Abstract
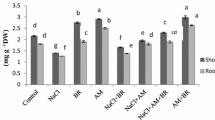
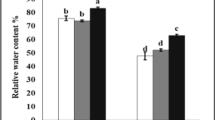
Savory (Saturja khuzistanica) is a precious medicinal plant. This species contains essential oil and its extract is widely used in various industries. This study investigate the simultaneous effects of Arbuscular mycorrhizal fungi (AMF) and methyl jasmonate (MeJA) on Satureja Khuzistanica tolerance, physiological and biochemical traits under salt stress. The experiment designed as a completely randomized factorial with two AMF levels, including the combination of AMF (Glomus intraradices and G. fasciculatum species) and without inoculation, four levels of salt (0, 3, 6, and 9 dS/m) and three concentrations of MeJA (0, 60 and 120 µM). The results indicated that salt stress had adverse effects on the savory plant. Salinity caused a significant impact on physiological and biochemical traits (including chlorophyll pigments, proline, sugar, protein, N, P+, K+, Ca2+, Mg2+, Na+) (P ≤ 0.01). The savory plant had the best performance under AMF + 60 µM MeJA treatments at saline and non-saline condition. The findings showed that the highest salt level (9 dS/m) caused a remarkable inhibition on the amounts of flavonoid, total phenol, and antioxidant activity in savory extract and essential oil, even under AMF and MeJA treatments. AMF symbiosis had better performance in the synthesis of all phytochemical parameters. MeJA at 60 µM concentration had the most positive effects on the phytochemical parameters of savory plants at all salt levels, such as AMF-treated and non-treated plants. The highest flavonoid content in the savory essential oil was induced at AMF + 60 µM MeJA + 6 dS/m salt treatment (1.02%). The highest amounts of total phenol (8.12%, 8.14%) and antioxidant activity (59.21%, 59.25%) in Satureja essential oil were observed at AMF + 60 µM MeJA + 3 and 6 dS/m salt treatments; there was no significant difference between 3 and 6 dS/m salt at this treatment. There was lower flavonoid content in the essential oil of S. khuzistanica than in its extract. But savory essential oil had higher amounts of total phenol rather than its extract. Applying AMF and MeJA seems to be a cost-effective way to improve physiological characteristics and antioxidant capacity in savory plants under saline conditions.



Similar content being viewed by others
Abbreviations
- AMF:
-
Arbuscular mycorrhizal fungi
- MeJA:
-
Methyl jasmonate
- Chl:
-
Chlorophyll
- H2SO4 :
-
Sulfuric acid
- H2O2 :
-
Hydrogen peroxide
- N:
-
Nitrogen
- P:
-
Phosphorous
- Na:
-
Sodium
- K:
-
Potassium
- Ca:
-
Calcium
- Mg:
-
Magnesium
- Mn:
-
Manganese
- EDTA:
-
Ethylenediaminetetraacetic acid
- PVPP:
-
Polyvinylpolypyrrolidone
- PMSF:
-
Phenylmethylsulfonyl fluoride
- DPPH:
-
2, 2-Diphenyl-1-picrylhydrazyl
- PAL:
-
Phenylalanine ammonia-lyase
References
Afkar S, Sharifi M (2015) Effect of foliar application with methyl jasmonate on physiological behavior of Mentha piperita. J Med Plants by-Products 2:155–160
Ahmadi FI, Karimi K, Struik PC (2018) Effect of exogenous application of methyl jasmonate on physiological and biochemical characteristics of Brassica napus L. cv. Talaye under salinity stress. S. Afr J Botany 115(2018):5–11. https://doi.org/10.1016/j.sajb.2017.11.018
Amanifar S (2020) Toghranegar Z (2020) The efficiency of arbuscular mycorrhiza for improving tolerance of Valeriana officinalis L. and enhancing valerenic acid accumulation under salinity stress. Ind Crops Prod 147:112234
Arnon AN (1967) Method of extraction of chlorophyll in the plants. Agron J 23:112–121
Banuelos J, Trejo D, Alarcon A, Lara L, Moreira C, Cruz S (2012) The reduction in proline buildup in mycorrhizal plants affected by nematodes. J Soil Sci Plant Nutr 12(2):263–270
Barraclough PB, Kuhlmann H, Weir AH (1980) The effects of prolonged drought and nitrogen fertilizer on root and shoot growth and water uptake by winter wheat. J Agron Crop Sci 163:352–360
Bates LS, Waldern RP, Teave ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Begum N, Qin Ch, Ahanger MA, Raza S, Khan MI, Ashraf M, Ahmed N, Zhang L (2019) Role of arbuscular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. Front Plant Sci 10(1068):1–15
Chandrasekaran M, Chanratana M, Kim K, Seshadri S, Sa T (2019) Impact of Arbuscular mycorrhizal fungi on photosynthesis, water status and gas exchange of plants under salt stress- a meta-analysis. Front Plant Sci 457(10):1–10. https://doi.org/10.3389/fpls.2019.00457
Chapman HD, Part PF (1961) Method of analysis for soils, plants and waters. University of California. Division of Agriculture Sciences, California, p 309
Chavoushi M, Manoochehri Kalantari Kh, Arvin MJ (2019) Effect of salinity stress and exogenously applied methyl jasmonate on growth and physiological traits of two Carthamus tinctorius varieties. Int J Hortic Sci Technol 6(1):39–49. https://doi.org/10.22059/ijhst.2019.277800.283
Creelman RA, Mullet JE (1997) Biosynthesis and action of jasmonates in plants. Annual Riview of Plant Biology 48: 355–81. https://www.researchgate.net/publication/8665829.
Eskandari M, Eskandari A (2013) Effects of 28-homobrassinolide on growth, photosynthesis and essential oil content of Satureja khuzestanica. Int J Plant Physiol Biochem 5(3):36–41. https://doi.org/10.5897/IJPPB11.064
Farhangi-Abriz S, Tavasolee A, Ghassemi-Golezani K, Torabian Sh, Monirifar H, Asadi Rahmani H (2020) Growth-promoting bacteria and natural regulators mitigate salt toxicity and improve rapeseed plant performance. Protoplasma. https://doi.org/10.1007/s00709-020-01493-1
Farsaraei S, Moghaddam M, Ghasemi Pirbalouti A (2020) Changes in growth and essential oil composition of sweet basil in response of salinity stress and superabsorbents application. Sci Hortic 271:109465. https://doi.org/10.1016/j.scienta.2020.109465
Fileccia V, Ruisi P, Ingraffia R, Giambalvo D, Frenda AS, Martinelli F (2017) Arbuscular mycorrhizal symbiosis mitigates the negative effects of salinity on durum wheat. PLoS ONE 12(9):1–15. https://doi.org/10.1371/journal.pone.0184158
Ghasemi K, Ghasemi Y, Ebrahimzadeh MA (2009) Antioxidant activity, phenol and flavonoid contents of 13 Citrus species peels and tissues. Pak J Pharm Sci 22(3):277–281
Golkar P, Bakhshi G, Vahabi MR (2020) Phytochemical, biochemical and growth changes in response to salinity in callus cultures of Nigella sativa L. In Vitro Cellular Dev Biol-Plant. https://doi.org/10.1007/s11627-020-10058-z
Guo A, Hu Y, Shi M, Wang H, Wu Y, Wang Y (2020) Effects of iron deficiency and exogenous sucrose on the intermediates of chlorophyll biosynthesis in Malus halliana. PLoS ONE 15(5):232–243
Hasanuzzaman M, Borhannuddin Bhuyan MHM, Zulfiqar F, Raza A, Mohsin SM, Al Mahmud J, Fujita M, Fotopoulos V (2020) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9(681):1–52. https://doi.org/10.3390/antiox9080681
Helaly AA, Dojima T, Craker L (2018) Effect of plant growth promoting bacteria on collard plants growth, yield production and nutritional compositions. 8th International Conference for Sustainable Agricultural Development held in Faculty of Agriculture. 5–7 March. Fayoum University, Egypt.1–16.
Hellubust JA, Craigie JS (1978) Handbook of Physiological and Biochemical Methods. Cambridge University Press, Cambridge
Hossein Jafari S, Mosleh Arani A, Tarkesh Esfahani S (2022) The combined effects of rhizobacteria and methyl jasmonate on rosmarinic acid production and gene expression profile in Origanum vulgare l. under salinity conditions. J Plant Growth Regul. https://doi.org/10.1007/s00344-022-10632-2
Huang B, Duan Y, Yi B, Sun L, Lu B, Yu X, Sun H, Zhang H, Chen W (2008) Characterization and expression profiling of cinnamate 4-hydroxylase gene from Salvia miltiorrhiza in rosmarinic acid biosynthesis pathway. Russ J Plant Physiol 55(3):390–399. https://doi.org/10.1134/S1021443708030163
Ilyas N, Mazhar R, Yasmin H, Khan W, Iqbal S, Dailin EHE, DJ, (2020) Rhizobacteria isolated from saline soil induce systemic tolerance in Wheat (Triticum aestivum L.) against salinity stress. Agron J 10:989–1009
Karagiannidis N, Thomidis T, Lazari D, Panou-Filtheou E, Karagiannidou C (2011) Effect of three greek arbuscular mycorrhizal fungi in improving the growth, nutrient concentration and production of essential oils of oregano and mint plants. Sci Hortic 129(2011):329–334. https://doi.org/10.1016/j.scienta.2011.03.043
Khaleghnezhad V, Yousefi AR, Tavakoli A, Farajmand B (2019) Interactive effects of abscisic acid and temperature on rosmarinic acid, total phenolic compounds, anthocyanin, carotenoid and flavonoid content of dragonhead (Dracocephalum moldavica L.). Sci Hortic 250(2019):302–309
Khalil SE (2020) Review: the effect of salt stress on some physiological and biochemical composition of some crops. Plant Arch 20(1):3573–3585
Khalvandi M, Amerian M, Pirdashti H, Keramati S, Hosseini J (2019) Essential oil of peppermint in symbiotic relationship with Piriformospora indica and methyl jasmonate application under saline condition. Ind Crops Prod 127(2019):195–202. https://doi.org/10.1016/j.indcrop.2018.10.072
Khatun S (2020) Effect of Glomus fasciculatum on nutrient uptake and growth of a medicinal plant, Coleus forskohlii. Eur J Med Plants 31(4):25–37. https://doi.org/10.9734/EJMP/2020/v31i430227
Konan YKF, Kouassi KM, Kouakou KL, Koffi E, Kouassi KN, Sekou D, Kone M, Kouakou TH (2014) Effect of methyl jasmonate on phytoalexins biosynthesis and induced disease resistance to Fusarium oxysporum f. sp. Vasinfectum in cotton (Gossypium hirsutum L.). Int J Agron. https://doi.org/10.1155/2014/806439
Krzyzanowska J, Czubacka A, Pecio L, Przybys M, Doroszewska T, Stochmal A, Oleszek W (2011) The effects of jasmonate acid and methyl jasmonate on rosmarinic acid production in Mentha piperita cell suspension cultures. Plant Cell Tiss Organ Cult. https://doi.org/10.1007/s11240-011-0014-8
Lamian A, Naghdi Badi H, Mehrafarin A, Seinf Sahandi M (2017) Changes in essential oil and morpho-physiological traits of tarragon (Artemisia dracuncalus L.) in response to arbuscular mycorrhizal fungus, AMF (Glomus intraradices N.C. Schenck & G.S. Sm.) inoculation under salinity. Acta Agric Slovenica 109(2):215–227. https://doi.org/10.14720/aas.2017.109.2.06
Li J, Hu L, Zhang L, Pan X, Hu X (2015) Exogemous sprmidine is enhancing tomato tolerance to salinity-alkanity stress by regulating chloroplast antioxidant system and chlorophyll metabolism. BMC Plant Biol 15:303–310
Lima dos Santos E, Alves da Silva F, Barbosa da Silva FS (2020) Arbuscular mycorrhizal fungi increase the phenolic compounds concentration in the bark of the stem of Libidibia ferrea in field conditions. Open Microbiol J 14:1874–2858
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275
Mahboubi M, Kazempour N (2018) Antioxidant and antimicrobial activity of Satureja khuzistanica Jamzad essential oil, ethanol and aqueous extracts. Biharean Biologist 12 (1): 37–39. http://biozoojournals.ro/bihbiol/index.html.
Maksimovic I, Ilin Z (2012) Effects of salinity on vegetable growth and nutrients uptake. In: Lee TS (ed) Irrigation systems and practices in challenging environments. IntechOpen, London, pp 169–190
Marinova D, Ribarova F, Atanasova M (2005) Total phenolics and flavonoids in Bulgarian fruits and vegetables. J Univ Chem Technol Metall 40:255–260
Mazhar R, Ilyas N, Saeed M, Bibi F, Batool N (2016) Biocontrol and salinity tolerance potential of Azospirillum lipoferum and its inoculation effect in wheat crop. Int J Agric Biol 18(3):494–500. https://doi.org/10.17957/IJAB/15.0115
Mojaddar Langroodi A, Tajik H, Mehdizadeh T (2019) Antibacterial and antioxidant characteristics of Zataria multiflora Boiss essential oil and hydroalcoholic extract of Rhus coriaria L. J Food Qual Hazards Cotrol 6(2019):16–24. https://doi.org/10.18502/jfqhc.6.1.454
Nehls U (2008) Mastering ectomycorrhizal symbiosis: the impact of carbohydrates. J Exp Bot 59(5):1097–1108
Oraei M, Tabatabaei SJ, Fallahi E, Imani A (2009) The effects of salinity stress and rootstock on the growth, photosynthetic rate, nutrient and sodium concentrations of almond. J Hortic Sci Biotechnol 23:131–140
Pessarakli M, Haghighi M, Sheibanirad A (2015) Plant responses under environmental stress conditions. Adv Plants Agric Res 2(6):276–286. https://doi.org/10.15406/apar.2015.02.00073
Raghu K, Rao SSR (2016) Effect of brassinosteroids on antioxidants content and radical scavenging activity of Tinospora cordifolia (Willd.) Miers ex Hook. F & Thoms. J Med Plants Stud 4: 117–121. www.scientiaresearchlibrary.com.
Ryan J, Estefan G, Rashid A (2007) Soil and plant analysis laboratory manual. ICARDA, Lebanon
Saleh AM, Abdel-Mawgoud M, Hassan AR, Habeeb TH, Yehia RS, AbdElgawad H (2020) Global metabolic changes induced by arbuscular mycorrhizal fungi in oregano plants grown under ambient and elevated levels of atmospheric CO2. Plant Physiol Biochem 151(2020):255–263. https://doi.org/10.1016/j.plaphy.2020.03.026
Sarabi V, Arjmand-Ghajur E (2020) Exogenous plant growth regulators/plant growth promoting bacteria roles in mitigating water-deficit stress on chicory (Cichorium pumilum Jacq.) at a physiological level. Agric Water Manag. https://doi.org/10.1016/j.agwat.2020.106439
Scagel CF, Lee J (2020) Salinity sensitivity and mycorrhizal responsiveness of polyphenolics in Siam Queen basil grown in soilless substrate. Sci Hortic 269:109394. https://doi.org/10.1016/j.scienta.2020.109394
Selvaraj T, Sumithra P (2011) Effect of Glomus aggregatum and plant growth promoting rhizomicroorganisms on growth, nutrition and content of secondary metabolites in Glycyrrhiza glabra L. Indian J Appl Pure Biol 26:283–290
Shariat A, Karimzadeh G, Assareh MH (2013) Karyology of Iranian endemic Satureja (Lamiaceae) species. Cytologia 78:305–312. https://doi.org/10.1508/cytologia.78.305
Sheyhakinia Sh, Bamary Z, Einali A, Valizadeh J (2020) The induction of salt stress tolerance by jasmonic acid treatment in roselle (Hibiscus sabdariffa L.) seedlings through enhancing antioxidant enzymes activity and metabolic changes. Biologia. https://doi.org/10.2478/s11756-020-00444-8
Siddiqi KhS, Husen A (2019) Plant response to jasmonates: current developments and their role in changing environment. Bull Natl Res Cent 43:153–164. https://doi.org/10.1186/s42269-019-0195-6
Thakur M, Sharma AD (2005) Salt stress induced proline accumulation in germinating embryos: evidence suggesting a role of proline in seed germination. J Arid Environ 62(3):517–523. https://doi.org/10.1016/j.jaridenv.2005.01.005
Thokchom SD, Gupta S (2020) Kapoor R (2020) Arbuscular mycorrhiza augment essential oil composition and antioxidant properties of Ocimum tenuiflorum L.- a popular green tea additive. Ind Crops Prod 153:112418
Torabi M, Halim RA (2010) Variation of root and shoot growth and free proline accumulation in Iranian alfalfa ecotypes under salt stress. J Food Agric Environ 8(3&4):323–327
Tuna AL, Kaya C, Ashraf M, Altunlu H, Yokas I, Yagmur B (2007) The effects of calcium sulfate on growth, membrane stability and nutrient uptake of tomato plants grown under salt stress. Environ Exp Bot 52(2):173–178
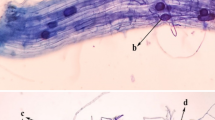
Vierheilig H, Coughlan AP, Wyss U, Piche Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol 12:5004–5007
Wu HH, Zou YN, Rahman MM, Ni QD, Wu Q (2017) Mycorrhizas alter sucrose and proline metabolism in trifoliate orange exposed to drought stress. Sci Rep 7(42389):1–10. https://doi.org/10.1038/srep42389
Zhang Sh, Yan Y, Wang B, Liang Z, Liu Y, Liu F, Qi Zh (2013) Selective responses of enzymes in the two parallel pathways of rosmarinic acid biosynthetic pathway to elicitors in Salvia miltiorrhiza hairy root cultures. J Biosci Bioeng. https://doi.org/10.1016/j.jbiosc.2013.10.013
Acknowledgements
This research has been supported by the Research and Technology Institute of Plant Production (RTIPP), Shahid Bahonar University of Kerman, Kerman, Iran. The authors are grateful for the financial support for this research project (Project number: 1401/965).
Author information
Authors and Affiliations
Contributions
AS designed and performed the experiment, collected data, and interpreted biochemical data. He is also the corresponding author. SHJ participated in statistically analyzing and interpreting phytochemical data and prepared the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Handling Editor: Mohammad Irfan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saadatfar, A., Hossein Jafari, S. Effects of Mycorrhizal Fungi and Methyl Jasmonate on Salt Stress Tolerance and Phytochemical Traits of Satureja Khuzistanica. J Plant Growth Regul 42, 7266–7279 (2023). https://doi.org/10.1007/s00344-023-11015-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-023-11015-x