns when stress conditions (HS or HL) were applied individually or simultaneously. No significant changes in SA content were recorded when plants were cultured under HS, HL or HS + HL (Fig. 7A). However, we hypothesized that this lack of changes may be due to the short duration (5 days) and longer period of stress exposition would turn on SA accumulation, as levels of the SA precursor CA increased under HL and followed an increasing pattern under combined stress conditions (Fig. 7B). It has been described the important role of CA and its derivatives (esters, amides and depsides) as photoprotectors in plants, acting as a radiation screen (Edreva 2005; Weremczuk-Jeżyna et al. 2021). CA concentration significantly increased in plants under HL alone or combined with HS (HS + HL) (Fig. 7B), probably, to improve plant performance under these adverse conditions. KL treatment induced a decrease in the content of this molecule under HS + HL + KL, which seems related to its mechanism of action. KL creates a particle film on the leaf epidermis which act as a photoprotective screen (Glenn 2012), allowing to the plant to divert all available resources to the synthesis of other metabolites involved in protection against stress. The role of SA and its precursor in plant acclimation mechanisms to changed abiotic conditions should be further studied.
Heat stress repressed ABA accumulation (Fig. 7C), which is in concordance with previous findings in citrus (Zandalinas et al. 2018a). This decrease in ABA content would prevent stomatal closure, enabling the plant to maintain high transpiration rates for leaf surface cooling, which is confirmed by significant increases in E and gs (Fig. 4) under HS conditions. Under HS + HL, E and gs values were similar to those recorded in control plants although ABA leaf content was significantly lower than in control plants, probably as a consequence of the convergence of HS and HL. Furthermore, data indicate that the ABA control of stomatal aperture is not absolute and other signals must be involved in the modulation of gas exchange in plants under combined stress conditions (Balfagón et al. 2022b). KL application did not have a strong effect on this hormone levels.
Auxin content significantly increased in plants under HL and HS + HL (Fig. 7D). Although increases in IAA endogenous content have been reported in other subtropical trees, such as Eriobotrya japonica, subjected to HS (Chen et al. 2022), up to date, there is no available information on how HL stress affects the accumulation of this phytohormone in plant tissues. Further studies are required needed to decipher the role of IAA and its crosstalk with other phytohormones under these adverse conditions. Contrary to that observed in the case of ABA (Fig. 7C), the effect of HL prevails on IAA accumulation when both stress conditions concur. KL treatment did not affect IAA leaf content, neither under control conditions nor under the combined stress conditions.
JA plays an important role during high irradiation conditions, since PhyB- and JA-based responsive pathways work together to protect the photosynthetic machinery, maintaining a high metabolic rate under this stressful condition (reviewed in Nguyen et al. 2022). Similarly, it has been observed that OPDA, the primary precursor of JA, accumulates in the chloroplast and has a crucial role in the detoxification of photosynthetic byproducts, increasing the thiol-based redox potential and regulating the expression of defense genes in the nucleus (Cheong et al. 2017). This increase in leaf content of JA and OPDA has been observed in citrus plants exposed to HL (Fig. 7). However, this situation is reverted when plants are exposed to HS + HL, pointing that the effect of HS overrides that of HL. It could be argued that the KL treatment is delaying the conversion of OPDA to JA, and longer periods of stress imposition could cause an increase in JA concentration in HL + KL plants.
Taking all results into consideration it can be concluded that Carrizo citrange plants under the combination of HL and HS stresses had a particular response at morphological, physiological and biochemical levels, different to that observed for each stress condition applied individually. Furthermore, this response cannot be considered as the addition of the responses to both single stresses as highlighted by the higher leaf abscission caused by the combined stress conditions. The determination of different non-destructive parameters allowed us to confirm the stress situation before symptoms of stress are evident in the plant: this is of a great practical interest since it allows using mitigating treatments sooner, what is relevant in an economically important crop worldwide as citrus. KL applied as a foliar spray is a suitable stress mitigating treatment against the damaging effects of the stress conditions studied here at different levels: (i) reduced the foliar damage, and leaf abscission in the case of HS + HL treatment, (ii) improved quantum efficiency as well as gas exchange parameters and (iii) enhanced chlorophyll a content under HS.
In conclusion, the simultaneous occurrence of high light (HL) and high temperature (HS) stresses on citrus plants results in significant physiological and biochemical damage that is not merely the sum of the effects of each stress applied individually. This study demonstrates that the application of KL as a foliar spray can effectively mitigate the negative impacts of combined stress conditions. KL treatment improved plant performance by reducing leaf damage and abscission, enhancing photosynthetic efficiency, increasing stomatal conductance and transpiration, and preserving chlorophyll content, as it is summarized in Fig. 8. Importantly, this study highlights the early physiological and biochemical alterations that occur in stressed plants before visible symptoms manifest. This early detection is crucial for implementing preventive measures and applying stress mitigating treatments in a timely manner, enhancing the plant resilience to adverse environmental conditions.
References
Abobatta WF (2021) Managing citrus orchards under climate change. MOJ Eco Environ Sci 6(2):43–44. https://doi.org/10.15406/mojes.2021.06.00212
Ahammed GJ, Xu W, Liu A, Chen S (2018) COMT1 silencing aggravates heat stress-induced reduction in photosynthesis by decreasing chlorophyll content, photosystem II activity, and electron transport efficiency in tomato. Front Plant Sci. https://doi.org/10.3389/fpls.2018.00998
Ahammed GJ, Guang Y, Yang Y, Chen J (2021) Mechanisms of elevated CO2-induced thermotolerance in plants: the role of phytohormones. Plant Cell Rep 40(12):2273–2286. https://doi.org/10.1007/s00299-021-02751-z
Arbona V, López-Climent MF, Pérez-Clemente RM, Gómez-Cadenas A (2009) Maintenance of a high photosynthetic performance is linked to flooding tolerance in citrus. Environ Exp Bot 66:135–142. https://doi.org/10.1016/j.envexpbot.2008.12.011
Balfagón D, Sengupta S, Gómez-Cadenas A et al (2019a) Jasmonic acid is required for plant acclimation to a combination of high light and heat stress. Plant Physiol 181:1668–1682. https://doi.org/10.1104/pp.19.00956
Balfagón D, Zandalinas SI, Gómez-Cadenas A (2019b) High temperatures change the perspective: integrating hormonal responses in citrus plants under co-occurring abiotic stress conditions. Physiol Plant 165:183–197. https://doi.org/10.1111/ppl.12815
Balfagón D, Terán F, de Oliveira T et al (2021) Citrus rootstocks modify scion antioxidant system under drought and heat stress combination. Plant Cell Rep. https://doi.org/10.1007/s00299-021-02744-y
Balfagon D, Gomez-Cadenas A, Rambla JL, Granell A, de Ollas C, Bassham DC, Mittler R, Zandalinas SI (2022a) Gamma-aminobutyric acid plays a key role in plant acclimation to a combination of high light and heat stress. Plant Phys 188(4):2026–2038. https://doi.org/10.1093/plphys/kiac010
Balfagón D, Rambla JL, Granell A et al (2022b) Grafting improves tolerance to combined drought and heat stresses by modifying metabolism in citrus scion. Environ Exp Bot. https://doi.org/10.1016/j.envexpbot.2022.104793
Balfagón D, Zandalinas SI, Dos Reis de Oliveira T, Santa-Catarina C, Gómez-Cadenas A (2022c) Reduction of heat stress pressure and activation of photosystem II repairing system are crucial for citrus tolerance to multiple abiotic stress combination. Physiol Plant 174:e13809. https://doi.org/10.1111/ppl.13809
Bates LS, Waldren RPTI (1973) Rapid determination of free proline for water-stress studies. Plant Soil 207:205–207
Bernardo S, Luzio A, Machado N et al (2021) Kaolin application modulates grapevine photochemistry and defence responses in distinct Mediterranean-type climate vineyards. Agronomy 11:477. https://doi.org/10.3390/agronomy11030477
Biswal AK, Pattanayak GP, Pandey SS, Leelavathi S, Reddy VS, Govindjee TBC (2012) Light intensity-dependent modulation of chlorophyll b biosynthesis and photosynthesis by overexpression of chlorophyllide a oxygenase in tobacco. Plant Phys 159(1):433–449. https://doi.org/10.1104/pp.112.195859
Blanke MM (2000) Repair mechanisms following photoinhibition in citrus. Acta Hortic 531:151–153. https://doi.org/10.17660/actahortic.2000.531.21
Braham M, Pasqualini E, Ncira N (2007) Efficacy of kaolin, spinosad and malathion against Ceratitis capitata in citrus orchards. Bull Insectol 60:39–47
Brito C, Dinis LT, Luzio A et al (2019) Kaolin and salicylic acid alleviate summer stress in rainfed olive orchards by modulation of distinct physiological and biochemical responses. Sci Hortic (amst) 246:201–211. https://doi.org/10.1016/j.scienta.2018.10.059
Buchner O, Stoll M, Karadar M et al (2015) Application of heat stress in situ demonstrates a protective role of irradiation on photosynthetic performance in alpine plants. Plant Cell Environ 38:812–826. https://doi.org/10.1111/pce.12455
Cabo S, Morais MC, Aires A et al (2020) Kaolin and seaweed-based extracts can be used as middle and long-term strategy to mitigate negative effects of climate change in physiological performance of hazelnut tree. J Agron Crop Sci 206:28–42. https://doi.org/10.1111/jac.12369
Carraro E, Di Iorio A (2022) Eligible strategies of drought response to improve drought resistance in woody crops: a mini-review. Plant Biotechnol Rep 16:265–282. https://doi.org/10.1007/s11816-021-00733-x
Chen Y, Deng C, Xu Q et al (2022) Integrated analysis of the metabolome, transcriptome and miRNome reveals crucial roles of auxin and heat shock proteins in the heat stress response of loquat fruit. Sci Hortic (amst) 294:110764. https://doi.org/10.1016/j.scienta.2021.110764
Cheong H, Barbosa dos Santos I, Liu W et al (2017) Cyclophilin 20–3 is positioned as a regulatory hub between light-dependent redox and 12-oxo-phytodienoic acid signaling. Plant Signal Behav. https://doi.org/10.1080/15592324.2017.1362520
Cirillo A, Conti S, Graziani G et al (2021) Mitigation of high-temperature damage by application of kaolin and pinolene on young olive trees (Olea europaea L.): a preliminary experiment to assess biometric, eco-physiological and nutraceutical parameters. Agronomy 11:1884. https://doi.org/10.3390/agronomy11091884
Devireddy AR, Zandalinas SI, Gómez-Cadenas A et al (2018) Coordinating the overall stomatal response of plants: rapid leaf-to-leaf communication during light stress. Sci Signal. https://doi.org/10.1126/scisignal.aam9514
Dinis LT, Ferreira H, Pinto G et al (2016) Kaolin-based, foliar reflective film protects photosystem II structure and function in grapevine leaves exposed to heat and high solar radiation. Photosynthetica 54:47–55. https://doi.org/10.1007/s11099-015-0156-8
Dongsansuk A, Lütz C, Neuner G (2013) Effects of temperature and irradiance on quantum yield of PSII photochemistry and xanthophyll cycle in a tropical and a temperate species. Photosynthetica 51:13–21. https://doi.org/10.1007/s11099-012-0070-2
Durgbanshi A, Arbona V, Pozo O et al (2005) Simultaneous determination of multiple phytohormones in plant extracts by liquid chromatography–electrospray tandem mass spectrometry. J Agric Food Chem 53:8437–8442. https://doi.org/10.1021/jf050884b
Edreva A (2005) The importance of non-photosynthetic pigments and cinnamic acid derivatives in photoprotection. Agric Ecosyst Environ 106:135–146. https://doi.org/10.1016/j.agee.2004.10.002
Ennab H, El-Sayed SA, El- Enien MA (2017) Effect of kaolin applications on fruit sunburn, yield, and fruit quality of Balady mandarin. Menoufia J Plant Prod 2(2):129–138
FAO (2020) Citrus fruit. Choice Rev Online 36:36–2167. https://doi.org/10.5860/choice.36-2167
Gerganova M, Popova AV, Stanoeva D, Velitchkova M (2016) Tomato plants acclimate better to elevated temperature and high light than to treatment with each factor separately. Plant Physiol Biochem 104:234–241. https://doi.org/10.1016/j.plaphy.2016.03.030
Gharaghani A, Mohammadi Javarzari A, Vahdati K (2018) Kaolin particle film alleviates adverse effects of light and heat stresses and improves nut and kernel quality in Persian walnut. Sci Hortic (amst) 239:35–40. https://doi.org/10.1016/j.scienta.2018.05.024
Glenn DM (2010) Canopy gas exchange and water use efficiency of “Empire” apple in response to particle film, irrigation, and microclimatic factors. J Am Soc Hortic Sci 135:25–32. https://doi.org/10.21273/jashs.135.1.25
Glenn M (2012) The mechanisms of plant stress mitigation by kaolin-based particle films and applications in horticultural and agricultural crops. HortScience 47:710–711. https://doi.org/10.21273/hortsci.47.6.710
Goh CH, Ko SM, Koh S, Kim YJ, Bae HJ (2012) Photosynthesis and environments: photoinhibition and repair mechanisms in plants. J Plant Biol 55:93–101. https://doi.org/10.1007/s12374-011-9195-2
Guha, A (2022) Protecting citrus trees from heat stress. In: Citrus industry, 5 July
Gullo G, Dattola A, Vonella V, Zappia R (2020) Effects of two reflective materials on gas exchange, yield, and fruit quality of sweet orange tree Citrus sinensis (L.) Osb. Eur J Agron 118:126071. https://doi.org/10.1016/j.eja.2020.126071
Haque MS, Husna MT, Uddin MN et al (2021) Heat stress at early reproductive stage differentially alters several physiological and biochemical traits of three tomato cultivars. Horticulturae. https://doi.org/10.3390/horticulturae7100330
Hasan MDK, Xing QF, Zhou CY, Wang KX, Xu T, Yang P, Qi ZY, Shao SJ, Ahammed GJ, Zhou J (2023) Melatonin mediates elevated carbon dioxide-induced photosynthesis and thermotolerance in tomato. J Pineal Res 74:e12858. https://doi.org/10.1111/jpi.12858
Hussain S, Khalid MF, Saqib M et al (2018) Drought tolerance in citrus rootstocks is associated with better antioxidant defense mechanism. Acta Physiol Plant 40:1–10. https://doi.org/10.1007/s11738-018-2710-z
Jifon JL, Syvertsen JP (2003) Photosynthesis and water use efficiency of ‘Ruby Red’ grapefruit leaves. J Am Soc Hortic Sci 128:107–112
Kavi Kishor PB, Sreenivasulu N (2014) Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ 37:300–311. https://doi.org/10.1111/pce.12157
Lo Verde G, Caleca V, Lo Verde V (2011) The use of kaolin to control Ceratitis cepitata in organic citrus groves. Bull Insectol 64(1):127–134
Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11:15–19. https://doi.org/10.1016/j.tplants.2005.11.002
Miura K, Tada Y (2014) Regulation of water, salinity, and cold stress responses by salicylic acid. Front Plant Sci 5:1–12. https://doi.org/10.3389/fpls.2014.00004
Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI (2007) Photoinhibition of photosystem II under environmental stress. Biochim Biophys Acta Bioenerg 1767:414–421. https://doi.org/10.1016/j.bbabio.2006.11.019
Nguyen TH, Goossens A, Lacchini E (2022) Jasmonate: a hormone of primary importance for plant metabolism. Curr Opin Plant Biol 67:102197. https://doi.org/10.1016/j.pbi.2022.102197
Pascual LS, Segarra-Medina C, Gomez-Cadenas A, Lopez-Climent MF, Vives-Peris V, Zandalinas SI (2022) Climate change-associated multifactorial stress combination: a present challenge for our ecosystems. J Plant Phys 276:153764. https://doi.org/10.1016/j.jplph.2022.153764
Pérez-Clemente RM, Vives V, Zandalinas SI et al (2013) Biotechnological approaches to study plant responses to stress. Biomed Res Int. https://doi.org/10.1155/2013/654120
Scheibe R, Backhausen JE, Emmerlich V, Holtgrefe S (2005) Strategies to maintain redox homeostasis during photosynthesis under changing conditions. J Exp Bot 56:1481–1489. https://doi.org/10.1093/jxb/eri181
Schreiber U, Berry JA (1977) Heat-induced changes of chlorophyll fluorescence in intact leaves correlated with damage of the photosynthetic apparatus. Planta 136:233–238. https://doi.org/10.1007/BF00385990
Suzuki N, Rivero RM, Shulaev V et al (2014) Abiotic and biotic stress combinations. N Phytol 203:32–43. https://doi.org/10.1111/nph.12797
Takahashi S, Tamashiro A, Sakihama Y, Yamamoto Y, Kawamitsu Y, Yamasaki H (2002) High-susceptibility of photosynthesis to photoinhibition in the tropical plant Ficus microcarpa L. f. cv. Golden Leaves. BMC Plant Biol 2(1):1–8. https://doi.org/10.1186/1471-2229-2-2
Vives-Peris V, Gómez-Cadenas A, Pérez-Clemente RM (2017) Citrus plants exude proline and phytohormones under abiotic stress conditions. Plant Cell Rep 36:1971–1984. https://doi.org/10.1007/s00299-017-2214-0
Vives-Peris V, Marmaneu D, Gómez-Cadenas A, Pérez-Clemente RM (2018) Characterization of Citrus WRKY transcription factors and their responses to phytohormones and abiotic stresses. Biol Plant 62:33–44. https://doi.org/10.1007/s10535-017-0737-4
Wani SH, Kumar V, Shriram V, Sah SK (2016) Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J 4:162–176. https://doi.org/10.1016/j.cj.2016.01.010
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Weremczuk-Jeżyna I, Hnatuszko-Konka K, Lebelt L, Grzegorczyk-Karolak I (2021) The protective function and modification of secondary metabolite accumulation in response to light stress in Dracocephalum forrestii shoots. Int J Mol Sci 22:7965. https://doi.org/10.3390/ijms22157965
Zafar SA, Hameed A, Khan AS, Ashraf M (2017) Heat shock induced morpho-physiological response in indica rice (Oryza sativa L.) at early seedling stage. Pak J Bot 49:453–463
Zandalinas SI, Balfagón D, Arbona V et al (2016a) ABA is required for the accumulation of APX1 and MBF1c during a combination of water deficit and heat stress. J Exp Bot 67:5381–5390. https://doi.org/10.1093/jxb/erw299
Zandalinas SI, Rivero RM, Martínez V et al (2016b) Tolerance of citrus plants to the combination of high temperatures and drought is associated to the increase in transpiration modulated by a reduction in abscisic acid levels. BMC Plant Biol 16:1–16. https://doi.org/10.1186/s12870-016-0791-7
Zandalinas SI, Balfagón D, Arbona V, Gómez-Cadenas A (2017a) Modulation of antioxidant defense system is associated with combined drought and heat stress tolerance in citrus. Front Plant Sci 8:1–10. https://doi.org/10.3389/fpls.2017.00953
Zandalinas SI, Sales C, Beltrán J et al (2017b) Activation of secondary metabolism in citrus plants is associated to sensitivity to combined drought and high temperatures. Front Plant Sci 7:1–17. https://doi.org/10.3389/fpls.2016.01954
Zandalinas SI, Balfagón D, Arbona V, Gómez-Cadenas A (2018a) Regulation of citrus responses to the combined action of drought and high temperatures depends on the severity of water deprivation. Physiol Plant 162:427–438. https://doi.org/10.1111/ppl.12643
Zandalinas SI, Mittler R, Balfagón D et al (2018b) Plant adaptations to the combination of drought and high temperatures. Physiol Plant 162:2–12. https://doi.org/10.1111/ppl.12540
Zandalinas SI, Balfagón D, Gómez-Cadenas A, Mittler R (2022) Plant responses to climate change: metabolic changes under combined abiotic stresses. J Exp Bot 73(11):3339–3354. https://doi.org/10.1093/jxb/erac073
Zhang D, Du Q, Zhang Z et al (2017) Vapour pressure deficit control in relation to water transport and water productivity in greenhouse tomato production during summer. Sci Rep 7:1–11. https://doi.org/10.1038/srep43461
Zhang H, Zhu J, Gong Z, Zhu JK (2022) Abiotic stress responses in plants. Nat Rev Genet 23:104–119. https://doi.org/10.1038/s41576-021-00413-0
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was supported by Grants PID2019-104062RB-I00 and TED2021-129795B-I00 funded by MCIN/AEI/10.13039/501100011033 and by the European Union-NextGenerationEU. Funding was also obtained from Generalitat Valenciana (CIAICO/2021/063). Fátima Terán was recipient of a Grant from MCIN (PRE2020-093757).
Author information
Authors and Affiliations
Contributions
FT and MFL-C performed all experiments, FT, VV-P, AG-C, and RMP-C conceived the study, the experimental design and wrote de manuscript. FT, VV-P, and MFL-C performed the sample and data analysis. AG-C, and RMP-C supervised the process. All the authors have revised the final version of this article.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling Editor: Golam Jalal Ahammed.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Terán, F., Vives-Peris, V., López-Climent, M.F. et al. Palliative Effects of Kaolin on Citrus Plants Under Controlled Stress Conditions of High Temperature and High Light Intensity. J Plant Growth Regul 43, 486–499 (2024). https://doi.org/10.1007/s00344-023-11103-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-023-11103-y