Abstract
Salinity is one of the most constraining environmental factors that limits plant growth and productivity because it disturbs mineral nutrition by triggering interactions at the interface soil roots. It implies a notable competition between sodium (Na+) and potassium (K+), with this last mineral being a key nutrient for plants. Using the halophyte Cakile maritima as a model plant grown in hydroponic conditions, this study was aimed to analyze how the simultaneous stressful conditions of high salinity (400 mM NaCl) and K+ deficiency (0 mM) for 15 days affect plant growth, ion balance, and antioxidant and NADPH-generating systems. Among the parameters analyzed, the most remarkable changes were observed in leaves, with drastic increases in the Na+/K+, Na+/Ca2+ and Na+/Mg2+ ratios, an enhanced accumulation of anthocyanins, and the induction of 3 new copper/zinc superoxide dismutase (CuZnSOD) isozymes in plants simultaneously exposed to both stresses. Taken together, the data revealed that the combination of both, high salinity and K+ deficiency, caused oxidative stress and modulated the whole antioxidative response of C. maritima in leaves and roots. Besides the differential response underwent by both organs, considering the different parameters analyzed under these stressful conditions, the most notable traits were that the effect of both stresses seems to be not additive and that salinity appears to improve C. maritima response to K+, a behavior not manifested in glycophyte species. Taken together our data support that, under extreme conditions that lead to an excess of ROS production, the induction of several CuZn-SODs in C. maritima may be one of the most outstanding strategies for the adaptation of this plant species to survive.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In their natural habitats, plants are prone to be exposed along their life cycle to several simultaneous constraints that impede their growth and development. Nevertheless, most of the studies dealing with responses to abiotic stresses tackle these issues separately. Thus, results obtained from individually applied stresses are too specific and fragmentary to deeply understand plant responses to simultaneous stresses (Martinez et al. 2019; Zhou et al. 2018). According to Pachauri et al. (2014) and Hoegh-Guldberg et al. (2019), the intensity of many climate change factors will increase in the future, and therefore, plants will be challenged not only by one stress but by a mix of adverse abiotic stressors termed as a multifactorial stress combination (Girme 1977; Zandalinas et al. 2021).
Salinity is among the most widespread environmental stresses which harms about 900 million ha of land worldwide (Velmurugan et al. 2020). The impact of salinity on plant responses is well documented either in glycophyte or halophyte species (Valderrama et al. 2006; Ellouzi et al. 2011, 2014; Hamed et al. 2013; Manai et al. 2014a; Flowers et al. 2015; Slama et al. 2015; Houmani et al. 2016). The continuous increase of the sea level in the framework of the current climate changes aggravates the soil salinity problem, particularly in sandy coastal regions (Srivastava et al. 2019). These ecosystems are naturally characterized by low nutrient availability for plants because of the dominance of salt toxic ions such as Na+ and Cl− and the low cationic exchange capacity (C.E.C < 15) (Radulov et al. 2011). Nevertheless, these areas are vegetation-rich biotopes and are considered precious ecosystems for several seashore herbaceous vegetation including halophytes (Sciandrello 2020). Because of the dominance of sodium ions, halophytes native to coastal areas have to deal not only with salinity but also with nutritional disruption, particularly potassium deficiency, because of the Na+/K+ antagonism.
Potassium (K+) is the seventh most abundant element on earth (Schroeder 2019) and the second most abundant macro-element in plant tissues. It must be maintained in the cytosol within a range of 100–200 mM to ensure efficient physiological events (Kader and Lindberg 2010; Wang and Wu 2013) such as plant growth and development (Tang et al. 2015; Johnson et al. 2022), and the activation of several enzymes of diverse metabolic pathways (White and Karley 2010; Kumar et al. 2020). In addition, K+ has a crucial role in mitigating plant responses to several abiotic constraints and, thus, it is considered a “vital regulator of plant tolerance to abiotic stresses” (Cakmak 2010; Hasanuzzaman et al. 2018; Kumari et al. 2021). Nevertheless, the availability of K+ to plants is unstable, owing to complex soil dynamics, which are strongly affected by root-soil interactions (Ashley et al. 2006). Besides, much of the total K+ in the soil is in a structural form, bound to primary minerals, often of minor contribution to plant nutrition (Alves et al. 2013).
In saline soils, K+ deficiency arises from low K+ availability in the soil solution and/or it can be triggered by high levels of salt elements because of the competition between K+ and Na+ (Waqas et al. 2021). At the chemical level, the competition between both ions for the absorption sites is located at the plasma membrane where low and high-affinity K+ transporters are responsible for the uptake and the distribution of K+ in plant tissues (Shabala and Cuin 2008; Ankit et al. 2022). This is due to a depolarization of the plasma membrane, an enhancement of reactive oxygen species (ROS) generation leading to activation of K+ efflux channels, and a fast K+ influx in the plant cell (Benito et al. 2014; Ragel et al. 2019).
Little is known about plant behavior under the interaction between salt stress and K+ availability. In this respect, a negative impact of abiotic stress combinations was documented, especially in the case of high salinity/K+ deficiency mixed stresses (Qu et al. 2012; Nieves-Cordones et al. 2019b). Very recently, using a typical salt-excluding halophytic grass (Puccinellia tenuiflora) under mild salinity and K+ deprivation, it has been shown the complex coordination of high-affinity K+-dependent Na+ transporters (PutHKT1;5, PutSOS1, PutHKT1;4, PutHKT2:1 and PutAKT1) that mediated the selective transport capacity for K+ over Na+ from root to shoot (Han et al. 2022).
In the last decade, increasing attention has been paid to investigating plant responses to several simultaneous stresses with respect to the ongoing climate changes, what intensifies the frequency of the occurrence, and the severity of such constraints. Hence, studies should be oriented to mimic the field and natural growing conditions. In fact, some studies investigating the interactions between abiotic or/and biotic stresses have lately been addressed (Kissoudis et al. 2016; García-Martí et al. 2019; Pandey et al. 2019; Nieves-Cordones et al. 2019b; Demirel et al. 2020; Talbi Zribi et al. 2020).
There is increasing evidence that abiotic stresses, either applied separately or combined, impact plant growth and productivity since they trigger oxidative stress via an increase in ROS production, a decrease of antioxidative defense, an imbalance in nutritional status, and modifications of cell anatomic structures (Tripathi et al. 2016; Zandalinas et al. 2021). For instance, salinity and nutrient deficiencies induce oxidative damage through the accumulation of ROS, mainly hydrogen peroxide (H2O2), superoxide anions (O2.−), hydroxyl radical (.OH), and singlet oxygen (1O2) (Zheng et al. 2009; Parida and Jha 2010). These species are produced in different plant organs including chloroplasts, mitochondria, peroxisomes, and apoplast (Kerchev et al. 2016; Takagi et al. 2016; Kohli et al. 2019; Palma and Corpas 2021; Corpas et al. 2020). In mitochondria, ROS generation is due to electron leakage from complex I to complex III, leading to the formation of O2.−. This is later converted into H2O2 due to the activity of Mn-SOD (Quan et al. 2008; Huang et al. 2016). In leaf peroxisomes, ROS are basically generated via the activity of the photorespiratory glycolate oxidase and other oxidases (Sarvajeet and Narendra 2010; Baishnab and Ralf 2012; Kerchev et al. 2016). At the apoplast, several mechanisms are responsible for O2.− and H2O2 generation. The most studied is the NADPH oxidase-dependent which results in the O2.− formation (Gilroy et al. 2016).
Plant responses to the combined effect of high salinity and K+ deficiency regarding their antioxidant defenses are still poorly understood, as few investigations emphasized the effect of moderate salinity (100 mM) on the antioxidative response of plants exposed to potassium shortage (Hafsi et al. 2010, 2017). But, more investigations are necessary to get deeper insights into the function of the antioxidant systems under the combination of salt stress and K+ deficiency.
Sea rocket (Cakile maritima L.) belongs to the mustard family Brassicaceae and is considered an excellent model for understanding biochemical traits of tolerance to many abiotic stresses in halophytes principally due to its adaptable antioxidant systems (Ben Amor et al. 2020; Debez et al. 2013; Houmani et al. 2018). The information available on the response of halophyte species under abiotic stresses is still related to individual effects, either osmotic (drought and salinity), mechanical wounding or heavy metals (Ibrahim et al. 2021; Derbali et al. 2021; Houmani et al. 2018; Amari et al. 2017; Belghith et al. 2022). On the other hand, the pre-treatment of halophytes during early development with salinity can have a priming effect allowing a more adequate response to subsequent stresses (Hamed et al. 2013). In previous studies, it has been independently investigated the effect of high salinity (Houmani et al. 2016; Arbelet-Bonnin et al. 2020; Farhat et al. 2021) and K+ deficiency (Houmani et al. 2022), but to our knowledge, there are no data regarding ROS metabolism of halophytes to the combined effect of both stresses. Consequently, the present study aims to decipher the metabolic antioxidant response in roots and leaves of C. maritima subjected to the simultaneous application of high salinity and K+ deficiency.
Material and Methods
Plant Material and Growth Conditions
C. maritima seeds were disinfected with sodium hypochlorite (50%; w/v) for 4 min, washed several times with distilled water, and then sown in Petri dishes containing two layers of filter paper soaked in distilled water. After 7 days, plantlets were hydroponically grown in half-strength Hoagland’s nutrient solution for 15 days (Houmani et al. 2022). To study the combined effects of salinity and K+ deficiency in C. maritima, three groups were made: (i) Control plants kept in the Hoagland nutrient solution (3 mM K+/0 mM NaCl); (ii) salt-treated plants (+NaCl in figures) maintained in the same Hoagland nutrient solution and supplemented with 400 mM NaCl (3 mM K+/400 mM NaCl); and (iii) plants subjected to the combined effects of K+ deficiency and salinity (0 mM K+/400 mM NaCl, −K/+NaCl in figures). After 15 days of treatment, the plants were harvested, and roots and leaves were separated and stored at − 80 °C for further biochemical analysis. The plant growth was carried out under greenhouse conditions (24/18 °C light/dark temperature; 80% relative humidity) for a total time of 37 days. Figure 1 illustrates the designed experiments to apply both stresses.
Experimental design to study simultaneous stresses, salinity (400 mM NaCl) plus potassium deficiency (0 mM K+), in the halophyte Cakile maritima L. Seeds were germinated in Petri dishes for 7 days. Then, the plantlets were hydroponically grown in half-strength Hoagland’s nutrient solution for additional 15 days. Thereafter, plants were separated into three lots: control (C) plants kept in the Hoagland nutrient solution containing 3 mM K+/0 mM NaCl; salinity stress (3 mM K+/400 mM NaCl); and simultaneous stresses by K+ deficiency and salinity (0 mM K+/400 mM NaCl). After 15 additional days, plants were harvested
Mineral Content
Plant material was washed with Milli-Q water and dried for 48 h at 80 °C. Then, it was pulverized to a fine powder from which 0.25 g of each sample was placed into a beaker. The digestion was carried out by adding 3 mL of HNO3 (65%, v/v), covered with a watch glass, and heated on a griddle set at 120 °C. When the solution began to turn brownish, 1 mL of HClO4 (70–72%, v/v) was added to each beaker, and the temperature was raised to 230 °C. After approximately 30 min, a white vapor could be observed inside the beaker indicating that the digestion had finished. The samples were removed, and 5 mL of boiling deionized water was added to each beaker. The content of each beaker was poured into a test tube, and hot deionized water was added again to a final volume of 15 mL. All elements were determined by atomic absorption spectrometry (Perkin Elmer 1100 B). In most cases, samples had to be diluted to obtain values within the range calibrated on the instrument.
Chlorophyll Content
Total chlorophyll content was monitored by measuring the SPAD (Soil Plant Analysis Development) value using a portable chlorophyll meter SPAD-502 (Minolta Camera Co., Ltd, Japan). This device measures leaf greenery by transmitting light from a light-emitting diode through a leaf at wavelengths 650 and 940 nm. The 650 nm light corresponds to a peak chlorophyll attenuation of red light (Ling et al. 2011).
Histochemical Detection of Superoxide Radical (O2 .−) in Leaves
Leaves from the different treatments were excised and vacuum-infiltrated with nitroblue tetrazolium (NBT) solution (0.5 mg · mL−1 in 100 mM phosphate buffer, pH 6.8) for 5 min. After infiltration, the samples were incubated for 1 h at 25 °C in darkness. Then, samples were illuminated until the appearance of dark spots, characteristic of blue formazan precipitates (Vargas et al. 2012).
Organ Crude Extracts
Leaves and roots were frozen in liquid N2 and ground to a powder in a mortar with a pestle. Two grams of the powder was suspended in 4 mL of 50 mM Tris–HCl buffer, pH 7.8, containing 0.1 mM EDTA, 0.02% (w/v) Triton X-100, 10% (w/v) glycerol, 1% (w/v) PVPP, and 5 mM dithiothreitol (DTT). The crude extracts were then filtered through one layer of Miracloth and centrifuged at 27,000 · g at 4 °C for 25 min. Finally, the supernatants were collected and used for assays.
Lipid Peroxidation
Lipid peroxidation products were estimated by measuring the concentration of malondialdehyde (MDA), the main thiobarbituric acid reactive substance, as described by Buege and Aust (1978).
Anthocyanin Content
Anthocyanin content was determined according to the Gould et al. (2000) method. Briefly, leaf samples were kept in 2 mL of a solution containing HCl/H2O/methanol (1/3/6; v/v/v) and stored at 4 ºC the dark conditions until the subsequent pigment extraction. The optical density (OD) was read at 530 and 653 nm, and the anthocyanin content was calculated according to: anthocyanins (µg mL−1) = OD530 − 0.24 OD653.
Activity Enzyme Assay
Glycolate oxidase (GOX; EC 1.1.3.1) activity was determined by measuring the formation of glyoxylate-phenylhydrazone as described by Kerr and Groves (1975). NADH-dependent hydroxypyruvate reductase (HPR; EC 1.1.1.29) activity was assayed according to Schwitzguébel and Siegenthaler (1984).
Catalase activity (EC 1.11.1.6) was assayed using the Aebi (1984) method by measuring the disappearance of H2O2. at 240 nm. Glutathione reductase (GR; EC 1.6.4.2) activity was measured by recording NADPH oxidation as the decrease in absorbance at 340 nm at 25 °C as described by Valderrama et al. (2006). Ascorbate peroxidase (APX; EC 1.11.1.11) was determined by monitoring the initial ascorbate oxidation by H2O2 at 290 nm (Hossain and Asada 1984). Monodehydroascorbate reductase (MDAR; EC 1.6.5.4) was assayed by measuring the monodehydroascorbate-dependent NADH oxidation at 340 nm, with monodehydroascorbate being generated by the ascorbate/ascorbate oxidase system (Hossain et al. 1984). The rate of monodehydroascorbate independent NADH oxidation (without ascorbate and ascorbate oxidase) was subtracted from the monodehydroascorbate-dependent reaction. Dehydroascorbate reductase (DHAR; EC 1.8.5.1) was determined by following the increase of ascorbate formation at 265 nm using an N2-saturated buffer (Dalton et al. 1993). The reaction rate was corrected by the non-enzymatic reduction of dehydroascorbate by reduced glutathione (GSH). A factor of 0.98, to account for the small contribution to the absorbance by oxidized glutathione (GSSG), was also considered.
NADP-dependent dehydrogenase (NADP-DH) activities were determined spectrophotometrically by recording the reduction of NADP at 340 nm. The assays were achieved at 25 °C in a reaction medium (1 mL) containing 50 mM HEPES, pH 7.6, 2 mM MgCl2, and 0.8 mM NADP. The reaction was started by the addition of a specific substrate for each enzyme. Thus, NADP-isocitrate dehydrogenase (NADP-ICDH; EC 1.1.1.42) activity was initiated by the addition of 10 mM 2R,3S-isocitrate; glucose-6-phosphate dehydrogenase (G6PDH; EC 1.1.1.49) activity was initiated by the addition of 5 mM glucose-6-phosphate; to determine 6-phosphogluconate dehydrogenase (6PGDH; EC 1.1.1.44) activity, the substrate was 5 mM 6-phosphogluconate; and, in the case of NADP-malic enzyme (NADP-ME; EC 1.1.1.40) activity, the reaction was initiated by the addition of 1 mM L-malate (Barroso et al. 1998; Leterrier et al. 2007; Mateos et al. 2009).
Non-denaturing PAGE
SOD (EC 1.15.1.1) isozymes were separated by non-denaturing polyacrylamide gel electrophoresis (PAGE) on 8% acrylamide gels and visualized by a photochemical NBT reduction method (Beauchamp and Fridovich 1971). Peroxidase (POX) isozymes were separated by non-denaturing PAGE on 6% acrylamide gels and detected as previously described by Ádám et al. (1995). Gels were incubated for 20 min in sodium acetate buffer 0.1 M, pH 5.5, containing 3,3-diaminobenzidine 1 mM and H2O2 (0.03%, v/v). Brown bands appeared over a colorless background at the end of the reaction.
Other Assays
Protein concentration was determined using the Bio-Rad protein assay with bovine serum albumin as standard. Relative gel band quantification was done using ImageJ software.
Statistical Analysis
Statistical analysis was performed using the Statgraphics program and data were analyzed by one-way ANOVA using the Duncan test. Different letters denote significantly different means at p < 0.05.
Results
Effects of Higher Salinity and K+ Deficiency on Biometrical Features and Mineral Content
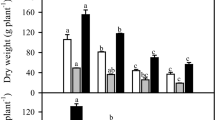
Figure 2 shows the appearance and the biometric analysis and chlorophyll content of 37-day-old C. maritima plants grown under different conditions of high salinity and K+ deficiency. Under both stressful conditions, the whole plants and leaves showed a smaller size (Fig. 2A, 2B). Thus, the whole plant fresh weight (Fig. 2C) and shoot length (Fig. 2D) were diminished 6- and 2.5-fold, respectively, under both stressful conditions. Interestingly, salinity applied alone (Houmani et al. 2016) or in combination with K+ deficiency (present work) decreased similarly the plant growth of C. maritima seedlings (exceeding 80%), thus suggesting that the combined effect of both stresses was not additive. The evaluation of chlorophyll content by monitoring the SPAD index at the end of the treatment also showed a decline between 16 and 19% in comparison to the control (Fig. 2E).
Growth parameters and chlorophyll content in Cakile maritima plants subjected to salinity and K+ deficiency stress conditions. (A) Appearance of 37-day-old whole plants grown in nutrient solutions containing either 3 mM K+/0 mM NaCl (control); high salt concentration (3 mM K+/400 mM NaCl, +NaCl); and simultaneous stresses by K+ deficiency and salinity (0 mM K+/400 mM NaCl, −K+NaCl). (B) Leaf appearance. (C) Whole plant fresh weight (FW). (D) Shoot length. E. Chlorophyll (Chl) content (SPAD). Results are the mean of at least three different experiments ± SEM. Different letters indicate that differences were statistically significant at p < 0.05 using the Duncan test
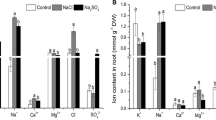
On the other hand, whereas the Na+ concentration increased as a consequence of the NaCl application to plants (Fig. 3A), the K+ content in leaves and roots decreased significantly under salinity stress, and this latter effect was amplified after the combination of both stresses (Fig. 3B). Likewise, a decline of Ca2+ and Mg2+ contents were observed in plants under the two stress conditions (Figs. 3C, 3D, respectively). In contrast, the Na+/K+, Na+/Mg2+ and Na+/Ca2+ ratios increased in leaves and roots of C. maritima exposed to both salinity and K+ deficiency, being higher in leaves when both stresses were combined (Fig. 3E, 3F and 3G). These results suggested that Na+ is preferentially allocated to leaves where it is stored in vacuoles leading to a disruption in ion acquisition.
Mineral content and ratios in leaves and roots of 37-day-old Cakile maritima plants grown in nutrient solutions containing either 3 mM K+/0 mM NaCl (control); high salt concentration (3 mM K+/400 mM NaCl, +NaCl); and simultaneous stresses by K+ deficiency and salinity (0 mM K+/400 mM NaCl, −K+NaCl). (A) Na+ content. B K+ content. C Ca2+ content. D Mg2+ content. (E) Na+/K+ ratio. (F) Na+/Ca2+ ratio. (G) Na+/Mg2+ ratio. Results are the mean of at least three different experiments ± SEM. Different letters indicate that differences were statistically significant at p < 0.05 using the Duncan test regarding their corresponding control, either leaves or roots. DW, dry weight
Oxidative Damage and Antioxidant Defenses
Figure 4A shows the histochemical detection of O2.− radicals (blue spots) in C. maritima leaves. Blue spots, characteristic of this assay, appeared in leaves from plants subjected to salt stress alone or in combination with K+ deficiency as compared to the control, being apparently stronger in this last treatment. The evaluation of MDA content is a recognized marker of oxidative damage, which stands for the unsaturated lipid peroxidation degree. MDA contents increased in all treatments compared to the control. In leaves, MDA content increased by 2.5- and 2.3- fold under salinity applied either alone or in combination with K+ deficiency, respectively (Fig. 4B). In roots, exposure to 400 mM NaCl either in the presence or absence of K+ stimulated MDA content by 1.8-and 2.2-fold as compared to control plants (Fig. 4B).
Detection of superoxide radicals and lipid peroxidation in Cakile maritima plants subjected to salinity (+NaCl) and K+ deficiency (−K/+NaCl) stress conditions. A Histochemical detection of superoxide radicals with NBT staining in leaves. Arrows indicate the precipitated blue formazan product. B Lipid peroxidation (MDA) in leaves and roots of 37-day-old Cakile maritima plants grown in nutrient solutions containing either 3 mM K+/0 mM NaCl (control); salinity stress (3 mM K+/400 mM NaCl, +NaCl); and simultaneous stresses by K+ deficiency and salinity (0 mM K+/400 mM NaCl, −K/+NaCl). Results are the mean of at least three different experiments ± SEM. Different letters indicate that differences were statistically significant at p < 0.05 using the Duncan test regarding their corresponding control. MDA, malondialdehyde
To overcome oxidative damages induced by numerous abiotic constraints, plants have developed many non-enzymatic and enzymatic ROS-scavenging systems. In our case, the determination of anthocyanin contents in leaves samples from plants subjected to salt NaCl alone revealed that salinity did not impact anthocyanin synthesis. By contrast, its interaction with K+ deficiency enhanced significantly the accumulation of such metabolite by 1.8-fold (Fig. 5).
Leaf anthocyanin content in of 37-day-old Cakile maritima plants grown in nutrient solutions containing either 3 mM K+/0 mM NaCl (control), salinity stress (3 mM K+/400 mM NaCl, +NaCl), and simultaneous stresses by K+ deficiency and salinity (0 mM K+/400 mM NaCl, −K/ +NaCl). Results are the mean of at least three different experiments ± SEM. Different letters indicate that differences were statistically significant at p < 0.05 using the Duncan test. FW, fresh weight
Figure 6A depicts the catalase activity in leaves and roots from C. maritima plants exposed to conditions assayed in this work. As compared to the control, leaf catalase activity decreased significantly upon salt treatment (28%), whereas it remained unaffected in plants subjected to the combined effects of salinity and K+ starvation. In roots, all treatments resulted in a significant increase in catalase activity as compared to the control, reaching 34%, and 24%, respectively in plants subjected to salt stress and its interaction with K+ shortage. Concerning GOX and HPR activities, two enzymes involved in the photorespiratory pathway and located in leaf peroxisomes, our results showed a significant decrease in both activities under salinity stress and its interaction with K+ deficiency (Fig. 6B, 6C). The activity of GOX was 2.6 and 1.7-fold lower under salt exposure either in the presence or absence of K+, respectively (Fig. 6B) suggesting that plants may cope with high salinity lowering this activity to avoid the over-accumulation of H2O2 into peroxisomes and, consequently, its harmful effects to other organelles. On the other hand, the activity of HPR was reduced by 1.6 and 2.25-fold as compared to the control in leaves of plants subjected to the individual effect of salinity and its combination with K+ deficiency (Fig. 6C).
Catalase activity in leaves and roots, and activities of photorespiration enzymes in leaves of 37-day-old Cakile maritima plants grown in nutrient solutions containing either 3 mM K+/0 mM NaCl (control), salinity stress (3 mM K+/400 mM NaCl, +NaCl), and simultaneous stresses by K+ deficiency and salinity (0 mM K+/400 mM NaCl, −K/+NaCl). A Catalase activity. B Glycolate oxidase (GOX) activity. C Hydroxypyruvate reductase (HPR) activity. Results are the mean of at least three different experiments ± SEM. Different letters indicate that differences were statistically significant at p < 0.05 using the Duncan test regarding their corresponding control
The analysis of the SOD isozyme patterns in leaves of 37-day-old C. maritima plants using non-denaturing electrophoresis indicated that SOD activity was induced by the presence of NaCl in the culture medium (Fig. 7A). Additionally, it was found that this increase was more pronounced upon the interactive effects of salinity and K+ deficiency, where we detected the disappearance of Fe-SOD isozyme and the induction of up to 7 CuZn-SOD isozymes (CuZn-SOD I-VII, according to their increasing electrophoretic mobility). In roots, up to 6 SOD isozymes could be detected in control plants: 2 Mn-SODs (designated I and II according to their increasing mobility), 1 Fe-SOD, and 3 CuZn-SODs (I-III). Salt treatment applied separately or in combination with K+ deficiency increased the activity of CuZn-SOD I–III isozymes (Fig. 7A). On the other hand, the quantification through the ImageJ software of the total SOD isozyme activity in leaves and roots showed increases by 2.9- and 2.3-fold under salinity conditions, respectively. This increase was even higher under combined salinity and K+ deprivation, with 4.2- and 3.2-fold, respectively (see data of T/C in Fig. 7A).
A Superoxide dismutase (SOD) and B peroxidase (POX) isoenzymatic activities in leaves and roots of 37-day-old Cakile maritima plants grown in nutrient solutions containing either 3 mM K+/0 mM NaCl (control), salinity stress (3 mM K+/400 mM NaCl, +NaCl), and simultaneous stresses by K+deficiency and salinity (0 mM K+/400 mM NaCl, −K/+NaCl). The different isozymes were separated by native PAGE (8% for SOD and 6% for POX). T/C indicates the relative level of the total active bands for each treatment (T) over the control (C) samples, and it expresses the fold change with respect to the control samples (value 1)
Figure 7B shows the analysis of POX isozymes of C. maritima plants exposed to high salinity either in the presence or absence of K+ in the culture medium. In both treatments, a general increase in POX activity was observed in leaves and roots with respect to control plants. In roots, POX activity was more prominent than in leaves, and a total of six POX isozymes were detected in C. maritima plants, designated as POXI-VI according to the increasing mobility in gels. Interestingly, in roots of plants under high salinity, POX IV was induced, and this isoenzyme was not detected either in control plants or under high salinity combined with K+ deficiency. In all root samples POX I and POX III were the most prominent isozymes. Furthermore, the POD VI was undetectable under high salinity and was significantly reduced under the combined effect of salinity and K+ deficiency in comparison with control plants. In the leaves of control plants, four POX isozymes (II to V) were found. Under high salinity, an increase in POD II, IV, and V was found whereas POD III was undetectable. Under the combined effect of salinity and K+ deficiency, an increase of POD III and V was observed, whereas the other two isozymes were nearly undetectable. Instead, the quantification of the total isozyme POX activity in C. maritima leaves and roots increased under salinity by 5.8- and 1.2-fold, respectively. The increase was lower under salinity and K+ deficiency with 1.9- and 1.3-fold, respectively (see data of T/C in Fig. 7B).
The enzymes involved in the ascorbate–glutathione cycle (APX, MDAR, DHAR, and GR) are responsible for cellular control of H2O2 levels and generally showed higher activity in both leaves and roots, following stress application in our experimental design (Fig. 8). Regarding APX, an increase of 2.7- and 4.6-fold was observed in leaves of plants subjected to salt stress alone or combined to K+ deficiency, respectively, while in roots these augmentations were 1.3- and 1.4-fold as compared to the control (Fig. 8A). MDAR activity was unaffected by salinity in leaves and increased by 1.54-fold when salinity was used besides K+ deficiency. This activity decreased in roots in both treatments (Fig. B). DHAR was raised by 1.5- and 2-fold in leaves of plants exposed to salt stress and supplemented or not with K+, respectively. The same tendency was observed in roots regardless of K+ availability with the highest increase being observed when salinity was combined with K+ deficiency (1.7-fold as compared to the control) (Fig. 8C). Finally, in leaves, GR activity increased on average by 3.3 in plants subjected to individual effects of salinity and its combination with K+ shortage. In roots, while salinity alone slightly reduced GR activity, its interaction with K+ deficiency stimulated it by 1.5-fold (Fig. 8D).
Ascorbate–glutathione cycle activities in leaves and roots of 37-day-old Cakile maritima plants grown in nutrient solutions containing either 3 mM K+/0 mM NaCl (control), salinity stress (3 mM K+/400 mM NaCl, +NaCl), and simultaneous stresses by K+ deficiency and salinity (0 mM K+/400 mM NaCl, −K/+NaCl). A Ascorbate peroxidase (APX) activity. B Monodehydroascrobate reductase (MDAR) activity. C Dehydroascorbate reductase (DHAR) activity. D Glutathione reductase (GR) activity. Results are the mean of at least three different experiments ± SEM. Different letters indicate that differences were statistically significant at p < 0.05 using the Duncan test regarding their corresponding control
In higher plants, there is a group of four NADP-dehydrogenases with the capacity for NADPH regeneration. They include NADP-ICDH, NADP-ME, and the enzymes of the oxidative pentose phosphate pathway, G6PDH and 6PGDH. Figure 9 summarizes the activity of these enzymes in leaves and roots of C. maritima plants subjected either to the individual effects of salinity or its interaction with K+ deficiency. In leaves, NADP-ICDH activity was significantly lower under salinity stress alone or in combination with K+ shortage. In roots, this activity was stimulated by salt stress (29%) and was unaffected under the combined treatments (Fig. 9A). Figure 9B shows the NADP-ME activity, and it was observed that in leaves the activity was lowered both under salinity conditions alone (29%) and combined to K+ shortage (34%). In roots, the activity was diminished by 29% and 30% under both situations. A significant increase in leaf G6PDH activity under salt stress was noted (38%), whereas it was unaffected by the interactive treatment. Oppositely, in roots, G6PDH activity was decreased by about 31% under salinity alone or combined with K+ shortage (Fig. 9C). In leaves, 6PGDH activity decreased by 44% and 50% under salt stress, either under optimal K+ supply or in absence of this element. In roots, 6PGDH activity decreased under salinity while remaining unaffected by its interaction with K+ deficiency (Fig. 9D).
NADP-dehydrogenase activities in leaves and roots of 37-day-old Cakile maritima plants grown in nutrient solutions containing either 3 mM K+/ 0 mM NaCl (control), salinity stress (3 mM K+/400 mM NaCl, + NaCl), and simultaneous stresses by K+ deficiency and salinity (0 mM K+/400 mM NaCl, -K/ + NaCl). A NADP-isocitrate dehydrogenase (ICDH) activity. B NADP-malic enzyme (ME) activity. C Glucose 6-phosphate dehydrogenase (G6PDH) activity. D 6-phosphogluconate dehydrogenase (6PGDH) activity. Results are the mean of at least three different experiments ± SEM. Different letters indicate that differences were statistically significant at p < 0.05 using the Duncan test regarding their corresponding control
Discussion
The current climate change enhances both frequency and intensity of stress factors’ combination (Gray et al. 2016), leading to further variations in soil properties (Rillig et al. 2019). Thus, plants have issue with several efficient mechanisms enabling them to survive under those potentially adverse environmental conditions (Roux et al. 2014; Mickelbart et al. 2015). In this regard, there are several reports documenting negative and positive interactions in conditions of combined abiotic stresses such as salinity-drought, potassium deficiency-heavy metals (Liu et al. 2013), salinity-phosphorus deficiency (Singh and Blanke 2000; Akhtari et al. 2019; Talbi Zribi et al. 2017, 2020), salinity-K+ deficiency (Hafsi et al. 2010, 2017; Nieves-Cordones et al. 2019b), drought-heat (Cohen et al. 2021), and salt-osmotic-heat (Sewelam et al. 2020) stresses. But various abiotic (heat and drought) combined with biotic (virus) stresses have been reported (Prasch and Sonnewald 2013). However, little is known about the halophyte’s response to multifactorial stress combination. These plants, continuously challenged by co-occurring environmental stresses, represent promising candidates for a deeper understanding of plant defense mechanisms to mixed stresses (Ozgur et al. 2013; Hamed et al. 2013; Ozfidan-Konakci et al. 2016; Zandalinas et al. 2021; Barros et al. 2021; Alam et al. 2022; Li et al. 2022).
The salinity tolerance of halophytes depends on the plant species. Thus, it has been documented that some species can survive under very high NaCl concentrations such as 600 mM in the case of Suaeda salsa and 1000 mM for Prosopis strombulifera (Reginato et al. 2012; Song and Wang 2015). Under our experimental conditions, the interaction of high salinity (400 mM) and K+ deficiency restricted similarly the plant growth of 37-day-old C. maritima seedlings grown only under saline conditions, thus demonstrating that both abiotic effects were not additive. The reduction in plant growth by salinity and K+ deficiency could be explained by the fact that K+ is required for many physiological and metabolic pathways (Houmani et al. 2016; Hasanuzzaman et al. 2018; Tighe-Neira et al. 2018; Kumar et al. 2020; Johnson et al. 2022). Moreover, the reduction in photosynthetic rate due to K+ deficiency, as suggested by the decrease in chlorophyll content, and the replacement of this ion by organic osmolytes or by sodium for osmotic adjustment purposes could result in a decrease in plant growth (Tsay et al. 2011; Tränkner et al. 2018). Contradictory to our findings, it has been reported an overall additive effect of many stress combinations (Vile et al. 2012; Shaar-Moshe et al. 2019; Zandalinas et al. 2021). Inhibition of plant growth and the photosynthesis process was previously described under salinity or low K+ availability (Zorb et al. 2014; Houmani et al. 2016, 2022). Besides, a combination of salinity and K+ starvation impacted biomass production in two barley species (Hordeum maritimum and H. vulgare) (Degl’Innocenti et al., 2009), Sulla carnosa (Hafsi et al., 2017), and more recently in tomato (Nieves-Cordones et al. 2019a, b). These data contrast with our data obtained from C. maritima in which the effect of both constraints was not additive. Such finding could be explained by the efficiency of Na+ to replacing K+ in many biological functions including osmoregulation, protein synthesis, stabilization of the chlorophyll molecule, stomatal movements, and maintaining the photosynthetic electron transport (Hedrich and Shabala 2018). According to Choudhary et al. (2016) and Ramu et al. (2016), the interactive effects of many abiotic stresses are not usually additive, given the interactions that occur between the multifactorial stress combinations, resulting in a unique response that is not similar to those observed when stresses are separately applied. Moreover, the effect of single stress can be negated or mitigated by another stress resulting in a positive or neutral impact on plants (Pandey et al. 2017). Moreover, according to Grime’s classification, plants having a slow growth rate are typical “stress tolerator” species. Our data demonstrated also that salinity resulted in an increase of Na+/K+, Na+/Mg2+, and Na+/Ca2+ ratios in both roots and leaves, being higher in the former organ. Indeed, ionic stress is one of the major induced salt stress effects in plants (Arif et al. 2020). NaCl causes an imbalance in cellular ion homeostasis associated with an altered K+ to Na+ ratio (Isayenkov and Maathuis 2019). This result indicates that C. maritma is a halophyte characterized by its capacity to accumulate Na+ in leaves, particularly in vacuoles, and its use for several functions mainly osmotic adjustment. Certainly, high tissue Na+/K+ ratio is a common characteristic of salinity tolerance in halophytes (Jouyban 2012). The reduction in plant growth caused either by a high salinity or its combination with K+ deficiency is a consequence of a high accumulation of Na+ and a loss of K+ and overproduction of ROS. The accumulation of ROS under stress conditions leads to further activation of both guard cell outward rectifying K+ channels (GORK) and nonselective cation channels (NSCC), and this results in a massive efflux of K+ from the cytosol to the apoplast. Such disruption affects the homeostasis of the cytosolic Na+ ratio (Hauser and Horie 2010). It has been reported that high salinity leads to nutritional deficiencies and oxidative stress (Isayenkov and Maathuis 2019; Malakar and Chattopadhyay 2021). Salinity and nutrient deficiency of almost all known macronutrients, including K+, gives rise to oxidative stress characterized by over-accumulation of ROS and damage to membrane lipids, proteins, and nucleic acids (Zhao et al. 2016; Hernández et al. 2017). Under K+ deficiency, ROS formation increases at the photosynthetic electron transport circuit and by a higher NADPH oxidase activity (Hasanuzzaman et al. 2018). Furthermore, under saline conditions, an imbalance of K+ status is susceptible to generate ROS provoking damage to plant cells (Gong et al. 2011). Yet, the metabolism of such components is poorly understood under mixed salinity/K+ deficiency stressing conditions. In the present study, high salinity either alone or in combination with K+ deficiency gave rise to O2.− accumulation, as it was histochemically detected in C. maritima leaves. This could be explained by the fact that both constraints increase NADPH oxidase activity, concomitant with NADPH-dependent O2.− generation. Our data support previous findings in the green alga Ulva pertusa (Goh et al. 2010). An accumulation of ROS was also reported under K+ deprivation in Arabidopsis thaliana and tomato (Nieves-Cordones et al. 2019a; Hernández et al. 2012), and more recently in quinoa subjected to the combined effect of moderate salinity (100 mM NaCl) and K+ deficiency (Waqas et al. 2021).
It is well known that an excessive accumulation of free radicals triggers lipid membrane peroxidation. MDA, a marker of oxidative damage resulting from the degradation of polyunsaturated fatty acids, showed a very strong increase in both organs of Cakile maritima plants exposed to high salinity and K+ deficiency, particularly in leaves. This is consistent with previous studies which showed a marked increase in foliar MDA content under salt stress, K+ deprivation, and especially when both stresses were combined (Hafsi et al. 2010; Gong et al. 2011; Waqas et al. 2021). In two soybean varieties and maize, the production of ROS was maximal under salinity stress and K+ deficiency as well (Abbasi et al. 2014; Parveen et al. 2021). MDA accumulation was also observed in potatoes exposed to different abiotic stress such drought (Nie et al. 2018; Demirel et al. 2020).
Photorespiration is among the H2O2 generating processes in plants exposed to various environmental challenges. The accumulation of this ROS is associated with oxidative damage to cells affecting membrane lipids, proteins, and nucleic acids. In leaf peroxisomes, H2O2 is mainly produced via the activity of GOX (Kerchev et al. 2016; Corpas et al. 2009, 2020). In our case, the activity of GOX and HPR, two key enzymes of the photorespiratory pathway, decreased upon salt exposure irrespective of the K+ status. The decrease in these activities by the addition of NaCl could be explained by the fact that salinity imposes an increase in the CO2 pressure resulting in a reduction of the photorespiratory activity. This eventuality contributes to minimize H2O2 production in peroxisomes and attenuating oxidative damages related to its excessive generation (Geissler et al. 2015). Our data are in line with those of Zhou et al. (2011), who found that salinity suppressed the activity of three key photorespiratory enzymes (phosphoglycolate phosphatase, HPR, and GOX) in wild tomato (Solanum chilense), and concluded that the reduction of these activities is among the mechanisms enabling this species to reduce the generation of ROS. Consequently, the inhibitory effect of salt ions on GOX activity is a strategy that favors decreased photosynthesis and avoids photooxidation to keep the functionality of enzymatic and non-enzymatic antioxidants.
Plants have a great ability to limit the over-accumulation of ROS in their tissues through very active enzymatic and non-enzymatic systems. Despite the abundant data focusing on the role of antioxidants in plant response to abiotic stresses, it is surprising that their contribution to the whole mechanisms underpinning tolerance to the combined effect of salinity and K+ deficiency has been rarely addressed. Moreover, reports to study the interactive effect of salinity and K+ deficiency concerning to oxidative defensive system in halophytes are still very limited. In the present study, C. maritma plants grown in the absence of K+ along with salinity stimulated several ROS scavengers, and this supports the idea that the establishment of a powerful antioxidant system is crucial to successfully deal with salinity under K+ limiting situations (Jithesh et al. 2006). Under our experimental conditions, the interactive effect of high salinity and K+ deficiency enhanced the accumulation of anthocyanins in leaves. Such compounds are defined as water-soluble vacuolar pigment flavonoids that arise as a plant’s defense mechanism to notably stimulate the detoxification of photoinhibition-induced ROS (Geilfus 2019). In fact, it has been reported that plants have evolved many strategies to deal with the excessive accumulation of ROS induced by salinity, including the overproduction and accumulation of anthocyanins (Eryılmaz 2006). Such secondary metabolites are known for their antioxidative properties (Landi et al. 2015) and their capacity to scavenge ROS.
Antioxidant enzymes are also of great importance regarding ROS detoxification. SOD, which is present in various subcellular compartments including cytosol, chloroplasts, mitochondria, peroxisomes, and apoplast is considered the first line of defense against ROS accumulation, via the dismutation of O2.− into H2O2 (Shehab et al. 2010; Sharma et al. 2012; del Río et al. 2018). The enhancement of SOD activity could be related to the special role of SOD in plant protection against oxidative damage (Mahanty et al. 2012; del Río et al. 2018). Our results revealed the induction of several CuZn-SODs under all treatments being higher under salinity in combination with low K+ supply, particularly in leaves, where up to six CuZnSODs were induced. This result supports the recent finding of Zandalinas et al. (2021), who indicated that the response of plants to multifactorial stress combination is stronger and more efficient in plant acclimated to mixed stresses. In two previous studies, we demonstrated in the same plant species used in this work that the Fe-SOD isozyme was strongly diminished upon both high long-term salinity stress exposure and K+ deficiency, while 7 CuZn-SODs isozymes were induced, thus suggesting that in this halophyte, CuZn-SODs play a vital protective role against both independently applied abiotic stresses (Houmani et al. 2016, 2022). Thus, taking into account the results shown here, it can be deduced that the induction of new SOD isozymes is necessary for C. maritima tolerance to the abiotic stresses salinity and K+ shortage, applied either single or simultaneously. Thus, SOD is considered a criterion of tolerance to abiotic stress since resistant plant species enhance their activity under unfavorable conditions (Wang et al. 2016). Particularly, the increase in the number of CuZnSOD isozymes could be one of the reasons to overcome the combined stress triggered by salinity and K+ deficiency, and this has been considered a tool for screening the stress-toleration capacities of plants elsewhere (Dreyer and Schippers 2019; Zhou et al. 2022). One possibility to explain this high number of CuZnSODs could be due to genome duplication events in these kinds of plant species, thus allowing the generation of multiple gene copies such as it has been described for rice CuZnSODs (Sanyal et al. 2022). In tomato plants, the analysis of SOD isozymes in leaves and roots showed the presence of one MnSOD and two CuZn-SODs, but, under salinity stress conditions, the MnSOD activity appeared unaffected whereas both CuZn-SODs increased their activity in the two organs (Manai et al. 2014b). There is conflicting literature about SOD activity modulation under K+ limiting conditions, either alone or by its interaction with salinity. In maize grown under K+ shortage conditions, an increase in SOD activity was observed in roots (Zhao et al. 2016). In H. vulgare, a slight decrease of the two SOD isozyme (CuZn-SOD I and II) activities was detected under the combined effect of moderate salinity and K+ starvation (Hafsi et al. 2010), while in Triticum aestivum, an increase in CuZn-SOD I and II activities was reported under K+ deprivation (Yilmaz et al. 2017). In quinoa, the increase in SOD activity was crucial for scavenging O2.− and reducing the probability of •OH generation under the interactive effect of salinity and K+ deficiency (Waqas et al. 2021).
The generation of H2O2 via SOD activity provokes a general increase in antioxidant scavenging enzymes since it has been reported that this species can be a key signaling molecule (Foyer et al. 2017). In this regard, the simultaneous action of catalase and the main components of the ascorbate–glutathione cycle can lessen oxidative damages under stress combinations. Catalase (CAT) is the main antioxidant enzyme present in peroxisomes and is a key barrier against a wide range of abiotic stress (Corpas et al. 2020). Data inferred from our study depicted a decrease in CAT activity in leaves of salt-treated plants, while it remained unaffected under salinity/K+ deficiency stress. In the root, all treatments resulted in a general increase in CAT activity. Plants exposed to environmental challenges show high catalytic activity to remove the excessive accumulation of H2O2. Under low phosphorus availability and salt stressors, CAT was the main enzyme ensuring detoxification of H2O2 in Brassica napus and maize, respectively (Neto et al. 2006; Chen et al. 2015). Given the functional plasticity of this antioxidant system, previous studies have included its modulation as an antioxidant defense under K+ deficiency and salt stress (Tewari et al. 2007; Hafsi et al. 2011; Mallik et al. 2011). However, the data obtained are not transversal among species. Actually, K+ deficiency induced CAT activity in Brassica juncea, (Ahmad et al. 2014) but reduced it in T. aestivum and Triticum durum (Yilmaz et al. 2017).
As shown above, salinity and K+ deficiency enhanced the H2O2-scavenging systems POX and APX activities in C. maritima leaves and roots. In T. aestivum and T. durum, POX and APX activities were induced by K+ deficiency (Yilmaz et al. 2017). Similarly, a significant increase in the activities of POX, SOD, and CAT was documented in all soybean genotypes either under salinity or K+ shortage conditions (Parveen et al. 2021). It has been demonstrated that the main antioxidant batteries including the enzymatic systems showed a specific and unique pattern under diverse stress combinations, but this was not similar to what it is observed when stresses were applied separately (Choudhury et al. 2017; Zandalinas et al. 2021). Contrary to our results, SOD, CAT, and APX activities were reduced in maize seedlings exposed to salt-stress and K+ deficiency, and the reduction was more accentuated as compared to the individual effect of each stress (Gong et al. 2011). Recently, a proteomic analysis suggested the increased abundance of peroxidase 1 (ApCPX1) in leaves from Alternanthera philoxeroides upon K+ starvation (Li et al. 2020). Other enzymes of the ascorbate–glutathione cycle (MDAR, DHAR, and GR) were stimulated by the interactive effect of salinity and K+ deficiency in C. maritima. These findings are in agreement with previous reports on barley grown under the interactive effects of salinity and K+ deprivation (Hafsi et al. 2010).
In the few studies investigating the interaction between abiotic stressors and ROS metabolism, a distinct response of the antioxidative components has been described. A stimulation of antioxidant enzymes, mainly CAT and SOD, was shown under the interactive effect of constraints such as boron toxicity and salinity in wheat (Naz et al. 2018). Similarly, the activity of CAT, SOD, and GR was enhanced under the interaction of salinity and Zn treatment in T. aestivum (Saeidnejad et al. 2016) or drought and heat stress in tomato (Raja et al. 2020). The interaction of other nutrients with salinity is susceptible to up-regulate the antioxidative response as in the case of phosphorus deficiency and salt stress. Thus, as demonstrated recently by Talbi Zribi et al. (2020), the combination of low P availability and salinity increased the total antioxidant capacity in Aeluropus littoralis.
NADPH is an essential cofactor necessary for cell growth and proliferation since it is involved in a wide range of metabolic pathways including fatty acid biosynthesis, biosynthesis of sugars through the Calvin cycle, biosynthesis of carotenoids and proline, shikimate pathway, biosynthesis of aromatic amino acids (Phe, Tyr, and Trp), conversion of ribonucleotide (RNA) to deoxy-ribonucleotide (DNA), regulation of chloroplast protein import, and biosynthesis of carbon monoxide, among other. Furthermore, NADPH is also needed for cellular detoxification and defense systems including the ascorbate–glutathione cycle, O2.−-generating NADPH oxidase, and NAPDH-dependent thioredoxin reductases (NTRs) (Corpas and Barroso 2014; Hu et al. 2020; Corpas et al. 2021). In our experimental design, a group of four dehydrogenase NADPH-generating enzymes has been evaluated (NADP-ICDH, NADP-ME, G6PDH, and 6PGDH) in leaves and roots from C. maritina. Remarkably, the enzymatic activity of these enzymes was higher in roots than in leaves, and the response under higher salinity plus K+ deficiency of the four enzyme systems in both organs was similar to the response under higher salinity alone. This again suggests that the salinity seems to prevent additional potential damages triggered by K+ deficiency. The NADP-dehydrogenases can be either positively or negatively regulated under multiple adverse environmental conditions such as salinity (Valderrama et al. 2006; Manai et al. 2014b; Bouthour et al. 2015), drought (Signorelli et al. 2013; Sánchez-McSweeney et al. 2021), low temperature (Airaki et al. 2012), heavy metal (Corpas et al. 2016; Kharbech et al. 2017; Ruíz-Torres et al. 2017), or herbicides (de Freitas-Silva et al. 2017), but the obtained results are still a matter of debate since the response of these systems depends on the plant species, the analyzed organs and stress intensity. Moderate salinity (100 mM NaCl) in tomato resulted in a significant decrease of NADP-ICDH, G6PDH, and 6PGDH in roots with the first enzyme being the most affected (94%). By contrast, in A. thaliana, NADP-ICDH activity was stimulated by the same NaCl concentration (Leterrier et al. 2012a, b). In rice, G6PDH was reduced upon salt exposure (Zhang et al. 2013). In C. maritima subjected to mechanical wounding, among the analyzed NADPH-regenerating enzymes, an induction of NADP-ICDH was noted in roots (Houmani et al. 2018). For instance, to the best of our knowledge, no information is available on any NADP-dehydrogenase under K+ deficiency in combination with salinity. This highlights the importance of studying their modulation under abiotic stressors separately or in combination.
Overall, the accumulation of superoxide anion and the increase in the antioxidative enzymes by high salinity and K+ deficiency suggest that C. maritima adopts two mechanisms to control oxidative stress via the reduction of ROS generation (decrease in the photorespiratory process) and the induction of the whole antioxidative response (enzymatic ROS-scavenging system) in leaves and roots. In fact, this halophyte has a powerful antioxidant system enabling it to maintain its redox homeostasis and prepare the cell ready for H2O2 signaling response as suggested by Waqas et al. (2021). This in turn will provide the beneficial effect of ROS during the acclimation to abiotic stress combination (Choudhury et al. 2017).
Conclusion
The ongoing climate change eases the co-occurrence of many abiotic stresses, resulting in increasing modifications in soil properties, and thus, imposing new views to get a deeper understanding of plant responses to multifactorial stress combinations (Zandalinas et al. 2021). Halophytes, native species of saline habitats where salinity is often associated with low nutrient availability (notably K+) represent suitable candidates for investigating the mechanisms involved in plant tolerance to salinity and K+ deficiency. In C. maritima, high salt stress and low K+ supply resulted in changes in SOD and POX isoenzyme patterns of isozymes, as well as the activity modulation of the ascorbate–glutathione cycle enzymes and the NADPH-generating systems, thus confirming the synergistic effect of the two constraints to stimulate the antioxidative response in this species.
The induction of until six CuZnSODs after the combination of high salinity with low K+ supply, particularly in leaves, suggests that there must be a well-modulated communication between the root, which is the organ that is in direct contact with these stresses (salinity and low K +), and the aerial parts. This is in good agreement with the idea that ROS content may participate in organelle-to-organelle, cell-to-cell, and among organs (root-stem-leaf) signaling events (Houmani et al. 2018; Mittler et al. 2022). The induction of several CuZn-SODs in C. maritima could be one of the most outstanding strategies for the adaptation of this species to survive under extreme conditions that lead to an excess of ROS production. Therefore, future research should be directed toward the molecular characterization and subcellular localization of the induced SODs, which would indicate which cellular compartments and metabolic pathways are the most affected by the stress response.
These powerful antioxidant systems enable this plant species to be a good candidate for future strategies aimed at increasing crop resistance to salinity and low nutrient availability through genetic engineering imposed by the new advances in plant biotechnology, as well as addressing new approaches focused on halophytes’ domestication.
References
Abbasi G, Akhtar J, Anwar-Ul-Haq M, Ali S, Chen Z, Malik W (2014) Exogenous potassium differentially mitigates salt stress in tolerant and sensitive maize hybrids. Pak J Bot 46:135–146
Ádám AL, Bestwick CS, Barna B, Mansfield JW (1995) Enzymes regulating the accumulation of active oxygen species during the hypersensitive reaction of bean to Pseudomonas syringae pv. Phaseolicola. Planta 7:240–249
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ahmad P, Hashem A, Abd-Allah EF, Alqarawi AA, John R, Egamberdieva D, Gucel S (2015) Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L) through antioxidative defense system. Front Plant Sci 6:868
Airaki M, Leterrier M, Mateos RM, Valderrama R, Chaki M, Barroso JB, del Río LA, Palma JM, Corpas FJ (2012) Metabolism of reactive oxygen species and reactive nitrogen species in pepper (Capsicum annuum L.) plants under low temperature stress. Plant Cell Environ 35:281–295
Akhtari A, Homaee M, Hosseini YA (2019) Predictive model for plant response to interactive effect of salinity and phosphorous. Int J Plant Prod 13:317–328
Alam H, Zamin M, Adnan M, Ahmad N, Nawaz T, Saud S, Basir A, Liu K, Harrison MT, Hassan S, Alharby HF, Alzahrani YM, Alghamdi SA, Majrashi A, Alharbi BM, Alabdallah NM, Fahad S (2022) Evaluating the resistance mechanism of Atriplex leucoclada (Orache) to salt and water stress; a potential crop for biosaline agriculture. Front Plant Sci 13:948736
Alves MJF, Melo VF, Reissmann CB, Kaseker JF (2013) Reserva mineral de potássio em Latossolo cultivado com Pinus taeda L. Rev Bras Cienc Solo 37:1599–1610
Amari T, Ghnaya T, Abdelly C (2017) Nickel, cadmium and lead phytotoxicity and potential of halophytic plants in heavy metal extraction. S Afr J Bot 111:99–110
Ankit A, Kamali S, Singh A (2022) Genomic & structural diversity and functional role of potassium (K+) transport proteins in plants. Int J Biol Macromol 208:844–857
Arbelet-Bonnin D, Blasselle C, Palm ER, Redwan M, Ponnaiah M, Laurenti P, Meimoun P, Gilard F, Gakière B, Mancuso S, El-Maarouf-Bouteau H (2020) Metabolism regulation during salt exposure in the halophyte Cakile maritima. Environ Exp Bot 177:104075
Arif Y, Singh P, Siddiqui H, Bajguz A, Hayat S (2020) Salinity induced physiological and biochemical changes in plants: an omic approach towards salt stress tolerance. Plant Physiol Biochem 156:64–77
Ashley MK, Grant M, Grabov A (2006) Plant responses to potassium deficiencies: a role for potassium transport proteins. J Exp Bot 57:425–436
Baishnab CT, Ralf O (2012) Reactive oxygen species generation and signaling in plants. Plant Signal Behav 7:1621–1633
Barros NLF, Marques DN, Tadaiesky LBA, de Souza CRB (2021) Halophytes and other molecular strategies for the generation of salt-tolerant crops. Plant Physiol Biochem 162:581–591
Barroso JB, Peragon J, Contreras-Jurado C, Garcia-Salguero L, Corpas FJ, Esteban FJ, Peinado MA, De La Higuera M, Lupiañez JA (1998) Impact of starvation-refeeding on kinetics and protein expression of trout liver NADPH-production systems. Am J Physiol 274:R1578–R1587
Beauchamp CO, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Analytical Biochem 44:276–287
Belghith I, Senkler J, Abdelly C, Braun HP, Debez A (2022) Changes in leaf ecophysiological traits and proteome profile provide new insights into variability of salt response in the succulent halophyte Cakile maritima. Funct Plant Biol 49:613–624
Ben Amor N, Jiménez A, Boudabbous M, Sevilla F, Abdelly C (2020) Chloroplast implication in the tolerance to salinity of the halophyte Cakile maritima. Russ J Plant Physiol 67:507–514
Benito B, Haro R, Amtmann A, Cuin TA, Dreyer I (2014) The twins K+ and Na+ in plants. J Plant Physiol 171:723–731
Bouthour D, Kalai T, Chaffei HC, Gouia H, Corpas FJ (2015) Differential response of NADP-dehydrogenases and carbon metabolism in leaves and roots of two durum wheat (Triticum durum Desf.) cultivars (Karim and Azizi) with different sensitivities to salt stress. J Plant Physiol 179:56–63
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Cakmak I (2010) Potassium for better crop production and quality. Plant Soil 335:1–2
Chen S, Zhao H, Ding G, Xu F (2015) Genotypic differences in antioxidant response to phosphorus deficiency in Brassica napus. Plant Soil 391:19–32
Choudhary A, Pandey P, Senthil-Kumar M (2016) Tailored responses to simultaneous drought stress and pathogen infection in plants. In: Hossain MA, Wani SH, Bhattacharjee S, Burritt DJ, Tran L-SP (eds) Drought stress tolerance in plants, vol 1. Springer, Cham, pp 427–438
Choudhury FK, Rivero RM, Blumwald E, Mittler R (2017) Reactive oxygen species, abiotic stress and stress combination. Plant J 90:856–867
Cohen I, Zandalinas SI, Huck C, Fritschi FB, Mittler R (2021) Meta-analysis of drought and heat stress combination impact on crop yield and yield components. Physiol Plant 171:66–76
Corpas FJ, Aguayo-Trinidad S, Ogawa T, Yoshimura K, Shigeoka S (2016) Activation of NADPH-recycling systems in leaves and roots of Arabidopsis thaliana under arsenic-induced stress conditions is accelerated by knock-out of Nudix hydrolase 19 (AtNUDX19) gene. J Plant Physiol 192:81–89
Corpas FJ, Barroso JB (2014) NADPH-generating dehydrogenases: their role in the mechanism of protection against nitro-oxidative stress induced by adverse environmental conditions. Front Environ Sci 2:55
Corpas FJ, González-Gordo S, Palma JM (2020) Plant peroxisomes: a factory of reactive species. Front Plant Sci 11:853
Corpas FJ, González-Gordo S, Palma JM (2021) Nitric oxide and hydrogen sulfide modulate the NADPH-generating enzymatic system in higher plants. J Exp Bot 72(3):830–847
Corpas FJ, Hayashi M, Mano S, Nishimura M, Barroso JB (2009) Peroxisomes are required for in vivo nitric oxide accumulation in the cytosol following salinity stress of Arabidopsis plants. Plant Physiol 151:2083–2094
Dalton DA, Baird LM, Langeberg L, Taugher CY, Anyan WR, Vance CV, Sarath G (1993) Subcellular localization of oxygen defense enzymes in soybean (Glycine max L. Merr) root nodules. Plant Physiol 102:481–489
de Freitas-Silva L, Rodríguez-Ruiz M, Houmani H, da Silva LC, Palma JM, Corpas FJ (2017) Glyphosate-induced oxidative stress in Arabidopsis thaliana affecting peroxisomal metabolism and triggers activity in the oxidative phase of the pentose phosphate pathway (OxPPP) involved in NADPH generation. J Plant Physiol 218:196–205
Debez A, Rejeb KB, Ghars MA, Gandour M, Megdiche W, Hamed KB, Amor NB, Brown SC, Savouré A, Abdelly C (2013) Ecophysiological and genomic analysis of salt tolerance of Cakile maritima. Environ Exp Bot 92:64–72
Degl’Innocenti E, Hafsi C, Guidi L, Navari-Izzo F (2009) The effect of salinity on photosynthetic activity in potassium-deficient barley species. J Plant Physiol 166:1968–1981
del Río LA, Corpas FJ, López-Huertas E, Palma JM (2018) Plant superoxide dismutases: function under abiotic stress conditions. In: Gupta DK, Palma JM, Corpas FJ (eds) Antioxidants and antioxidant enzymes in higher plants. Springer, Cham, pp 1–26
Demirel U, Morris WL, Ducreux LJM, Yavuz C, Asim A, Tindas I, Campbell R, Morris JA, Verrall SR, Hedley PE, Gokce ZNO, Caliskan S, Aksoy E, Caliskan ME, Taylor MA, Hancock RD (2020) Physiological, biochemical, and transcriptional responses to single and combined abiotic stress in stress-tolerant and stress-sensitive potato genotypes. Front Plant Sci 11:169
Derbali W, Manaa A, Spengler B, Goussi R, Abideen Z, Ghezellou P, Abdelly C, Forreiter C, Koyro HW (2021) Comparative proteomic approach to study the salinity effect on the growth of two contrasting quinoa genotypes. Plant Physiol Biochem 163:215–229
Dreyer BH, Schippers JHM (2019) Copper-zinc superoxide dismutases in plants: evolution, enzymatic properties, and beyond. Ann Plant Rev Online 2:1–36
Ellouzi H, Hamed KB, Cela J, Munné-Bosch S, Abdelly C (2011) Early effects of salt stress on the physiological and oxidative status of Cakile maritima (halophyte) and Arabidopsis thaliana (glycophyte). Physiol Plant 142:128–143
Ellouzi H, Ben Hamed K, Hernández I, Cela J, Müller M, Magné C, Abdelly C, Munné-Bosch S (2014) A comparative study of the early osmotic, ionic, redox and hormonal signaling response in leaves and roots of two halophytes and a glycophyte to salinity. Planta 240:1299–1317
Eryılmaz F (2006) The relationships between salt stress and anthocyanin content in higher plants. Biotechnol Biotechnol Equip 20:47–52
Farhat N, Kouas W, Braun HP, Debez A (2021) Stability of thylakoid protein complexes and preserving photosynthetic efficiency are crucial for the successful recovery of the halophyte Cakile maritima from high salinity. Plant Physiol Biochem 166:177–190
Flowers TJ, Munns R, Colmer TD (2015) Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann Bot 115:419–431
Foyer CH, Ruban AV, Nixon PJ (2017) Photosynthesis solutions to enhance productivity. Philos Trans r Soc B Biol Sci 372:3–6
García-Martí M, Piñero MC, García-Sanchez F, Mestre TC, López-Delacalle M, Martínez V, Rivero RM (2019) Amelioration of the oxidative stress generated by simple or combined abiotic stress through the K+ and Ca2+ supplementation in tomato plants. Antioxidants 8:81
Geilfus CM (2019) Controlled environment horticulture: improving quality of vegetables and medicinal plants. Springer, Cham. ISBN 978-3-030-23196-5
Geissler N, Hussin S, El-Far MMM, Koyro HW (2015) Elevated atmospheric CO2 concentration leads to diferent salt resistance mechanisms in a C3 (Chenopodium quinoa) and a C4 (Atriplex nummularia) halophyte. Environ Exp Bot 118:67–77
Gilroy S, Białasek M, Suzuki N, Górecka M, Devireddy AR, Karpiński S, Mittler R (2016) ROS, calcium and electric signals: key mediators of rapid systemic signaling in plants. Plant Physiol 171:1606–1615
Goh CH, Oh SJ, Jun SS, Han T (2010) External K+ deficiency inhibits photosynthetic activity through superoxide anion production in protoplasts isolated from the thallus of Ulva pertusa. J Plant Biol 53:155–164
Gong XL, Chao L, Zhou M, Hong MM, Luo LY, Wang L, Ying W, Cai JW, Gong SJ, Hong FS (2011) Oxidative damages of maize seedlings caused by exposure to a combination of potassium deficiency and salt stress. Plant Soil 340:443–452
Gould KS, Markham KR, Smith RH, Goris JJ (2000) Functional role of anthocyanins in the leaves of Quintinia serrata A. Cunn J Exp Bot 51:1107–1115
Gray SB, Dermody O, Klein SP, Locke AM, McGrath JM, Paul RE, Rosenthal DM, Ruiz-Vera UM, Siebers MH, Strellner R, Ainsworth EA, Bernacchi CJ, Long SP, Ort DR, Leakey AD (2016) Intensifying drought eliminates the expected benefits of elevated carbon dioxide for soybean. Nat Plants 2:16132
Grime JP (1977) Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am Nat 111:1169–1194
Hafsi C, Romero-Puertas MC, del Río LA, Sandalio LM, Abdelly C (2010) Differential antioxidative response in barley leaves subjected to the interactive effects of salinity and potassium deprivation. Plant Soil 334:449–460
Hafsi C, Romero-Puertas MC, del Rio LA, Abdelly C, Sandalio LM (2011) Antioxidative response of Hordeum martitimum L. to potassium deficiency. Acta Physiol Plantarum 33:193–202
Hafsi C, Falleh H, Saada M, Ksouri R, Abdelly C (2017) Potassium deficiency alters growth, photosynthetic performance, secondary metabolites content, and related antioxidant capacity in Sulla carnosa grown under moderate salinity. Plant Physiol Biochem 118:609–617
Han QQ, Wang YP, Li J, Li J, Yin XC, Jiang XY, Yu M, Wang SM, Shabala S, Zhang JL (2022) The mechanistic basis of sodium exclusion in Puccinellia tenuiflora under conditions of soil salinity and potassium deprivation. Plant J. https://doi.org/10.1111/tpj.15946
Hamed KB, Ellouzi H, Talbi OZ, Hessini K, Slama I, Ghnaya T, Bosch SM, Savour A, Abdelly C (2013) Physiological response of halophytes to multiple stresses. Funct Plant Biol 40:883–896
Hasanuzzaman M, Bhuyan M, Nahar K, Hossain M, Mahmud J, Hossen M, Masud A, Fujita M (2018) Potassium: a vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 8:31
Hauser F, Horie T (2010) A conserved primary salt tolerance mechanism mediated by HKT transporters: a mechanism for sodium exclusion and maintenance of high K+/Na+ ratio in leaves during salinity stress. Plant Cell Environ 33:552–565
Hedrich R, Shabala S (2018) Stomata in a saline world. Curr Opinion Plant Biol 46:87–95
Hernández M, Fernandez-García N, Garcia-Garma J, Rubio-Asensio J, Rubio F, Olmos E (2012) Potassium starvation induces oxidative stress in Solanum lycopersicum L. roots. J Physiol 169:1366–1374
Hernández JA, Barba-Espín G, Clemente-Moreno MJ, Díaz-Vivancos P (2017) Plant responses to salinity through an antioxidative metabolism and proteomic point of view. In: Sarwat M, Ahmad A, Abdin M, Ibrahim M (eds) Stress signaling in plants: genomics and proteomics perspective, vol 2. Springer, Berlin, pp 173–200
Hoegh-Guldberg O, Jacob D, Taylor M, Guillén Bolaños T, Bindi M, Brown S, Camilloni IA, Diedhiou A, Djalante R, Ebi K, Engelbrecht F, Guiot J, Hijioka Y, Mehrotra S, Hope CW, Payne AJ, Pörtner HO, Seneviratne SI, Thomas A, Warren R, Zhou G (2019) The human imperative of stabilizing global climate change at 1.5°C. Science 365:1263
Hossain MA, Asada K (1984) Inactivation of ascorbate peroxidase in spinach chloroplasts on dark addition of hydrogen peroxide: its protection by ascorbate. Plant Cell Physiol 25:1285–1295
Hossain MA, Nakano Y, Asada K (1984) Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol 25:385–395
Houmani H, Debez D, de Freitas-Silva S, Abdelly C, Palma JM, Corpas FJ (2022) Potassium (K+) starvation-induced oxidative stress triggers a general boost of antioxidant and NADPH-generating systems in the halophyte Cakile maritima. Antioxidants 11:401
Houmani H, Rodríguez-Ruiz M, Palma JM, Corpas FJ (2018) Mechanical wounding promotes local and long distance response in the halophyte Cakile maritima through the involvement of the ROS and RNS metabolism. Nitric Oxide 74:93–101
Houmani H, Rodríguez-Ruiz M, Palma JM, Abdelly C, Corpas FJ (2016) Modulation of superoxide dismutase (SOD) isozymes by organ development and high long-term salinity in the halophyte Cakile maritima. Protoplasma 3:885–894
Hu CH, Wang PQ, Zhang PP, Nie XM, Li BB, Tai L, Liu WT, Li WQ, Chen KM (2020) NADPH oxidases: the vital performers and center hubs during plant growth and signaling. Cells 9(2):437
Huang S, Van Aken O, Schwarzländer M, Belt K, Millar A (2016) The roles of mitochondrial reactive oxygen species in cellular signaling and stress responses in plants. Plant Physiol 171:1551–1559
Ibrahim Y, Neji M, Taamalli W, Abdelly C, Gandour M (2021) The genetic variation in response to drought in Tunisian populations of Brachypodium hybridum (Poaceae): an interplay between natural selection and phenotypic plasticity. Environ Exp Bot 179:104234
Isayenkov SV, Maathuis FJ (2019) Plant salinity stress: many unanswered questions remain. Front Plant Sci 10:80
Jithesh MN, Prashanth SR, Sivaprakash KR, Parida AK (2006) Antioxidative response mechanisms in halophytes: their role in stress defence. J Genet 85:237–254
Johnson R, Vishwakarma K, Hossen MS, Kumar V, Shackira AM, Puthur JT, Abdi G, Sarraf M, Hasanuzzaman M (2022) Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol Biochem 172:56–69
Jouyban Z (2012) The Effects of Salt stress on plant growth Technical. J Eng Appl Sci 2:7–10
Kader MA, Lindberg S (2010) Cytosolic calcium and pH signaling in plants under salinity stress. Plant Signal Behav 5:233–238
Kerchev P, Waszczak C, Lewandowska A, Willems P, Shapiguzov A, Li Z, Alseekh S, Mühlenbock P, Hoeberichts FA, Huang J, Van Der Kelen K, Kangasjärvi J, Fernie AR, De Smet R, Van de Peer Y, Messens J, Van Breusegem F (2016) Lack of GLYCOLATE OXIDASE 1, but not GLYCOLATE OXIDASE 2, attenuates the photorespiratory phenotype of CATALASE2-deficient Arabidopsis. Plant Physiol 171:1704–1719
Kerr MW, Groves D (1975) Purification and properties of glycollate oxidase from Pisum sativum leaves. Phytochemistry 14:359–362
Kissoudis C, Sunarti S, van de Wiel C, Visser RG, van der Linden CG, Bai Y (2016) Responses to combined abiotic and biotic stress in tomato are governed by stress intensity and resistance mechanism. J Exp Bot 67:5119–5132
Kohli SK, Khanna K, Bhardwaj R, Abd Allah EF, Ahmad P, Corpas FJ (2019) Assessment of subcellular ROS and NO metabolism in higher plants: multifunctional signaling molecules. Antioxidants 8(12):641
Kharbech O, Houmani H, Chaoui A, Corpas FJ (2017) Alleviation of Cr(VI)-induced oxidative stress in maize (Zea mays L.) seedlings by NO and H2S donors through differential organ-dependent regulation of ROS and NADPH-recycling metabolisms. J Plant Physiol 219:71–80
Kumar P, Kumar T, Singh S, Tuteja N, Prasad R, Singh J (2020) Potassium: a key modulator for cell homeostasis. J Biotechnol 324:198–210
Kumari S, Chhillar H, Chopra P, Khanna RR, Khan MIR (2021) Potassium: a track to develop salinity tolerant plants. Plant Physiol Biochem 167:1011–1023
Landi M, Tattini M, Gould KS (2015) Multiple functional roles of anthocyanins in plant-environment interactions. Environ Exp Bot 119:4–17
Leterrier M, del Río LA, Corpas FJ (2007) Cytosolic NADP isocitrate dehydrogenase of pea plants: genomic clone characterization and functional analysis under abiotic stress conditions. Free Radic Res 41:191–199
Leterrier M, Barroso JB, Valderrama R, Palma JM, Corpas FJ (2012a) NADP-dependent isocitrate dehydrogenase from arabidopsis roots contributes in the mechanism of defence against the nitro-oxidative stress induced by salinity. Scientific World Journal, Singapore
Leterrier M, Airaki M, Palma JM, Chak M, Barroso JB, Corpas FJ (2012b) Arsenic triggers the nitric oxide (NO) and S-nitrosoglutathione (GSNO) metabolism in Arabidopsis. Environ Pollut 166:136–143
Li LQ, Lyu CC, Li JH, Wan CY, Liu L, Xie MQ, Zuo RJ, Ni S, Liu F, Zeng FC, Lu YF, Yu LP, Huang XL, Wang XY, Lu LM (2020) Quantitative proteomic analysis of alligator weed leaves reveals that cationic peroxidase 1 plays vital roles in the potassium deficiency stress response. Int J Mol Sci 21(7):2537
Ling Q, Huang W, Jarvis P (2011) Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana. Photosynth Res 107(2):209–214
Liu CH, Chao YY, Kao CH (2013) Effect of potassium deficiency on antioxidant status and cadmium toxicity in rice seedlings. Bot Studies 54:2
Mahanty S, Kaul T, Pandey P, Reddy RA, Mallikarjuna G, Reddy CS, Sopory SK, Reddy MK (2012) Biochemical and molecular analyses of copper-zinc superoxide dismutase from a C4 plant Pennisetum glaucum reveals an adaptive role in response to oxidative stress. Gene 505:309–317
Malakar P, Chattopadhyay D (2021) Adaptation of plants to salt stress: the role of the ion transporters. J Plant Biochem Biotechnol 30:668–683
Mallik S, Nayak M, Sahu BB, Panigrahi AK, Shaw BP (2011) Response of antioxidant enzymes to high NaCl concentration in different salt-tolerant plants. Biol Plant 55:191–195
Manai J, Kalai T, Gouia H, Corpas FJ (2014a) Exogenous nitric oxide (NO) ameliorates salinity-induced oxidative stress in tomato (Solanum lycopersicum) plants. J Soil Sci Plant Nutr 14:433–446
Manai J, Gouia H, Corpas FJ (2014b) Redox and nitric oxide homeostasis are affected in tomato (Solanum lycopersicum) roots under salinity-induced oxidative stress. J Plant Physiol 171:1028–1035
Martinez V, Nieves-Cordones M, Lopez-Delacalle M, Rodenas R, Mestre TC, Garcia-Sanchez F, Rubio F, Nortes PA, Mittler R, Rivero RM (2019) Tolerance to stress combination in tomato plants: new insights in the protective role of melatonin. Molecules 23:535
Mateos RM, Bonilla-Valverde D, del Río LA, Palma JM, Corpas FJ (2009) NADP-dehydrogenases from pepper fruits: effect of maturation. Physiol Plant 135:130–139
Mickelbart MV, Hasegawa PM, Bailey-Serres J (2015) Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat Rev Genet 16:237–251
Mittler R, Zandalinas SI, Fichman Y, Van Breusegem F (2022) Reactive oxygen species signalling in plant stress responses. Nat Rev Mol Cell Biol 23(10):663–679
Naz T, Akhtar J, Anwar-Ul-Haq, Saqib M, Iqbal MM, Shahid M (2018) Interaction of salinity and boron in wheat affects physiological attributes, growth and activity of antioxidant enzymes. Pak J Agric Sci 55:339–347
Neto ADDA, Prisco JT, Enéas-Filho J, Abreu CEBD, Gomes-Filho E (2006) Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt tolerant and salt-sensitive maize genotypes. Environ Exp Bot 56:87–94
Nie LZ, Yu XX, Ma YH, Fang YY, Li LM, Yu Z (2018) Enhanced drought and osmotic stress tolerance in transgenic potato plants expressing AtCDPK1, a calcium-dependent protein kinase. Russ J Plant Physiol 65:865–873
Nieves-Cordones M, López-Delacalle M, Ródenas R, Martínez V, Rubio F, Rivero RM (2019a) Critical responses to nutrient deprivation: a comprehensive review on the role of ROS and RNS. Environ Exp Bot 161:74–85
Nieves-Cordones M, Ródenas R, Lara A, Martínez V, Rubio F (2019b) The combination of K+ deficiency with other environmental stresses: what is the outcome? Physiol Plantarum 165:264–276
Ozfidan-Konakci C, Uzilday B, Ozgur R, Yildiztugay E, Sekmen AH, Turkan I (2016) Halophytes as a source of salt tolerance genes and mechanisms: a case study for the Salt Lake area, Turkey. Funct Plant Biol 43:575–589
Ozgur R, Uzilday B, Sekmen AH, Turkan I (2013) Reactive oxygen species regulation and antioxidant defence in halophytes. Funct Plant Biol 40:832–847
Li C, Duan C, Zhang H, Zhao Y, Meng Z, Zhao Y, Zhang Q (2022) Adaptative mechanisms of halophytic Eutrema salsugineum encountering saline environment. Front Plant Sci 13:909527
Pachauri RK , Allen MR , Barros VR , Broome J, Cramer W, Christ R, Church JA, Clarke L, Dahe Q, Dasgupta P, Dubash NK, Edenhofer O, Elgizouli I, Field CB, Forster P, Friedlingstein P, Fuglestvedt J, Gomez-Echeverri L, Hallegatte S, Hegerl G, Howden M, Jiang K, Jimenez Cisneroz B, Kattsov V, Lee H, Mach KJ, Marotzke J, Mastrandrea MD , Meyer L, Minx J, Mulugetta Y, O'Brien K, Oppenheimer M, Pereira JJ , Pichs-Madruga R, Plattner GK, Pörtner HO, Power SB, Preston B, Ravindranath NH, Reisinger A, Riahi K, Rusticucci M, Scholes R, Seyboth K, Sokona Y, Stavins R, Stocker TF, Tschakert P, van Vuuren D, van Ypserle JP (2014) Climate change 2014: synthesis report. In: R Pachauri and L Meyer (eds) Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. Geneva, Switzerland, p 151
Palma JM, Corpas FJ (2021) Editorial: Subcellular Compartmentalization of plant antioxidants and ROS generating systems. Front Plant Sci 12:643239
Pandey P, Irulappan V, Bagavathiannan MV, Senthil-Kumar M (2017) Front. Plant Sci 8:537
Pandey A, Khan MK, Hakki EE, Gezgin S, Hamurcu M (2019) Combined boron toxicity and salinity stress—an insight into its interaction in plants. Plants 8:364
Parida AK, Jha B (2010) Antioxidative defense potential to salinity in the euhalophyte Salicornia brachiata. J Plant Growth Regul 29:137–148
Parveen A-U-H, Aziz T, Aziz O, Maqsood L (2021) Potassium induces carbohydrates accumulation by enhancing morpho-physiological and biochemical attributes in soybean under salinity. Arch Agron Soil Sci 67:946–959
Prasch CM, Sonnewald U (2013) Simultaneous application of heat, drought, and virus to Arabidopsis plants reveals significant shifts in signaling networks. Plant Physiol 162:1849–1866
Quan LJ, Zhang B, Shi WW, Li HY (2008) Hydrogen peroxide in plants: a versatile molecule of the reactive oxygen species network. J Integr Plant Biol 50:2–18
Qu C, Liu C, Gong X, Li C, Hong M, Wang L, Hong F (2012) Impairment of maize seedling photosynthesis caused by a combination of potassium deficiency and salt stress. Environ Exp Bot 75:134–141
Radulov I, Berbecea A, Sala F, Crista F, Alina Lato A (2011) Mineral fertilization influence on soil pH, cationic exchange capacity and nutrient content. Res J Agric Sci 43:160
Ragel P, Raddatz N, Leidi EO, Quintero FJ, Pardo JM (2019) Regulation of K+ nutrition in plants. Front Plant Sci 10:281
Raja V, Qadir SU, Alyemeni MN, Ahmad P (2020) Impact of drought and heat stress individually and in combination on physio-biochemical parameters, antioxidant responses, and gene expression in Solanum lycopersicum. Biotech 10:208
Ramu VS, Paramanantham A, Ramegowda V, Mohan-Raju B, Udayakumar M, Senthil-Kumar M (2016) Transcriptome analysis of sunflower genotypes with contrasting oxidative stress tolerance reveals individual- and combined- biotic and abiotic stress tolerance mechanisms. PLoS ONE 11:e0157522
Reginato M, Sgroy V, Llanes A, Cassán F, Luna V (2012) The American halophyte Prosopis strombulifera, a new potential source to confer salt tolerance to crops. Crop production for agricultural improvement. Springer, Dordrecht, pp 115–143. https://doi.org/10.1007/978-94-007-4116-4_5
Rillig MC, Lehmann A, de Souza Machado AA, Yang G (2019) Microplastic effects on plants. New Phytol 223:1066–1070
Roux F, Voisin D, Badet T, Balagué C, Barlet X, Huard-Chauveau C, Roby D, Raffaele S (2014) Resistance to phytopathogens e tutti quanti: placing plant quantitative disease resistance on the map. Mol Plant Pathol 15:427–432
Ruíz-Torres C, Feriche-Linares R, Rodríguez-Ruíz M, Palma JM, Corpas FJ (2017) Arsenic-induced stress activates sulfur metabolism in different organs of garlic (Allium sativum L.) plants accompanied by a general decline of the NADPH-generating systems in roots. J Plant Physiol 211:27–35
Saeidnejad AH, Kafi M, Pessarakli M (2016) Interactive effects of salinity stress and Zn availability on physiological properties, antioxidants activity and micronutrients’ content of wheat (Triticum Aestivum) plants. Commun Soil Sci Plant Anal 47:1048–1057
Sánchez-McSweeney A, González-Gordo S, Aranda-Sicilia MN, Rodríguez-Rosales MP, Venema K, Palma JM, Corpas FJ (2021) Loss of function of the chloroplast membrane K+/H+ antiporters AtKEA1 and AtKEA2 alters the ROS and NO metabolism but promotes drought stress resilience. Plant Physiol Biochem 160:106–119
Sanyal RP, Prashar V, Jawali N, Sunkar R, Misra HS, Saini A (2022) Molecular and biochemical analysis of duplicated cytosolic CuZn superoxide dismutases of rice and in silico analysis in plants. Front Plant Sci 13:864330
Sarvajeet SG, Narendra T (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Schwitzguébel JP, Siegenthaler PA (1984) Purification of peroxisomes and mitochondria from spinach leaf by percoll gradient centrifugation. Plant Physiol 75:670–674
Sciandrello S (2020) Coastal saltmarsh vegetation in Sicily (Italy): phytosociological insights and plant diversity. Plant Biosyst Int J Dealing All Asp Plant Biol 154:860–876
Schroeder D (2019) Structure and weathering of potassium containing minerals. IPI Res Top 2013:43–63
Sewelam N, Brilhaus D, Bräutigam A, Alseekh S, Fernie AR, Maurino VG (2020) Molecular plant responses to combined abiotic stresses put a spotlight on unknown and abundant genes. J Exp Bot 71:5098–5112
Shaar-Moshe L, Hayouka R, Roessner U, Peleg Z (2019) Phenotypic and metabolic plasticity shapes life-history strategies under combinations of abiotic stresses. Plant Direct 3:e00113
Shabala S, Cuin TA (2008) Potassium transport and plant salt tolerance. Physiol Plant 133:651–669
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Botany 2012:217037
Shehab GG, Ahmed OK, EL-Beltagi HS, (2010) Effects of various chemical agents for alleviation of drought stress in rice plants (Oryza sativa L.). Not Bot Hort Agrobot Cluj 38:139–148
Signorelli S, Corpas FJ, Borsani O, Barroso JB, Monza J (2013) Water stress induces a differential and spatially distributed nitro-oxidative stress response in roots and leaves of Lotus japonicas. Plant Sci 201–202:137–146
Singh P, Blanke M (2000) Deficiency of potassium but not phosphorus enhances root respiration. Plant Growth Regul 32:77–81
Slama I, Abdelly C, Bouchereau A, Flowers T, Savouré A (2015) Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann Bot 115:433–447
Song J, Wang B (2015) Using euhalophytes to understand salt tolerance and to develop saline agriculture: Suaeda salsa as a promising model. Ann Bot 115:541–553
Srivastava AK, Shankar A, Chandran AKN, Sharma M, Jung KH, Suprasanna P, Pandey GK (2019) Emerging concepts of potassium homeostasis in plants. Oxford University Press on behalf of the Society for Experimental Biology
Takagi D, Takumi S, Hashiguchi M, Sejima T, Miyake C (2016) Superoxide and singlet oxygen produced within the thylakoid membranes both cause photosystem I photoinhibition. Plant Physiol 171:1626–1634
Talbi Zribi O, Hessini K, Trabelsi N, Zribi F, Hamdi A, Ksouri R, Chedly A (2017) Aeluropus littoralis maintains adequate gas exchange, pigment composition and phenolic contents under combined effects of salinity and phosphorus deficiency. Australian J Bot 65:453–462
Talbi Zribi O, Mbarki S, Metoui O, Trabelsi N, Zribi F, Ksouri R, Abdelly C (2020) Salinity and phosphorus availability differentially affect plant growth, leaf morphology, water relations, solutes accumulation and antioxidant capacity in Aeluropus littoralis. Plant Biosyst Int J Dealing All Asp Plant Biol 155:935–943
Tang ZH, Zhang AJ, Wei M, Chen XG, Liu ZH, Li HM, Ding YF (2015) Physiological response to potassium deficiency in three sweet potato (Ipomoea batatas [L.] Lam.) genotypes differing in potassium utilization efficiency. Acta Physiol Plant 37:1–10
Tewari RK, Kumar P, Sharma PN (2007) Oxidative stress and antioxidant responses in young leaves of mulberry plants grown under nitrogen, phosphorus or potassium deficiency. J Integr Plant Biol 49:313–322
Tighe-Neira R, Alberdi M, Arce-Johnson P, Romero J, Reyes-Díaz M, Rengel Z, Inostroza-Blancheteau C (2018) Role of potassium in governing photosynthetic processes and plant yield. Plant nutrients and abiotic stress tolerance. Springer, Berlin, pp 191–203
Tränkner M, Tavakol E, Jákli B (2018) Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol Plant 163:414–431
Tripathi DK, Singh S, Singh VP, Prasad SM, Chauhan DK, Dubey NK (2016) Silicon nanoparticles more efficiently alleviate arsenate toxicity than silicon in maize cultiver and hybrid differing in arsenate tolerance. Front Environ Sci 4:46
Tsay Y-F, Ho CH, Chen HY, Lin SH (2011) Integration of nitrogen and potassium signaling. Annu Rev Plant Biol 62:207–226
Valderrama R, Corpas FJ, Carreras A, Gómez-Rodríguez MV, Chaki M, Pedrajas JR, Fernández-Ocaña A, del Río LA, Barroso JB (2006) The dehydrogenase-mediated recycling of NADPH is a key antioxidant system against salt-induced oxidative stress in olive plants. Plant Cell Environ 29:1449–1459
Vargas WA, Martín JM, Rech GE, Rivera LP, Benito EP, Díaz-Mínguez JM, Thon MR, Sukno SA (2012) Plant defense mechanisms are activated during biotrophic and necrotrophic development of Colletotricum graminicola in maize. Plant Physiol 158:1342–1358
Velmurugan A, Swarnam P, Subramani T, Meena B, Kaledhonkar MJ (2020) Water Demand and Salinity. In: Desalination—challenges and opportunities. IntechOpen
Vile D, Pervent M, Belluau M, Vasseur F, Bresson J, Muller B, Granier C, Simonneau T (2012) Arabidopsis growth under prolonged high temperature and water deficit: independent or interactive effects? Plant Cell Environ 35:702–718
Wang Y, Wu WH (2013) Potassium transport and signaling in higher plants. Ann Rev Plant Biol 64:451–476
Wang W, Xia MX, Chen J, Yuan R, Deng FN, Shen FF (2016) Gene expression characteristics and regulation mechanisms of superoxide dismutase and its physiological roles in plants under stress. Biochem Mosc 81:465–480
Waqas M, Yaning C, Iqbal H, Shareef M, Rehmane H, Bilal HM (2021) Synergistic consequences of salinity and potassium deficiency in quinoa: linking with stomatal patterning, ionic relations and oxidative metabolism. Plant Physiol Biochem 159:17–27
White PJ, Karley AJ (2010) Potassium. In: Hell R, Mendel RR (eds) Cell biology of metals and nutrients. Plant cell monographs, vol 17. Springer, Berlin
Yilmaz O, Kahraman K, Ozgur R, Uzilday B, Turkan I, Ozturk L (2017) Growth performance and antioxidative response in bread and durum wheat plants grown with varied potassium treatments under ambient and elevated carbon dioxide. Environ Exp Bot 137:26–35
Zandalinas SI, Sengupta S, Fritschi FB, Azad RK, Nechushtai R, Mittler R (2021) The impact of multifactorial stress combination on plant growth and survival. New Phytol 230:1034–1048
Zhang L, Liu J, Wang X, Bi Y (2013) Glucose-6-phosphate dehydrogenase acts as a regulator of cell redox balance in rice suspension cells under salt stress. Plant Growth Regul 69:139–148
Zhao XH, Yu HQ, Wen J, Wang XG, Du Q, Wang J, Wang Q (2016) Response of root morphology, physiology and endogenous hormones in maize (Zea mays L.) to potassium deficiency. J Integr Agric 15:785–794
Zheng C, Jiang D, Liu F, Dai T, Jing Q, Cao W (2009) Effects of salt and water logging stresses and their combination on leaf photosynthesis, chloroplast ATP synthesis, and antioxidant capacity in wheat. Plant Sci 176:575–582
Zhou S, Sauvé RJ, Liu Z, Reddy S, Bhatti S (2011) Identification of salt-induced changes in leaf and root proteomes of the wild tomato, Solanum chilense. J Amer Soc Hort Sci 136:288–302
Zhou R, Kong L, Wu Z, Rosenqvist E, Wang Y, Zhao L, Zhao T, Ottosen CO (2018) Physiological response of tomatoes at drought, heat and their combination followed by recovery. Physiol Plant 165:144–154
Zhou G, Liu C, Cheng Y, Ruan M, Ye Q, Wang R, Yao Z, Wan H (2022) Molecular evolution and functional divergence of stress-responsive Cu/Zn superoxide dismutases in plants. Int J Mol Sci 23:7082
Zorb C, Senbayram M, Peiter E (2014) Potassium in agriculture – status and perspectives. J Plant Physiol 171:656–669
Acknowledgements
FJC and JMP research work is supported by a European Regional Development Fund cofinanced grants from the Ministry of Economy and Competitiveness (PID2019-103924GB-100) and the Plan Andaluz de Investigación, Desarrollo e Innovación (P18-FR-1359), Spain. The valuable technical assistance of Carmelo Ruiz-Torres is deeply acknowledged.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
H.H. and F.J.C. conceived and designed the experiments. H.H. carried out experiments and collected, analyzed, and interpreted the data. H.H., J.M.P, F.J.C., interpreted the data, revised and edited the article. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors have no conflict of interest.
Additional information
Handling Editor: Padmanabh Dwivedi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Houmani, H., Palma, J.M. & Corpas, F.J. High Salinity Stimulates the Adaptive Response to Potassium Deficiency Through the Antioxidant and the NADPH-Generating Systems in the Roots and Leaves of the Halophyte Cakile maritima. J Plant Growth Regul 42, 6286–6306 (2023). https://doi.org/10.1007/s00344-022-10819-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-022-10819-7