Abstract
Contamination of agricultural soils with heavy metals present lethal consequences in terms of diverse ecological and environmental problems that entail entry of metal in food chain, soil deterioration, plant growth suppression, yield reduction and alteration in microbial community. Metal polluted soils have become a major concern for scientists around the globe. In more recent times, armed with new knowledge and understanding, removal of heavy metals using different applications has emerged as a solution for waste treatment and contaminant remediation in water and soil. However, the description of metal toxicity to the plants and its removal and degradation from the soil is limited. There are a number of reports in the literature where PGP bacterial inoculation and various chelating agents improves metal accumulation and it’s detoxification in different plant parts without influencing plant growth. Therefore, there is a need to select some useful chemicals which possess the potential to improve plant growth as well as expedite the phytoremediation of metals. In this review, we have discussed the mechanisms possessed by different chelating agents to promote plant growth and phytoremediation of metals. We anticipate that this analysis of interconnected systems will lead to the discovery of new research fields.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Most heavy metals are produced by pollution and their presence causes many ecological, evolutionary, and nutritional problems. Many risks are created due to heavy metal contamination, like soil pollution as well as security of food and its quality (Christophoridis et al. 2020; Zaheer et al. 2022). Heavy metals cause many harmful effects for living things, including plants. They decrease the growth and development of plants even at low concentrations of heavy metals with respect to other metals (Kamran et al. 2019; Kour et al. 2021; Muhammad et al. 2009). Excess amounts of other metals or elements do not damage the tissues/cells of the plant, and their accumulation can even increase the growth of the plant (Ahmad et al. 2022, Ali and Chaudhury 2016). The metals which are lethal or harmful for plants include: lead (Pb), cadmium (Cd), cobalt (Co), iron (Fe), silver (Ag), platinum (Pt), nickel (Ni), chromium (Cr), copper (Cu), and zinc (Zn) (Chen and Lu 2018; Pescatore et al. 2022). Agricultural soil is befouled by many heavy metals, which is a major issue. Many anthropogenic activities effect soil and the effectiveness of the plant, like urbanization, smelting, sludge, military operations, mining, dumping, and excess amounts of pesticide and insecticide applications (Chowdhary et al. 2018; Rehman et al. 2020; Saleem et al. 2020e; Shahid et al. 2015). These activities inhibit the growth of the plants and cause many toxic symptoms in the plants due to heavy metal contamination, and these metals destroy human health by entering into the food chain (AFZAL et al. 2021; Gill et al. 2021; Saleem et al. 2020i). However, the metal contaminants are useful in fertilization as well as in pesticides, fungicides, and nematicides for the better production and development of the plant. These applications are a major source for the growth of the plants. Metal deposition in soil is produced due to biomass and an increase in plant growth (Alsafran et al. 2021; Hassan et al. 2021; Sufyan et al. 2021). The chemical composition, plant type and their species, and the pH of heavy metals all impact the phytotoxicity of the heavy metals in the plants (Al Jabri et al. 2022, Saleem et al. 2022d). A large amount of reacting oxygen species and oxidative stress are involved through direct and indirect toxicity of heavy metals and produced in the following ways: (a) reduction of electrons, (b) the irregular management of metabolic pathways, (c) the activities of anti-oxidative enzymes are reduced, and (d) depletion of lower molecular weight compounds (Ali et al. 2013; Farid et al. 2019; Zaheer et al. 2015). Crop yield and productivity can be improved and the health risk decreased acutely by removing the harmful effects of these heavy metals (Ahmad et al. 2022; Ehsan et al. 2014; Najeeb et al. 2009; Rehman et al. 2016).
Heavy metals are natural components of the terrestrial ecosystem. However, their presence in excess is harmful to humans and the environment (Farid et al. 2019; Saleem et al. 2020h). Therefore, remediation is necessary to alleviate the negative effects caused by the heavy metals incorporated to ecosystems (Mahar et al. 2016; Saini et al. 2021). Research has been continuing to develop effective methods of remediation to treat contaminated lands (Madhu and Sadagopan 2020; Saleem et al. 2020d; Tela et al. 2012). Remediation could be done by immobilization, removal, sequestration, active mixing, and phytoextraction (Hashmat et al. 2021; Kamran et al. 2020; Murtaza et al. 2021; Saleem et al. 2020l, 2021; Zaheer et al. 2020b). However, selection and applicability of the remediation methods depend on a number of factors including cost, duration of effectiveness, commercial level availability, general acceptance, applicability to high metal contents, and applicability to mixed metal and organic wastes as well as toxicity, mobility, and volume reduction (Abbar et al. 2017; Aziz et al. 2021; Tariq et al. 2021; Zhang et al. 2013). Heavy metal toxicity can be minimized by reducing their availability using organic and inorganic amendments (Al Jabri et al. 2022; Alam et al. 2022; Lone et al. 2022; Mahar et al. 2016; Saleem et al. 2020a, c, 2022c). Keeping in view the excessive release of heavy metals into environment and its toxic effects especially on plants, in this review, we aimed to discuss heavy metal sources, essentiality, bioaccumulation, homeostasis and possible methods to remediate it from the soil and also the context of their responses toward both heavy metal deficient and toxic soil conditions and to identify remediation measures with future directions for research.
Sources of Heavy Metals in the Soil
Heavy metals are significant environmental pollutants, and their toxicity is a problem of increasing significance for ecological, evolutionary, nutritional, and environmental reasons. The term ‘‘heavy metals’’ refers to any metallic element that has a relatively high density and is toxic or poisonous even at low concentration (Nagajyoti et al. 2010). ‘‘Heavy metals’’ in a general collective term, which applies to the group of metals and metalloids with atomic density greater than 4 g cm−3, or 5 times or more, greater than water (Riaz et al. 2020; Saini et al. 2021). All these factors not only reduce plant growth but also make the environment more vulnerable to the threat of plant population survival. In plants, metal stress disturbs the plant metabolism and decreases plant growth by reducing essential macro and micronutrient uptake (Ubando et al. 2020; Vardhan et al. 2019). In recent years, there has been an increasing ecological and global public health concern associated with environmental contamination by these metals. Also, human exposure has risen dramatically as a result of an exponential increase of their use in several industrial, agricultural, domestic, and technological applications (Cristaldi et al. 2017; Tangahu et al. 2011). Reported sources of heavy metals in the environment include geogenic, industrial, agricultural, pharmaceutical, domestic effluents, and atmospheric sources (Cristaldi et al. 2017; Hasanpour and Hatami 2020). Additionally, sources of heavy metals include mining, industrial production (foundries, smelters, oil refineries, petrochemical plants, pesticide production, chemical industry), untreated sewage sludge and diffuse sources such as metal piping, traffic, and combustion by-products from coal-burning power stations (Laghlimi et al. 2015; Saleem et al. 2020b; Zaheer et al. 2020a). Environmental pollution is very prominent in point source areas such as mining, foundries, and smelters, and other metal-based industrial operations (Nagajyoti et al. 2010; Shahid et al. 2020; Vardhan et al. 2019).
Although heavy metals are naturally occurring elements that are found throughout the earth’s crust, most environmental contamination and human exposure result from anthropogenic activities such as mining and smelting operations, industrial production and use, and domestic and agricultural use of metals and metal-containing compounds (Grčman et al. 2001; Griga et al. 2003; Wan et al. 2016). Environmental contamination can also occur through metal corrosion, atmospheric deposition, soil erosion of metal ions, and leaching of heavy metals, sediment re-suspension and metal evaporation from water resources to soil and ground water (Evangelou et al. 2007; Wuana and Okieimen 2011). Natural phenomena such as weathering and volcanic eruptions have also been reported to significantly contribute to heavy metal pollution (Sun et al. 2014; Zhu et al. 2020). Industrial sources include metal processing in refineries, coal burning in power plants, petroleum combustion, nuclear power stations and high tension lines, plastics, textiles, microelectronics, wood preservation, and paper processing plants (Şenkal et al. 2019; Wan et al. 2016). Heavy metals are also considered as trace elements because of their presence in trace concentrations (ppb range to less than 10 ppm) in various environmental matrices (Soliman and Moustafa 2020; Vardhan et al. 2019). Their bioavailability is influenced by physical factors such as temperature, phase association, adsorption, and sequestration. It is also affected by chemical factors that influence speciation at thermodynamic equilibrium, complexation kinetics, lipid solubility, and octanol/water partition coefficients (Shahzad et al. 2018). Biological factors such as species characteristics, trophic interactions, and biochemical/physiological adaptation, also play an important role (Riaz et al. 2020). Heavy metal-induced toxicity and carcinogenicity involves many mechanistic aspects, some of which are not clearly elucidated or understood. However, each metal is known to have unique features and physic-chemical properties that confer to its specific toxicological mechanisms of action. Sources of different heavy metals in the environment and their toxic effects to the plants are presented in Fig. 1.
In biological systems, heavy metals have been reported to affect cellular organelles and components such as cell membrane, mitochondrial, lysosome, endoplasmic reticulum, nuclei, and some enzymes involved in metabolism, detoxification, and damage repair (Rana et al. 2020; Saleem et al. 2020f). A reliable strategy and stable treatment systems are urgently needed to address the issue of increasing heavy metals contaminated by domestic and industrial processes due to the expanding human population. Indeed, many countries do not have adequate water resources to sustain their agricultural needs. To ensure agricultural productivity, inadequately treated with metal polluted sources are extensively applied to food crops; however, the quality and safety of food crops grown in soils irrigated with poorly treated reclaimed water cannot be guaranteed (Vardhan et al. 2019; Wang et al. 2020).
Fertilizers as a Source of Heavy Metals
Soil contamination with heavy metals is becoming a worldwide threat due to its adverse impacts on environmental safety (Saleem et al. 2020g; Zhang et al. 2013). Anthropogenic activities especially application of agrochemicals are the major sources of heavy metals accumulation in soil (Antonkiewicz et al. 2018; Ashraf et al. 2017; Tombarkiewicz et al. 2022; Yang et al. 2018). The inorganic and organic fertilizers (Fertilizer is a substance added to soil to improve plants growth and yield) are the most important sources of heavy metals to agricultural soil include liming, sewage sludge, irrigation waters, and pesticides, sources of heavy metals in the agricultural soils (Kour et al. 2021; Wan et al. 2016). Others, particularly fungicides, inorganic fertilizers, and phosphate fertilizers have variable levels of Cd, Cr, Ni, Pb, and Zn depending on their sources. Soil is one of the key resources that are being polluted with excess of Cu and is derived from parental rocks and anthropogenic activities. The potential industrial and agricultural sources for Cu includes batteries, pigments and paints, alloys, fuel, catalysts, fertilizers, and pesticides (Rehman et al. 2019b; Saleem et al. 2019, 2020j). Cd is of particular concern in plants since it accumulates in leaves at very high levels, which may be consumed by animals or human being. Cd enrichment also occurs due to the application of sewage sludge, manure, and limes (Afzal et al. 2020; Alatawi et al. 2022a; Heile et al. 2021; Imran et al. 2020, 2021; Javed et al. 2020; Saleem et al. 2022b). Because some of the compounds used to provide these elements contain tiny quantities of heavy metals (such as Cd and Pb) as impurities i.e., their concentration in the soil rises dramatically with repeated fertilizer applications (Yang et al. 2018). Fertilizers provide a variety of essential nutrients to plants to promote growth and productivity while also increasing soil organic matter. Anaerobic digestion (AD) produces organic or biofertilizers, which are in the form of ammonium fertilizers (sulfate and nitrate) following the fermentation process (Zhang et al. 2013). Chemically manufactured/synthetic fertilizers, often known as inorganic fertilizers, blend inorganic and chemical ingredients (Yang et al. 2011).
Higher quantities of As, Cd, and Pb were found in various phosphate and micronutrient fertilizers and other materials (e.g., nitrogen, potash, gypsum) (Ahmad et al. 2022). A few waste-derived fertilizer products have also been discovered to contain high levels of dioxins (measured in parts per trillion) in their composition (Abubakari et al. 2017). Phosphorus is widely used in fertilizer production; nevertheless, its widespread use contributes significantly to the accumulation of heavy metals in soil (Alam et al. 2022; Alatawi et al. 2022b; Sarwar et al. 2022; Ullah et al. 2022). It has been demonstrated that water-insoluble phosphorus fertilizers cause the formation of Phosphate rocks, which play a key role in the immobilization of metals in soils via metal phosphate precipitation (Saleem et al. 2022a). Long-term misuse of fertilizers, which results in a heavy metal deposition in agricultural soils, impacts soil fertility, plant development, and production while also boosting fertility (Griga et al. 2003). The most frequent forms of inorganic fertilizers include phosphate fertilizers, liming materials, and bio-fertilizers, which lead to the buildup of heavy metals in soils, which are subsequently ingested by plants (Shahzad et al. 2018).
The Use of Pesticides as a Source of Heavy Metals
Pesticides are used worldwide in agriculture, industry, public health, and for domestic applications: as a consequence, a great part of the population may be exposed to these compounds. In spite of this extensive use, knowledge on the health risks associated with prolonged exposure is rather poor, and major uncertainties still exist (Antle and Pingali 1994; Husak 2015). Epidemiological observations in man have so far produced little conclusive information, mainly because of weaknesses in exposure assessment (Chhipa 2017). Several common pesticides that were historically widely used in agriculture and horticulture contains high concentration of metals in it (Tariq et al. 2007). For example, nearly 10% of the insecticides and fungicides permitted in the United Kingdom in the recent past were based on compounds containing Cd, Cr, Ni, Pb, and Zn depending on their sources. Although the levels of heavy metals in agricultural soil are very small, but repeated use of phosphate fertilizer and the long persistence, time for metals, there may be dangerously high accumulation of some metals (Raj et al. 2021). For example, Cu-containing fungicidal sprays like Bordeaux combination (CuSO4) and Cu-oxychloride are employed to limit fungus infection which inhibit the plant growth (Hussain et al. 2022; Rehman et al. 2019b). Contamination might be an issue, especially if the sites are refurbished for other agricultural or non-agricultural activities. According to (Zubrod et al. 2019), the world population increases to approximately 97 million people each year. Around 50,000 plant pathogen species, 9000 insect and mite species, and 8000 weed species cause agricultural loss worldwide, with most of the damage happening in the United States (Ali et al. 2022b). Plant infections are estimated to have caused a 13% loss in agricultural production, insect pests a 14% loss in crop production, and weeds a 13% loss in crop production. According to prediction research, pesticides are estimated to safeguard approximately one-third of agricultural products worldwide (Benelli et al. 2018). Pesticides are toxic compounds or mixtures of harmful substances that are naturally occurring or chemically produced. These are frequently used in the agricultural area to control weeds (herbicides), fungi (fungicides), bacteria (bactericides), and insect infestations (insecticides) that are hazardous to the crops and the environment (Naha et al. 2020). These can also be employed against some disease carriers and pests (e.g., ticks, rodents, mosquitoes, and lice), which can be used throughout the ecosystem to combat disease (Kamal et al. 2022). Agricultural regions utilize many pesticides, accounting for over 85% of worldwide pesticide production. In wet areas (carpets, freezers, cabinets), these can also help reduce and prevent insect infestation breakouts, fungus, and germs (Hayles et al. 2017).
Biosolids and Manures as a Source of Heavy Metals
The application of biosolids to agricultural soils can be a beneficial agricultural practice as it provides essential nutrients and organic matter that can help to improve soil properties (Mohajerani and Karabatak 2020). Biosolids can provide a waste management route for sewage sludge; however, biosolids are also considered a sink for industrial and domestic chemicals that become sequestered in solids during wastewater treatment (Spinosa and Vesilind 2001). The presence of detectable concentrations of dioxins and pharmaceuticals in biosolids has been well documented (Clarke and Cummins 2015), and has led to concerns that land applications may result in the accumulation of dioxins and a series of emerging pollutants in soil with subsequent translocation through the human food chain (Girovich 1996). While the environmental occurrence of these contaminants is usually low (mg kg−1 down to sub ng kg−1), toxicologists, epidemiologists, and risk assessment experts advise that there may still be significant and widespread adverse environmental and human health consequences (e.g., cancer risk and adverse reproductive development) at the detected levels (Mohajerani and Karabatak 2020). Heavy metals such as As, Pb, Cu, Hg, Cd, Se, Ni, and Mo accumulate in biosolids (composts, livestock manures, and municipal sewage sludge) when they are applied to the soil (Basta et al. 2001). Animal wastes generated in agriculture, such as chicken, pig, and cattle manure, are commonly put on crops and pastures as solids or slurries, depending on the kind of animal waste. Even though most manures are good fertilizers, Cu and Zn are added to feeds in the pig and poultry industries as growth promoters. As a result of their presence in poultry, health products can potentially contaminate the soil with heavy metals (Machete and Chabo 2020). The manures produced by animals fed on such diets have significant As, Cu, and Zn quantities. If this method is used regularly on restricted land areas, it can significantly accumulate these metals Biosolids are solid finished products formed from wastewater treatment and subsequent treatment of the resulting sludge to limit the risk of pollution to humans and the environment (López-Rayo et al. 2016). Biosolids (BS), on the other hand, contain HMs such as Ni, Cu, Pb, Cd, and Zn which are not required for plant growth (De Bhowmick and Sarmah 2022). A significant difficulty is the generation of vast quantities of biosolids formed during wastewater treatment operations. Biosolids have traditionally been dealt with by using typical biosolids disposal procedures such as ocean dumping, landfilling, and incineration (Bucknall 2020). Due to more onerous environmental restrictions and the high economic expenses associated with these disposal methods, these choices are becoming less practical. Recently, researchers have looked into the possibility of using biosolids for positive reasons in various applications (Drechsel et al. 2022). Land application of biosolids has been proposed as a sustainable approach for resource recovery because of their high concentrations of organic matter and nutrients. The amendment of soils with biosolids has emerged as the most common method of utilizing biosolids. Most biosolids are applied as a soil amendment to agricultural land and pastures (Widyastuti et al. 2021; Wu et al. 2021; Yang et al. 2012; Zamora-Ledezma et al. 2021). It is also possible to use biosolids for home purposes by adding them to potting mix and other soil additives. However, the amount of biosolids used for domestic reasons is far less than that used for agricultural purposes (Roopnarain et al. 2022).
Wastewater and Metal Mining as a Source of Heavy Metal
Triggered by exorbitantly increasing population rapid industrialization and urbanization posed the menace of air water and soil pollution which is a potential threat to mankind. Among industries tanneries played a leading role in polluting the soil and water bodies. The wastewater discharged from the tanning industry contains numerous pollutants including heavy metals (Hashem et al. 2020; Kamran et al. 2019; Maqbool et al. 2018). Globally, wastewater irrigates around 20 million hectares of arable land. More than half of the vegetables supplied to urban areas in Asian and African cities are grown with the help of wastewater irrigation (Chowdhary et al. 2018). Environmental advantages and dangers are often overlooked by farmers, who are more concerned with boosting their yields and profits than anything else (Hao et al. 2019). As a result, even though wastewater effluents typically contain modest levels of heavy metal contamination, repeated irrigation with wastewater effluents may cause heavy metal deposition in the soil. There is a legacy of metal pollution in soil throughout many countries due to the mining and processing of metal ores and the existence of industrial activities. Heavy metal concentrations rise due to mining tailings discharges (Particles that have settled to the bottom of the flotation cell are heavier and larger) into nearby wetlands and other natural depressions. Due to extensive Pb and Zn ore mining and smelting, human and environmental health and well-being are at risk (Sandeep et al. 2019). Many restoration approaches used on these sites are time-consuming, expensive, and ineffective in restoring soil productivity. Human exposure to HMs in the environment depends on their bioavailability in soil. Consumption of plant material grown in (the food chain) or oral bioavailability (contaminated soil ingestion) are two techniques of food chain absorption. In addition to textiles and tanning and petrochemicals, insecticides and pharmaceutical manufacturing facilities all produce materials with highly changeable compositions, such as those resulting from spilled oil or petroleum-based products. Textiles, tanning, petrochemicals, insecticides, and pharmaceuticals are only a few examples. Many of these wastes are put on land, but they offer little benefit to farming or forestry. In addition, many of them contain heavy metals (Pb, Cr, and Zn) or dangerous organic compounds; hence thus are rarely used on the ground. Some are deficient in plant nutrients or lack soil-conditioning qualities (Fig. 2) (Jayakumar et al. 2021; Khanna et al. 2021; Kumar et al. 2021, 2022).
Toxic Effect of Metals on the Plants
Fe, Zn, Mn, Cu, Ni, Co, and Mo are all transition-metal elements that are vital to plant nutrition (which is required for nitrogen fixation in legumes). Metallic heavyweights include non-essential cadmium, lead, and mercury (Flora et al. 2008; Mehta et al. 2021).When present in high concentrations in agricultural plants' tissues, all metals are toxic to the plants. Deficiencies of important heavy-metal elements are more widespread in agriculture than their toxicity, which is surprising considering their importance in food production (Daulta et al. 2022). This is even though Mn and Fe poisoning can impair crop yields on acidic soils and soils that have been waterlogged or otherwise flooded. As a result of weathering or human activity, some heavy metals can accumulate in soils, resulting in toxicities that can harm humans (Sandeep et al. 2019). Although the molecular biology of heavy metal uptake and transport in plants has been thoroughly characterized, researchers still strive to determine how plant cells and tissues maintain heavy metal homeostasis.
Furthermore, excessive levels of heavy metals have been shown to trigger cellular reactions in laboratory animals. The production of antioxidant molecules and enzymes is among the many responses caused by reactive oxygen species (Gavrilescu 2022). By preventing the entry of heavy metals into roots and their migration to xylem; and by chelating heavy metals that enter the cytoplasm and sequestering them in non-vital compartments such as the apoplast and vacuole, plants may tolerate high concentrations of heavy metals in their environment (Khan et al. 2021). In addition, knowing how specific plant species might accumulate excessive levels of heavy metals will also help us learn how plants can avoid and tolerate heavy metals in their tissues, as shown in Fig. 3 (Raza et al. 2021).
Toxic Effect of Zn
Zn is a plant micronutrient which is involved in many physiological functions its inadequate supply will reduce crop yields. Zn deficiency is the most wide spread micronutrient deficiency problem, almost all crops and calcareous, sandy soils, peat soils, and soils with high phosphorus and silicon are expected to be deficient (Shi et al. 2015; Zvezdanović et al. 2007). Zn deficiencies can affect plant by stunting its growth, decreasing number of tillers, chlorosis, and smaller leaves, increasing crop maturity period, spikelet sterility, and inferior quality of harvested products (Liu et al. 2007; Umair Hassan et al. 2020). Beside its role in crop production Zn plays a part in the basic roles of cellular functions in all living organisms and is involved in improving the human immune system (Tang et al. 2020; Tanveer et al. 2022; Zhang et al. 2021). The threshold of Zn toxicity varies among plant species, time of exposure to Zn stress and composition of the nutrient growth medium. Plant growth inhibition extends in T. aestivum after addition of ZnSO4 (Ahmad et al. 2022; Sarwar et al. 2014), whereas Pisum sativum became inhibited after Zn application (Mukherjee et al. 2014). Photosynthesis is strongly affected in plants exposed to heavy metals excess. High Zn concentrations in plants can cause phytotoxicity. The yield may be reduced when plant leaf Zn concentrations reaches about 300–1000 µg Zn g−1 (Ullah et al. 2020). The best way to identify Zn deficiency in crops is the determination of Zn concentrations in tissues, however, the results should be interpreted in full recognition of the interaction of Zn with other nutrients because the deficiency of one nutrient may result in excess accumulation of other nutrients by a plant (Rajput et al. 2018; Rizwan et al. 2019; Zhang et al. 2021).
Toxic Effect of Cd
Cd is a highly toxic metal that affects terrestrial and aquatic organisms, including humans. Due to agricultural and industrial growth, Cd concentrations in agricultural soils have increased (Priyadarshanee et al. 2022a, b). A diverse spectrum of human endeavors and environmental contaminants contribute to the introduction of Cd into ecosystems (Iftikhar et al. 2022). For animals and people alike, the high mobility of cesium in polluted soils poses a significant threat to health (Paul and Saha 2022). Cyanide toxicity affects many human organs, but the kidneys are particularly vulnerable, where it can induce renal tubular damage, kidney stones, and pulmonary emphysema (Gupta 2019). As a result of its identical charge, same ionic radius, and chemical activity, Cd substitutes for calcium in minerals (Kong et al. 2021). Cd toxicity in plants manifests as chlorosis and growth stunting. Higher toxicity slows down plant growth, resulting in plant necrosis (Pramanick et al. 2022). Poisoning plants with cadmium prevents carbon fixation and lowers chlorophyll concentrations and photosynthetic activity, negatively affecting plants (Sperdouli et al. 2022). Soil Cd reduces physiological activity such as stomatal conductance, leaf water content, and transpiration, resulting in plants' osmotic stress and physiological impairment (Javad et al. 2022). Plant membranes are damaged, and cell macromolecules and organelles die due to a buildup of reactive oxygen species (ROS) caused by cadmium poisoning (Sun et al. 2022). Many studies have been conducted on the impact of Cd toxicity on crop yield, ROS generation, the formation of lipid peroxidation, and the many remediation techniques available (Haider et al. 2022). As illustrated in Fig. 4, only a little research on the effects of Cd on plants and Cd remediation approaches to avoid Cd mobilization in the rhizosphere have been conducted (Ai et al. 2020).
Toxic Effects of Cr
Cr is the seventeenth most abundant element on the planet (Matrosova et al. 2021). Plating, alloying, animal hide tanning, water corrosion prevention, textile colors, pigments, ceramic glaze, refractory brick, and pressure-treated wood are examples of Cr's industrial applications (Dongre 2021). This broad anthropogenic usage of Cr has resulted in environmental contamination, which has grown in recent years and now poses an increasing hazard (Hojjati-Najafabadi et al. 2022). Cr (0), trivalent Cr (III), and hexavalent Cr (VI) are the most stable and frequent chromium species; however, additional oxidation states exist as well. Steel and other alloys can be made from Cr (0), a metallic form of Cr generated in industry and has a high fusing point. Cr can reach natural streams by weathering Cr-containing rocks, industrial activities, and soil leaching (Aharchaou et al. 2022). Cr can be reduced, oxidized, sorption, desorption, dissolution, and precipitation in the aquatic environment. Cr (III) aqueous solubility is affected by the pH of the water (Ibrahim et al. 2019). Cr III can be exposed to higher concentration and lower nutritional concentration. Which effect plant totals biomass, roots growth, shoot growth, and metabolic activities, as shown in Fig. 5.
Cr (III) tends to precipitate at neutral to basic pH while solubilizing at acidic ph. Divalent cations can precipitate even though Cr (VI) chromate and dichromate are extremely soluble at any pH (Prasad et al. 2021; Sandeep et al. 2019). The Cr concentration of underlying soil rocks and sediments fluctuates as the natural composition of the rocks and sediments changes (Hussain et al. 2021; Javed et al. 2021; Ma et al. 2022a, b, Saleem et al. 2022d). Soil chromium levels can be increased by disposing chromium-bearing liquids and solid wastes, such as chromium byproducts, ferrochromium slag, or chromium plating baths. Cr (III) and Cr(II) are commonly found in soil (VI) (Prasad et al. 2021). As of this writing, the mechanism underlying plant uptake of Cr is yet unknown. Instead of being necessary, Cr is a non-essential element whose absorption relies on Cr speciation and lacks a specialized mechanism (Sterckeman and Thomine 2020). Because Cr (III) uptake by plants is a passive process, they do not have to put any effort. It is hypothesized that carriers utilize Cr (VI) absorption to carry essential components such as sulfate (Khan et al. 2021).
Toxic Effects of As
As is one of the toxic environmental pollutants which has recently attracted attention because of its chronic and epidemic effects on human health through widespread water and crop contamination due to the natural release of this toxic element from aquifer rocks in many countries (Farooq et al. 2015; Uddin Nizam et al. 2016). As is a highly toxic and carcinogenic element (Siddiqui et al. 2020b) and the most widespread sources of arsenic in soil and water are natural sources, such as volcanic activities, weathering, and erosion of minerals and rocks, and geothermal waters (Islam et al. 2013; Saleem et al. 2022a). In addition, use of pesticides, fertilizers, industrial wastes, agricultural chemicals, and Cu chromate-arsenate (CCA) wood preservative are the major anthropogenic sources of arsenic contamination in soil and water (Tanveer et al. 2022). There exist abundant evidences that As negatively interferes with several biochemical and physiological processes inside plant causing reduced plant growth and yield (Farooq et al. 2015; Kalve et al. 2011). Inside the plant cell, heavy metals including arsenic induce oxidative stress by enhanced production of reactive oxygen species (ROS), which may cause cell death via oxidative processes such as protein oxidation, enzyme inhibition, DNA and RNA damage, and lipid peroxidation (Gomes et al. 2014; Merrington 2018; Shahid et al. 2017). The sites contaminated with As need immediate attention due to the associated severe health risks. As has significant effects on different activities, considerable changes were noted in both shoot and roots systems, as shown in Fig. 6.
Toxic Effect of Hg
As a heavy metal in the periodic table's transition element series, mercury is a toxic substance. Because it may be found in three forms (elemental, organic, and inorganic), each with a distinct toxicity profile, it is notable (Barik et al. 2021). When mercuric Hg is methylated by soil and water microorganisms, methylmercury is the most common organic form of Hg in the environment (Barkay and Gu 2021). In addition to altering biological tissues, mercury is a prevalent environmental pollutant linked to numerous health problems (Maghsoudi et al. 2021). Multiple chemical forms of Hg are present in the environment, exposing people and animals. It is made up of mercuric (Hg+1), inorganic mercurous (Hg+1), and organic mercury compounds (Hg+2). Humans, plants, and animals all come into contact with mercury since it is so common in the environment (Priyadarshanee et al. 2022a, b). Hg is used in various industrial processes, including the production of caustic soda and nuclear reactors, antifungal agents for wood processing, a solvent for reactive and valuable metals, and a preservative for medicinal products (Cai et al. 2019; Haider et al. 2022).
Toxic Effects of Pb
Pb is naturally occurring, and human activities such as burning fossil fuels, mining, and manufacturing contribute to releasing excessive amounts of metal into the environment. There are several industrial, agricultural, and residential uses for Pb (Sharma and Sharma 2022). Pb -acid batteries, ammunition, metal products (such as solder and pipes), and X-ray shielding devices are now all made using it. An estimated 1.52 million metric tons of lead were consumed (Anderson 2012). Over the last several years, the usage of lead in paints, ceramic tiles, caulking, and pipe solder has declined dramatically. A quarter of the 16.4 million households in the United States with more than one kid under six still have high levels of Pb-contaminated deteriorating paint, dust, or bare soil (Sandil and Kumar 2022). Despite this development resulting from children playing in bare, contaminated dirt, lead in dust and soil re-contaminates cleansed residences (Naqhiyah Farhan 2018); and boosts blood Pb levels in those youngsters. The most prevalent source of lead poisoning in children is dust and chips from deteriorating lead paint on interior surfaces (Hauptman et al. 2017). Children who live in homes where Pb paint is deteriorating have been shown to have blood lead levels of 20 g dL−1 or higher (Marino 2003). Pb's toxicological effects are likely to affect one or more of a plant's physiologically active tissues engaged in the growth, maintenance, and photosynthesis (Jaishankar et al. 2014). There has not been any indication that Se is necessary for plants. Seed germination and growth can be compromised in plants with an excess of Se. The physiological significance of se in plants, despite several investigations, remains a mystery (Pour and Makkawi 2021). The physiological reactions of plants to Se and their capacity to accumulate Se in tissue were highly variable (Kumbhakar et al. 2022).
Approaches for Remediation of Heavy Metals
HMs remediation from contaminated soil, water, or sediment has been the subject of numerous treatment procedures, including physical, chemical, and biological. For example, thermal treatment, adsorption, and chlorination are examples of adsorption and chlorination operations (Singh et al. 2021). As described earlier in this article, most of the steps mentioned above are only suggested as a single remediation method. However, despite their success, these methods have some downsides, including inefficiency and high costs (Sreejith et al. 2022). Several benefits can be gained by repurposing these problems as integrated processes, including increased efficiency, reduced cost and time, increased flexibility, less environmental impact, and the potential for large-scale treatment options (Feng et al. 2018). Because of these considerations, many researchers worldwide have found that combining or integrating treatment options is more beneficial (Selvi et al. 2019). An in-depth understanding of the objectives of both processes is necessary for the integration process. For large-scale applications, it is essential to combine two approaches to be tested and compared to their solo equivalents in terms of cost and efficiency (Wibowo et al. 2021). It has been shown that integrated strategies for heavy metal removal from various environments are becoming more popular (Azimi et al. 2017).
Chemical-Biological Remediation Approach
Chemical–biological integrated treatment method is claimed to be a cost-effective and eco-friendly solution to remove heavy metals from wastewater. According to several researchers worldwide, adopting this treatment rather than individual chemical or biological therapies is good and has shown significant advantages in HMs elimination (Greenwell et al. 2016; Pradhan et al. 2017). A combination of both therapies' advantages and disadvantages is recommended. One of the most popular repair procedures is chemical remediation because of its ease of use and rapid results. However, metal precipitates and toxic by-products have significantly impeded this method (Crini and Lichtfouse 2019).
In contrast, biological treatment is increasingly popular due to its little impact on the environment and high return on investment. The downsides include a long acclimation period, alterations in the isolate's biodegradability, and sludge production (Marzuki et al. 2021). However, these limitations can be circumvented by combining both approaches and thoroughly knowing the workings of each method. Because of its efficiency and cost viability, some experts believe that this integrated system often includes biological therapy followed by chemical treatment and the other way around (Kaasa et al. 2018). An environmentally acceptable choice of non-toxic chemicals would unquestionably enhance the efficacy of this procedure, even though academics have already adopted it (Khanna et al. 2021).
Phytobial Remediation Approach
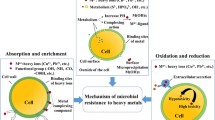
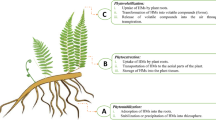
Plants can be used to remove heavy metals from soil and water, which is both cost-effective and environmentally friendly. In phytobial remediation, both plants and bacteria clean up soil and water. The literature says that plants and microbes are used to break down heavy metals in phytobial-based remediation (Khanna et al. 2021; Kumar et al. 2022). Figure 7 shows how metals can get into cells like metal bioprecipitation, metal bioaccumulation, metal binding on the cell surface, metal biotransformation, and metal methylation bioaccumulation. Operations such as solubilization, reduction, biosorption, and siderophores are ways that metals can be removed from the body. Heavy metals can also be removed from the body through DNA-mediated interactions (Ahemad 2015). Integrating the right bacteria that can make a lot of plant growth-promoting substances (PGPS) can help these mechanisms work better (Banov et al. 2020). People use organic acids, siderophores, biosurfactants, and other chemicals to make metals easier for us to use (Roy et al. 2015).
Phytobial remediation is widely recognized as the cleanest and most cost-effective option compared to other invasive technologies. Large areas of polluted groundwater, soil, and sediment can also be treated. It is also an in situ treatment option that has been shown to aid in topsoil preservation by minimizing heavy metal dispersion in the soil. Despite these advantages, this method is restricted to shallow aquifers and soils due to plant root length restrictions, the risk of heavy metals entering the food chain, regular monitoring (due to little rainfall), the long duration (which can last several seasons), safety lack, complicated metal recovery procedures, proper disposal method, and a high recycle economy. A study by (Roy et al. 2015) proposed many solutions to address these issues, including the use of deep-rooted plants, the development of herbivore-distracting transgenic plants, the development of appropriate evaluation methods, and the integration of other methods such as bioremediation, EK, and bioaugmentation, among others. The various microorganisms involved in phytobial cleanup are explained in depth here (Chaudhari 2021).
Phytobial Remediation Using Free Living Organism
Phytoremediation is helped by the movement, immobilization, and volatilization of free-living microorganisms, which allow the process (Waigi et al. 2017). Redox transformation, volatilization, chelation, and leaching are ways metals may be transferred. As is eliminated using bacteria such as Sulfur spirillum barnesii, Geobacter, and Bacillus selenatarsenatis. A new hybrid method uses anaerobic bioleaching and electro kinetics (Lee et al. 2009). Heavy metals build up in the plant's harvested tissue, which can be thrown away—adding microorganisms that move around to contaminated water speeds up the buildup of heavy metals (Quarshie et al. 2021). The immobilization process changes the physical and chemical properties of the pollutant so that it cannot move. The metals are oxidized by the enzymes found in bacteria, making them more challenging to move and less harmful. To remove heavy metals from the soil, bacteria such as Sporosarcina ginsengisoli, Candida glabrata, Bacillus cereus, and Aspergillus Niger were utilized in an immobilization approach (Jiang et al. 2022). It used the process of biomethylation to remove HMs from the environment. There were a lot of bacteria, fungi, and algae used in the biotransformation process (Ai et al. 2020).
Endophyte and Rhizo Microbe Remediation
Endophytes are bacteria or fungi that live inside plants. They spend at least part of their life cycle without hurting the plant. They can be found in practically every plant, and some of them have the power to promote plant growth. A few fungal endophytes produce secondary metabolites. Heavy metal tolerance in Methylo bacteria strains from the Pteris vittata plant has been identified. However, further research is needed to fully understand the unstudied endo Phyto biome possibilities. The root zone of a plant is referred to as the rhizosphere. Some bacteria in this region have a symbiotic connection with the plant by secretions, lysates mucigel, secreting exudates, and mucilage to help plant development. Microbe-secreted siderophores, for example, will aid in metal chelation and solubilization (Selvi et al. 2019). Rhizo-remediation uses these secretions to stimulate plant growth, immobilize heavy metals, and prevent metal buildup. Different metals can bind to siderophores with other ligand-binding groups. Pseudomonas azotosomes siderophores have been shown the arsenic to mobilize and remove. The higher pH in the rhizosphere zone assists in the mobilization and absorption of heavy metals because root bacteria are aerobic. The cation and anion uptake ratio is connected to the increased pH in the rhizosphere. According to researchers, the plant-microbial partnership secretes biosurfactants that aid with metal immobilization by raising the pH of the rhizosphere (Ali et al. 2022a, b; Manghwar et al. 2021).
Fungal and Algal Phytoremediation
Mycorrhizal fungi are associated with many plants, increasing the root surface area and aiding in water and nutrient uptake. Several recent studies have shown that mycorrhizal fungus can help plants better absorb and store heavy metals. A few researchers have found that mycorrhizal fungi present in Plantago lanceolata L promote As buildup, although other researchers have not confirmed this. Algae are considered an essential component of the aquatic system that contributes significantly to the biogeochemical cycle. Their outstanding absorption and sequestration potential has piqued the interest of researchers all over the world. It also has a high tolerance for heavy metals, the ability to remove them selectively, grows both autotrophically and heterotrophically, synthesizes metallothionein and phytochelatin, and the ability to cause genetic changes. Some micro- and macroalgae such as Enteromorpha sp., Ulva sp., Cladophora sp., Chaetomorpha sp., and Fucus serratus have all been reported to collect substantial levels of heavy metals.
Enhanced Phytoremediation Approaches
Significant technology must be employed to remove or reduce heavy metals to tolerable levels. Although it may be accomplished by various integrated strategies, including recombinant genetic engineering of bacteria and plants, heavy metal removal applications have shown recombinant genetic engineering to be worthwhile (Wu et al. 2021). Gene-modified microorganisms perform better than their wild counterparts, with enormous restorative potential. Genetic engineering can also activate phytoremediation and boost heavy metal accumulation and uptake. The "ars" operon in the "arsR" gene encodes a regulatory protein that helps detect arsenic pollution. A recombinant E. coli strain with the "ars" gene was produced by (Kaur and Garg 2014), which accumulated 60 times as much arsenic as the control strain. "Ars" recombinant strains are best appropriate for in situ bioremediation in a real-world context (Saravi and Dehpour 2016). When Enterobacter cloacae CAL2 was added to the canola plants, heavy metal accumulation was four times higher than in control cells. Transgenic plants were introduced to boost the plant's capacity for heavy metal removal from the soil (Fahrenfeld et al. 2019).
Hyperaccumulators for Remediation of Heavy Metals
HM ions are actively removed from plants by excluders, which make up the majority of plant species capable of living in soils high in dangerous trace elements (Sun et al. 2022; Tang et al. 2021, 2022). An HM ion is only toxic to the roots of an excluder plant, while the aerial parts are mainly unaffected by the toxin. When exposed to large concentrations of HM, hyperaccumulators, on the other hand, may store the toxin above ground without showing any symptoms of phytotoxicity (Iftikhar et al. 2022). Sebartia acuminata (Sapotaceae), a New Caledonian Ni-accumulating tree, was the first to be referred to as a hyperaccumulator because of its 26 per cent dry weight nickel concentration in its latex. The translocation factor (TF) and bioaccumulation factor (BCF) are also important in screening hyperaccumulators for phytoremediation of heavy metals. Screening of hyperaccumulators depend on BCF and TF values (both of them are greater than 1) for evaluation and selection of plants for phytoremediation (Parveen et al. 2020; Saleem et al. 2020j). The TF is the capacity of plants to transfer metals from roots to shoots and BCF express the ability of plants to accumulate metals from soils to tissues (Deng et al. 2021; Saleem et al. 2020k, 2021). Another requirement for criteria of plants whether it is Cu hyperaccumulator species or not is Cu accumulation in shoots. Cu accumulation in shoots should be greater than 1000 mg kg−1 dry weight when grown on metals rich soils (Javed et al. 2020; Rehman et al. 2019a). Caryophylacales and other hyperaccumulator-rich groupings include Asteroideae, Euphorbiaceae, Rubiaceae, Fabaceae, Scrophulariaceae, Myrtaceae, Proteaceae, and Caryophyllaceae, and other hyperaccumulator-rich groups. Hyperaccumulator species appear to predominate in some plant genera; for example, 48 of 170 species in the genus Alyssum were found to hyper accumulate Ni in a thorough survey. Metal specificity and accumulation may vary between populations, discovering that hyperaccumulation ability differs among species. Hyperaccumulators differ from non-hyperaccumulators in various essential physiological processes of HM detoxification, according to (Sajid et al. 2018), who researched the Zn, Cd, and Ni model hyperaccumulator alpine pennycress (Noccaea caerulescens, originally Thlaspi caerulescens). As a result of these changes in the root cell plasma membrane, root vacuole sequestration of Hm ions increased xylem transport of Hm to shoots. It increased Hm influx into the plasma membrane of leaf cells and (v) sequestration in the leaf vacuole. The plant can absorb more Hm ions. According to (Sun et al. 2022), the plant's improved active metal transport rather than enhanced intracellular ligands play the most important role in hyperaccumulation (such as glutathione, photoheating, or metallothionein). When compared to the non-accumulating sister species Arabidopsis lyrate and the closely related reference model Arabidopsis thaliana, a Zn and Cd hyperaccumulator, Arabidopsis helleri, showed an abundance of HMA4 gene copies and corresponding transcripts, as well as other transition metal homeostasis and biotic factors (Banov et al. 2020).
Chelating Agents
Even though chelation is based on fundamental coordination chemistry, creating a perfect chelator and chelation treatment that fully removes a toxic metal from a desired site in the body necessitates a thorough drug design strategy (Cappai 2020). Chelating agents are organic or inorganic substances that may bind metal ions and create chelates, complex ring-like structures that bind metal ions. In bidentate chelates, the "ligand" binding atoms form two covalent links, one covalent and one co-ordinate linkage, or two co-ordinate couplings. S, N, and O atoms are commonly used as ligand atoms in chemical groups such as –SH, –S–S, –NH2, =NH, –OH, –OPO3H, or > C=O. The metal ion and the two-ligand atoms linked to the metal create ring structures in bidentate or multidentate ligands (Fig. 8). Several donors use bidentate ligands. Inorganic chelate ligands, for example, create a five-membered ring with metal ions. Other chelating ligands, such as EDTA 4, a hexadentate ligand, are also conceivable. However, the proton retains its positive charge because no electrons are lost or gained (Holbein et al. 2021).
These complexes' pharmacokinetics and their toxicological behavior are determined by this feature, which is referred to as the 'net ionic charge' of the complex. It is measured in nanograms. Complexes of this nature can be produced in the biological environment by metal cations such as Na+, Mg+, Cu+, Cu2+, and Zn2+ and transition metals such as Mn, Fe, and Co. Metal cations such as Na+, Mg+, Cu+, Cu2+, and Zn2+ are all found in the biological environment (Blackman et al. 2019). However, the chelating agent and the chelated metal's properties are the most important factors determining their stability. Using equilibrium equations that depend on the atomic structure of chelated metal atoms, one can get quantitative information about the strength constant in compounds. The chelating agent can remove the metal with a lower equilibrium constant from the equation because of its higher stability stable (Treviño et al. 2019).
The number of ring formations is another issue to consider regarding heterocyclic rings. Despite Pb's more excellent stability consistent, Ca2+ is readily available. It preferentially binds to Na2EDTA because of the number of heterocyclic rings produced and the relative concentrations of the two EDTA salts in the bodily fluid (Machete and Chabo 2020; Treviño et al. 2019). Furthermore, even if an ideal chelator possesses all the required characteristics, the results are unpredictable. Toxic factors or endogenous compounds like hemoglobin, cytochromes, and other chelating agents may prevent a chemical entity from being an ideal chelator in vivo, despite its ability to chelate metals in vitro (Hajeb et al. 2014). It is also essential to consider the pH of the solution when it comes to complexes. This is because metals prefer to form hydroxides at high pH, making them more difficult for chelating agents to access (Gao et al. 2022, Zhang and Zhou 2019). As a result of these conditions, this feature becomes critical. Cleavage of metal ions and their complexes can be most effectively performed when these properties are combined with those of the metal complex itself (Schattschneider et al. 2019). A more stable complex will be formed most of the time (but not always) when a chelating agent occupies a more significant percentage of a metal ion's coordination sites. When it comes to the absorption, distribution, and ability to find and bind to metal ions, chelators' net ionic charge dictates their absorption, distribution, and ability to get to the metal ion (Selvi et al. 2019). First, transport toxic metal ions across physiological barriers to areas where they are concentrated; then form stable complexes with these ions, which are non-toxic and aid in their excretion. Finally, chelation complexes are formed that are non-toxic and aid in their excretion from the site of deposition (Sperdouli et al. 2022).
Common Chelating Agents: Pharmacology and Toxicology
An ideal chelator must be highly bio transformable, maintain chelating activity at bodily fluid pH, be soluble in water, reach metal storage sites, and form metal complexes that are less dangerous than the free metal ion.
During World War II, dimercaprol (also known as British Anti-Lewisite or BAL) was created as a test antidote to the arsenic-based poison gas Lewisite (BAL). As a result of their work painting the hulls of ships following World War II, many navy personnel suffered lead poisoning (Tang et al. 2021, 2022). This was the first time EDTA had been used medically to chelate lead. BAL has dominated physician prescriptions for general metal intoxication for decades because of its excellent ability to treat human arsenic and mercury poisoning. This dithiol is structurally similar to BAL but has fewer harmful effects than BAL had in the 1960s (Flora et al. 2008, Flora and Pachauri 2010). In the former Soviet Union, scientists created novel dithiol sodium 2,3-dimercaptopropane 1-sulfonate (DMPS) as a mercury chelating agent. Chelation therapy has been used to reduce the body's hazardous metal levels for patients with severe symptoms and high biological indicators. Chelating medicines can influence metal toxicity by mobilizing the poisonous metal, primarily eliminated in the urine. Metal poisoning can be reduced by chelating drugs that form a stable complex with the metal ion, thus protecting biological targets from the metal's harmful effects. Deferoxamine (DFOA), which includes complexes covering Fe3surface + 's entirely, prevents iron from catalyzing free radical reactions(Gerhardsson 2022). However, the metal exposed by the chelator may become more toxic in other situations due to the biological context of handling it (Fig. 9). To put it simply, unlike EDTA, which cannot completely cover the surface of Fe3+, it produces an open complex (basket complex) that enhances Fe3potential + 's to create oxidative stress, which is suitable for the treatment of oxidative stress. Various chelating chemical structures are shown in Fig. 9.
Conclusion and Future Prospective
Heavy metals discharged into the environment due to numerous human activities have caused variable levels of soil contamination across the world. As a result, careful and stringent monitoring of these operations is recommended as a viable solution to heavy metal contamination. However, a detailed understanding of the origin and sources of different heavy metals, the potential risks to the environment and individuals, and their chemistry is mandatory to determine an effective remedial technique. Other remediation techniques, including thermal processes, physical, biological, chemical, and electrical, with advantages or disadvantages to rectify the soil contamination by immobilizing, containing, and extracting the heavy metal contaminants were discussed in additional research. Heavy metal resources (Mining, pesticides, fertilizers, herbicides, and irrigation of agriculture fields with industrial and sewage water, Biosolids, manures, pesticides), phytotoxicity of some contaminants (such as Pb, As, Hg, Zn, and Cd), removal/recovery from the contaminated environment using different remediation approaches such as the integrated option summarized in this literature review. Single remediation techniques, despite their effectiveness, have some significant disadvantages, such as high costs and inefficiency. The advantages of integrated approaches are less cost and time, a high level of flexibility, lower environmental effect, and the large-scale treatment possibility and high efficiency. Due to these mentioned factors, numerous studies have discovered combining or integrating treatment approaches (Chemical-Biological, Electro-Kinetic Microbial, Electrokinetic-Phytoremediation, and Phytobial Remediation) are more helpful and beneficial. Cost and efficiency are the determinative factors for large-scale applications of the integration process in the severely polluted site. Biotechnological techniques are overgrowing in remediation, and the recombinant genetic engineering of bacteria and plants is one of the promising biotechniques that can enhance the Phytoremediation Approaches. As a result, due to the high demand for integrated processes, future remediation should be able to estimate the ecological impact, depth understanding of the objectives, and be more innovative. As a remediation technique, chelate extraction, chemical soil washings, and phytobial remediation require more study assessments due to their economic feasibility, extensive use, and efficacy. Future research should include creating new remediation technologies and developing the assessment methodologies for determining remediation efficacy. Governments' strict definition and implementation of new standards are also vital to environmental protection and undoubtedly significantly reduce dangerous heavy metal levels in the environment.
An in-depth understanding of the objectives of both processes is necessary for the integration process. For large-scale applications, it is essential to combine two approaches so that they can be tested and compared to their solo equivalents in terms. It has been shown that integrated strategies for heavy metal removal from various environments are becoming more popular. More analysis of the method is required to doubt the in-place operational parameters.
References
Abbar B, Alem A, Marcotte S, Pantet A, Ahfir N-D, Bizet L, Duriatti D (2017) Experimental investigation on removal of heavy metals (Cu2+, Pb2+, and Zn2+) from aqueous solution by flax fibres. Process Saf Environ Prot 109:639–647
Abubakari M, Moomin A, Nyarko G, Dawuda M (2017) Heavy metals concentrations and risk assessment of roselle and jute mallow cultivated with three compost types. Ann Agric Sci 62:145–150
Afzal J, Saleem MH, Batool F, Elyamine AM, Rana MS, Shaheen A, El-Esawi MA, Tariq Javed M, Ali Q, Arslan Ashraf M, Hussain GS, Hu C (2020) Role of ferrous sulfate (FeSO4) in resistance to cadmium stress in two rice (Oryza sativa L.) genotypes. Biomolecules 10:1693
Afzal J, Wang X, Saleem M-H, Sun X, Hussain S, Khan I, Rana M-S, Ahmed S, Awan S-A, Fiaz S, Aziz O, Kubar K-A, Ali S, Hu C (2021) Application of ferrous sulfate alleviates negative impact of cadmium in rice (Oryza sativa L.). Biocell 45:1631–1649
Aharchaou I, Maul A, Pons M-N, Pauly D, Poirot H, Flayac J, Rodius F, Rousselle P, Beuret M, Battaglia E (2022) Effects and bioaccumulation of Cr (III), Cr (VI) and their mixture in the freshwater mussel Corbicula fluminea. Chemosphere 297:134090–134090
Ahemad M (2015) Phosphate-solubilizing bacteria-assisted phytoremediation of metalliferous soils: a review. 3 Biotech 5:111–121
Ahmad S, Mfarrej MFB, El-Esawi MA, Waseem M, Alatawi A, Nafees M, Saleem MH, Rizwan M, Yasmeen T, Anayat A, Ali S (2022) Chromium-resistant Staphylococcus aureus alleviates chromium toxicity by developing synergistic relationships with zinc oxide nanoparticles in wheat. Ecotoxicol Environ Saf 230:113142
Ai P, Jin K, Alengebawy A, Elsayed M, Meng L, Chen M, Ran Y (2020) Effect of application of different biogas fertilizer on eggplant production: Analysis of fertilizer value and risk assessment. Environ Technol Innov 19:101019–101019
Al Jabri H, Saleem MH, Rizwan M, Hussain I, Usman K, Alsafran M (2022) Zinc oxide nanoparticles and their biosynthesis: overview. Life 12:594
Alam A, Tariq M, Haq I, Ali J, Adnan M, Fahad S, Ahmad DM, Romman M, Saleem MH, Ahmad S, Gul F, Durrishahwar, Subhan F, Wahid F, Ur-Rahman I, Yasin G (2022) Co-application of phosphorus and sulfur improve yield, quality, and nutrients uptake in Nicotiana tabaccum L. Philippine Agricultural Scientist
Alatawi A, Wang X, Maqbool A, Saleem MH, Usman K, Rizwan M, Yasmeen T, Arif MS, Noreen S, Hussain A, Ali S (2022a) S-fertilizer (elemental sulfur) improves the phytoextraction of cadmium through Solanum nigrum L. Int J Environ Res Public Health 19:1655
Alatawi A, Wang X, Saleem MH, Mohsin M, Rehman M, Usman K, Fahad S, Mfarrej MFB, Hefft DI, Ali S (2022b) Individual and synergic effects of phosphorus and gibberellic acid on organic acids exudation pattern, ultra-structure of chloroplast and stress response gene expression in Cu-stressed jute (Corchorus capsularis L.). J Plant Growth Regul 1–26
Ali SY, Chaudhury S (2016) EDTA-enhanced phytoextraction by Tagetes sp. and effect on bioconcentration and translocation of heavy metals. Environ Process 3:735–746
Ali B, Wang B, Ali S, Ghani M, Hayat M, Yang C, Xu L, Zhou W (2013) 5-Aminolevulinic acid ameliorates the growth, photosynthetic gas exchange capacity, and ultrastructural changes under cadmium stress in Brassica napus L. J Plant Growth Regul 32:604–614
Ali M, Wang X, Haroon U, Chaudhary HJ, Kamal A, Ali Q, Saleem MH, Usman K, Alatawi A, Ali S, Hussain Munis MF (2022a) Antifungal activity of Zinc nitrate derived nano Zno fungicide synthesized from Trachyspermum ammi to control fruit rot disease of grapefruit. Ecotoxicol Environ Saf 233:113311
Ali Q, Zheng H, Rao MJ, Ali M, Hussain A, Saleem MH, Nehela Y, Sohail MA, Ahmed AM, Kubar KA, Ali S, Usman K, Manghwar H, Zhou L (2022b) Advances, limitations, and prospects of biosensing technology for detecting phytopathogenic bacteria. Chemosphere 133773
Alsafran M, Usman K, Al Jabri H, Rizwan M (2021) Ecological and health risks assessment of potentially toxic metals and metalloids contaminants: a case study of agricultural soils in Qatar. Toxics 9:35
Anderson CG (2012) The metallurgy of antimony. Geochemistry 72:3–8
Antle JM, Pingali PL (1994) Pesticides, productivity, and farmer health: a Philippine case study. Am J Agr Econ 76:418–430
Antonkiewicz J, Pełka R, Bik-Małodzińska M, Żukowska G, Gleń-Karolczyk K (2018) The effect of cellulose production waste and municipal sewage sludge on biomass and heavy metal uptake by a plant mixture. Environ Sci Pollut Res 25:31101–31112
Ashraf MA, Hussain I, Rasheed R, Iqbal M, Riaz M, Arif MS (2017) Advances in microbe-assisted reclamation of heavy metal contaminated soils over the last decade: a review. J Environ Manag 198:132–143
Azimi A, Azari A, Rezakazemi M, Ansarpour M (2017) Removal of heavy metals from industrial wastewaters: a review. ChemBioEng Rev 4:37–59
Aziz H, Wang X, Murtaza G, Ashar A, Hussain S, Abid M, Murtaza B, Saleem MH, Fiaz S, Ali S (2021) Evaluation of compost and biochar to mitigate chlorpyrifos pollution in soil and their effect on soil enzyme dynamics. Sustainability 13:9695
Banov M, Rousseva S, Pavlov P (2020) Sustainable management and restoration of the fertility of damaged and contaminated lands and soils. Soil Health Restor Manag 113–159
Barik A, Biswal D, Arun A, Balasubramanian V (2021) Biodetoxification of heavy metals using biofilm bacteria. Environ Agric Microbiol 39–61
Barkay T, Gu B (2021) Demethylation—the other side of the mercury methylation coin: a critical review. ACS Environ Au
Basta N, Gradwohl R, Snethen K, Schroder J (2001) Chemical immobilization of lead, zinc, and cadmium in smelter-contaminated soils using biosolids and rock phosphate. J Environ Qual 30:1222–1230
Benelli G, Maggi F, Pavela R, Murugan K, Govindarajan M, Vaseeharan B, Petrelli R, Cappellacci L, Kumar S, Hofer A (2018) Mosquito control with green nanopesticides: towards the One Health approach? A review of non-target effects. Environ Sci Pollut Res 25:10184–10206
Blackman LD, Gunatillake PA, Cass P, Locock KES (2019) An introduction to zwitterionic polymer behavior and applications in solution and at surfaces. Chem Soc Rev 48:757–770
Bucknall DG (2020) Plastics as a materials system in a circular economy: plastics in the circular economy. Philos Trans R Soc A 378:20190268–20190268
Cai LM, Wang QS, Wen HH, Luo J, Wang S (2019) Heavy metals in agricultural soils from a typical township in Guangdong Province, China: occurrences and spatial distribution. Ecotoxicol Environ Saf 168:184–191
Cappai R (2020) Integrate approach to the study of chelating agents for the effects of toxic metal ions.
Chaudhari SK (2021) Soil and water management in India: challenges and opportunities. Springer, New York, pp 751–764
Chen X, Lu X (2018) Contamination characteristics and source apportionment of heavy metals in topsoil from an area in Xi’an city, China. Ecotoxicol Environ Saf 151:153–160
Chhipa H (2017) Nanofertilizers and nanopesticides for agriculture. Environ Chem Lett 15:15–22
Chowdhary P, Yadav A, Singh R, Chandra R, Singh D, Raj A, Bharagava RN (2018) Stress response of Triticum aestivum L. and Brassica juncea L. against heavy metals growing at distillery and tannery wastewater contaminated site. Chemosphere 206:122–131
Christophoridis C, Evgenakis E, Bourliva A, Papadopoulou L, Fytianos K (2020) Concentration, fractionation, and ecological risk assessment of heavy metals and phosphorus in surface sediments from lakes in N. Greece. Environ Geochem Health 1–23
Clarke RM, Cummins E (2015) Evaluation of “classic” and emerging contaminants resulting from the application of biosolids to agricultural lands: a review. Hum Ecol Risk Assess Int J 21:492–513
Crini G, Lichtfouse E (2019) Advantages and disadvantages of techniques used for wastewater treatment. Environ Chem Lett 17:145–155
Cristaldi A, Conti GO, Jho EH, Zuccarello P, Grasso A, Copat C, Ferrante M (2017) Phytoremediation of contaminated soils by heavy metals and PAHs. A brief review. Environ Technol Innov 8:309–326
Daulta R, Prakash M, Goyal S (2022) Metal content in soils of Northern India and crop response: a review. Int J Environ Sci Technol 1–28
De Bhowmick G, Sarmah AK (2022) Microplastics contamination associated with land-application of biosolids: a perspective. Curr Opin Environ Sci Health 26:100342–100342
Deng G, Yang M, Saleem MH, Rehman M, Fahad S, Yang Y, Elshikh MS, Alkahtani J, Ali S, Khan SM (2021) Nitrogen fertilizer ameliorate the remedial capacity of industrial hemp (Cannabis sativa L.) grown in lead contaminated soil. J Plant Nutr 1–9
Dongre RS (2021) Chromium & lead as soil pollutants: insights on toxicity profiles and their remediation. J Adv Biotechnol Bioeng 9:1–16
Drechsel P, Otoo M, Hanjra MA (2022) Resource recovery from wastewater and the consumer point of view: social, cultural and economic aspects. Resour Recov Water 383–414
Ehsan S, Ali S, Noureen S, Mahmood K, Farid M, Ishaque W, Shakoor MB, Rizwan M (2014) Citric acid assisted phytoremediation of cadmium by Brassica napus L. Ecotoxicol Environ Saf 106:164–172
Evangelou MW, Bauer U, Ebel M, Schaeffer A (2007) The influence of EDDS and EDTA on the uptake of heavy metals of Cd and Cu from soil with tobacco Nicotiana tabacum. Chemosphere 68:345–353
Fahrenfeld NL, Arbuckle-Keil G, Naderi Beni N, Bartelt-Hunt SL (2019) Source tracking microplastics in the freshwater environment. TrAC 112:248–254
Farid M, Ali S, Saeed R, Rizwan M, Bukhari SAH, Abbasi GH, Hussain A, Ali B, Zamir MSI, Ahmad I (2019) Combined application of citric acid and 5-aminolevulinic acid improved biomass, photosynthesis and gas exchange attributes of sunflower (Helianthus annuus L.) grown on chromium contaminated soil. Int J Phytorem 21:760–767
Farooq MA, Li L, Ali B, Gill RA, Wang J, Ali S, Gill MB, Zhou W (2015) Oxidative injury and antioxidant enzymes regulation in arsenic-exposed seedlings of four Brassica napus L. cultivars. Environ Sci Pollut Res 22:10699–10712
Feng Y, Gao Y, Tian G, Li Z, Hu H, Zheng H (2018) Flexible process planning and end-of-life decision-making for product recovery optimization based on hybrid disassembly. IEEE Trans Autom Sci Eng 16:311–326
Flora SJS, Pachauri V (2010) Chelation in metal intoxication. Int J Environ Res Public Health 7:2745–2788
Flora SJS, Mittal M, Mehta A (2008) Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J Med Res 128:501–501
Gao S, Zhang R, Zhang H, Zhang S (2022) The seasonal variation in heavy metal accumulation in the food web in the coastal waters of Jiangsu based on carbon and nitrogen isotope technology. Environ Pollut 297:118649–118649
Gavrilescu M (2022) Enhancing phytoremediation of soils polluted with heavy metals. Curr Opin Biotechnol 74:21–31
Gerhardsson L (2022) Diagnosis and treatment of metal poisoning general aspects. Elsevier, New York, pp 663–684
Gill RA, Ahmar S, Ali B, Saleem MH, Khan MU, Zhou W, Liu S (2021) The role of membrane transporters in plant growth and development, and abiotic stress tolerance. Int J Mol Sci 22:12792
Girovich MJ (1996) Biosolids treatment and management: processes for beneficial use. CRC Press, Boca Raton
Gomes M, Soares A, Garcia Q (2014) Phosphorous and sulfur nutrition modulate antioxidant defenses in Myracrodruom urundeuva plants exposed to arsenic. J Hazard Mater 276:97–104
Grčman H, Velikonja-Bolta Š, Vodnik D, Kos B, Leštan D (2001) EDTA enhanced heavy metal phytoextraction: metal accumulation, leaching and toxicity. Plant Soil 235:105–114
Greenwell M, Sarker M, Rahman PKSM (2016) Biosurfactant production and biodegradation of leather dust from tannery. Open Biotechnol J 10
Griga M, Bjelkova M, Tejklová E (2003) Potential of flax (Linum usitatissimum L.) for heavy metal phytoextraction and industrial processing of contaminated biomass—a review. Risk assessment and sustainable land management using plants in trace element-contaminated soils. Centre INRA Bordeaux-Aquitaine, Villenave d’Ornon, France 174–180
Gupta PK (2019) Toxic effects of metals and micronutrients. Springer, New York, pp 83–119
Haider FU, Wang X, Farooq M, Hussain S, Cheema SA, ul Ain N, Virk AL, Ejaz M, Janyshova U, Liqun C (2022) Biochar application for the remediation of trace metals in contaminated soils: implications for stress tolerance and crop production. Ecotoxicol Environ Saf 230:113165–113165
Hajeb P, Sloth JJ, Shakibazadeh SH, Mahyudin NA, Afsah-Hejri L (2014) Toxic elements in food: occurrence, binding, and reduction approaches. Compr Rev Food Sci Food Saf 13:457–472
Hao Z, Chen L, Wang C, Zou X, Zheng F, Feng W, Zhang D, Peng L (2019) Heavy metal distribution and bioaccumulation ability in marine organisms from coastal regions of Hainan and Zhoushan, China. Chemosphere 226:340–350
Hasanpour M, Hatami M (2020) Application of three dimensional porous aerogels as adsorbent for removal of heavy metal ions from water/wastewater: a review study. Adv Colloid Interface Sci 102247
Hashem IA, Abbas AY, Abd El-Hamed AE-NH, Salem HMS, El-hosseiny OEM, Abdel-Salam MA, Saleem MH, Zhou W, Hu R (2020) Potential of rice straw biochar, sulfur and ryegrass (Lolium perenne L.) in remediating soil contaminated with nickel through irrigation with untreated wastewater. PeerJ 8:e9267
Hashmat S, Shahid M, Tanwir K, Abbas S, Ali Q, Niazi NK, Akram MS, Saleem MH, Javed MT (2021) Elucidating distinct oxidative stress management, nutrient acquisition and yield responses of Pisum sativum L. fertigated with diluted and treated wastewater. Agric Water Manag 247:106720
Hassan A, Amjad SF, Saleem MH, Yasmin H, Imran M, Riaz M, Ali Q, Joyia FA, Ahmed S, Ali S (2021) Foliar application of ascorbic acid enhances salinity stress tolerance in barley (Hordeum vulgare L.) through modulation of morpho-physio-biochemical attributes, ions uptake, osmo-protectants and stress response genes expression. Saudi J Biol Sci
Hauptman M, Bruccoleri R, Woolf AD (2017) An update on childhood lead poisoning. Clin Pediatr Emerg Med 18:181–192
Hayles J, Johnson L, Worthley C, Losic D (2017) Nanopesticides: a review of current research and perspectives. New Pestic Soil Sensors 193–225
Heile AO, Zaman Qu, Aslam Z, Hussain A, Aslam M, Saleem MH, Abualreesh MH, Alatawi A, Ali S (2021) Alleviation of cadmium phytotoxicity using silicon fertilization in wheat by altering antioxidant metabolism and osmotic adjustment. Sustainability 13:11317
Hojjati-Najafabadi A, Mansoorianfar M, Liang T, Shahin K, Karimi-Maleh H (2022) A review on magnetic sensors for monitoring of hazardous pollutants in water resources. Sci Total Environ 824:153844–153844
Holbein BE, Ang MTC, Allan DS, Chen W, Lehmann C (2021) Exploiting the Achilles’ heel of iron dependence in antibiotic resistant bacteria with new antimicrobial iron withdrawal agents. Springer, New York, pp 251–311
Husak V (2015) Copper and copper-containing pesticides: metabolism, toxicity and oxidative stress. J Vasyl Stefanyk Precarpathian Natl Univ 39–51
Hussain I, Saleem MH, Mumtaz S, Rasheed R, Ashraf MA, Maqsood F, Rehman M, Yasmin H, Ahmed S, Ishtiaq M (2021) Choline chloride mediates chromium tolerance in Spinach (Spinacia oleracea L.) by restricting its uptake in relation to morpho-physio-biochemical attributes. J Plant Growth Regul 1–21
Hussain I, Afzal S, Ashraf MA, Rasheed R, Saleem MH, Alatawi A, Ameen F, Fahad S (2022) Effect of metals or trace elements on wheat growth and its remediation in contaminated soil. J Plant Growth Regul
Ibrahim ATA, Banaee M, Sureda A (2019) Selenium protection against mercury toxicity on the male reproductive system of Clarias gariepinus. Comp Biochem Physiol Part C 225:108583–108583
Iftikhar A, Abbas G, Saqib M, Shabbir A, Amjad M, Shahid M, Ahmad I, Iqbal S, Qaisrani SA (2022) Salinity modulates lead (Pb) tolerance and phytoremediation potential of quinoa: a multivariate comparison of physiological and biochemical attributes. Environ Geochem Health 44:257–272
Imran M, Hussain S, El-Esawi MA, Rana MS, Saleem MH, Riaz M, Ashraf U, Potcho MP, Duan M, Rajput IA (2020) Molybdenum supply alleviates the cadmium toxicity in fragrant rice by modulating oxidative stress and antioxidant gene expression. Biomolecules 10:1582
Imran M, Hussain S, Rana MS, Saleem MH, Rasul F, Ali KH, Potcho MP, Pan S, Duan M, Tang X (2021) Molybdenum improves 2-acetyl-1-pyrroline, grain quality traits and yield attributes in fragrant rice through efficient nitrogen assimilation under cadmium toxicity. Ecotoxicol Environ Saf 211:111911
Islam MS, Wahid-Uz-Zaman M, Rahman MM (2013) Phytoaccumulation of arsenic from arsenic contaminated soils by Eichhornia crassipes L., Echinochloa Crusgalli L. and Monochoria Hastata L. in Bangladesh. Int J Environ Prot 3, 17
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7:60–60
Javad S, Shah AA, Ramzan M, Sardar R, Javed T, Al‐Huqail AA, Ali HM, Chaudhry O, Yasin NA, Ahmed S (2022) Hydrogen sulphide alleviates cadmium stress in Trigonella foenum‐graecum by modulating antioxidant enzymes and polyamine content. Plant Biol
Javed MT, Saleem MH, Aslam S, Rehman M, Iqbal N, Begum R, Ali S, Alsahli AA, Alyemeni MN, Wijaya L (2020) Elucidating silicon-mediated distinct morpho-physio-biochemical attributes and organic acid exudation patterns of cadmium stressed Ajwain (Trachyspermum ammi L.). Plant Physiol Biochem
Javed MT, Tanwir K, Abbas S, Saleem MH, Iqbal R, Chaudhary HJ (2021) Chromium retention potential of two contrasting Solanum lycopersicum Mill. cultivars as deciphered by altered pH dynamics, growth, and organic acid exudation under Cr stress. Environ Sci Pollut Res 1–13
Jayakumar M, Surendran U, Raja P, Kumar A, Senapathi V (2021) A review of heavy metals accumulation pathways, sources and management in soils. Arab J Geosci 14:1–19
Jiang M, Wang K, Wang Y, Zhao Q, Wang W (2022) Technologies for the cobalt-contaminated soil remediation: a review. Sci Total Environ 813:151908–151908
Kaasa S, Loge JH, Aapro M, Albreht T, Anderson R, Bruera E, Brunelli C, Caraceni A, Cervantes A, Currow DC (2018) Integration of oncology and palliative care: a lancet oncology commission. Lancet Oncol 19:e588–e653
Kalve S, Sarangi BK, Pandey RA, Chakrabarti T (2011) Arsenic and chromium hyperaccumulation by an ecotype of Pteris vittata–prospective for phytoextraction from contaminated water and soil. Curr Sci 888–894
Kamal A, Saleem MH, Alshaya H, Okla MK, Chaudhary HJ, Munis MFH (2022) Ball-milled synthesis of maize biochar-ZnO nanocomposite (MB-ZnO) and estimation of its photocatalyticability against different organic and inorganic pollutants. Journal of Saudi Chemical Society, 101445
Kamran M, Malik Z, Parveen A, Huang L, Riaz M, Bashir S, Mustafa A, Abbasi GH, Xue B, Ali U (2019) Ameliorative effects of biochar on rapeseed (Brassica napus L.) growth and heavy metal immobilization in soil irrigated with untreated wastewater. J Plant Growth Regul 1–16
Kamran M, Danish M, Saleem MH, Malik Z, Parveen A, Abbasi GH, Jamil M, Ali S, Afzal S, Riaz M (2020) Application of abscisic acid and 6-benzylaminopurine modulated morpho-physiological and antioxidative defense responses of tomato (Solanum lycopersicum L.) by minimizing cobalt uptake. Chemosphere 128169
Kaur H, Garg H (2014) Pesticides: environmental impacts and management strategies. Pesticides 8:187–187
Khan I, Awan SA, Rizwan M, Ali S, Hassan MJ, Brestic M, Zhang X, Huang L (2021) Effects of silicon on heavy metal uptake at the soil-plant interphase: a review. Ecotoxicol Environ Saf 222:112510–112510
Khanna N, Agrawal C, Pimenov DY, Singla AK, Machado AR, da Silva LRR, Gupta MK, Sarikaya M, Krolczyk GM (2021) Review on design and development of cryogenic machining setups for heat resistant alloys and composites. J Manuf Process 68:398–422
Kong X, Ge R, Liu T, Xu S, Hao P, Zhao X, Li Z, Lei X, Duan H (2021) Super-stable mineralization of cadmium by calcium-aluminum layered double hydroxide and its large-scale application in agriculture soil remediation. Chem Eng J 407:127178–127178
Kour J, Kohli SK, Khanna K, Bakshi P, Sharma P, Singh AD, Ibrahim M, Devi K, Sharma N, Ohri P, Skalicky M, Brestic M, Bhardwaj R, Landi M, Sharma A (2021) Brassinosteroid signaling, crosstalk and, physiological functions in plants under heavy metal stress. Front Plant Sci 12
Kumar S, Islam ARMT, Islam HMT, Hasanuzzaman M, Ongoma V, Khan R, Mallick J (2021) Water resources pollution associated with risks of heavy metals from Vatukoula Goldmine region, Fiji. J Environ Manag 293:112868–112868
Kumar V, Dwivedi SK, Oh S (2022) A review on microbial-integrated techniques as promising cleaner option for removal of chromium, cadmium and lead from industrial wastewater. J Water Process Eng 47:102727–102727
Kumbhakar SK, Chauhan R, Jadhav SK, Quraishi A (2022) Lead induced-toxicity in vegetables, its mitigation strategies, and potential health risk assessment: a review. Int J Environ Sci Technol 1–26
Laghlimi M, Baghdad B, El Hadi H, Bouabdli A (2015) Phytoremediation mechanisms of heavy metal contaminated soils: a review. Open J Ecol 5:375
Lee K-Y, Yoon I-H, Lee B-T, Kim S-O, Kim K-W (2009) A novel combination of anaerobic bioleaching and electrokinetics for arsenic removal from mine tailing soil. Environ Sci Technol 43:9354–9360
Liu HJ, Zhang JL, Christie P, Zhang FS (2007) Influence of external zinc and phosphorus supply on Cd uptake by rice (Oryza sativa L.) seedlings with root surface iron plaque. Plant Soil 300:105–115
Lone J, Shikari A, Sofi N, Ganie S, Sharma M, Sharma M, Kumar M, Saleem MH, Almaary KS, Elshikh MS, Dwiningsih Y, Raza MA (2022) Screening technique based on seed and early seedling parameters for cold tolerance of selected F2-derived F3 rice genotypes under controlled conditions. Sustainability 14:8447
López-Rayo S, Laursen KH, Lekfeldt JDS, Delle Grazie F, Magid J (2016) Long-term amendment of urban and animal wastes equivalent to more than 100 years of application had minimal effect on plant uptake of potentially toxic elements. Agr Ecosyst Environ 231:44–53
Ma J, Saleem MH, Alsafran M, Jabri HA, Mehwish, Rizwan M, Nawaz M, Ali S, Usman K (2022a) Response of cauliflower (Brassica oleracea L.) to nitric oxide application under cadmium stress. Ecotoxicol Environ Saf 243:113969
Ma J, Saleem MH, Yasin G, Mumtaz S, Qureshi FF, Ali B, Ercisli S, Alhag SK, Ahmed AE, Vodnar DC, Hussain I, Marc RA, Chen F (2022b) Individual and combinatorial effects of SNP and NaHS on morpho-physio-biochemical attributes and phytoextraction of chromium through Cr-stressed spinach (Spinacia oleracea L.). Front Plant Sci 13
Machete JB, Chabo RG (2020) A Review of piggery manure management: generally, across western, Asian and African countries. Botsw J Agric Appl Sci 14:17–27
Madhu PM, Sadagopan RS (2020) Effect of heavy metals on growth and development of cultivated plants with reference to cadmium, chromium and lead–a review. J Stress Physiol Biochem 16:84–102
Maghsoudi AS, Hassani S, Mirnia K, Abdollahi M (2021) Recent advances in nanotechnology-based biosensors development for detection of arsenic, lead, mercury, and cadmium. Int J Nanomed 16:803–803
Mahar A, Wang P, Ali A, Awasthi MK, Lahori AH, Wang Q, Li R, Zhang Z (2016) Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: a review. Ecotoxicol Environ Saf 126:111–121
Manghwar H, Hussain A, Ali Q, Saleem MH, Abualreesh MH, Alatawi A, Ali S, Munis MFH (2021) Disease severity, resistance analysis, and expression profiling of pathogenesis-related protein genes after the inoculation of Fusarium equiseti in wheat. Agronomy 11:2124
Maqbool A, Ali S, Rizwan M, Ishaque W, Rasool N, ur Rehman MZ, Bashir A, Abid M, Wu L (2018) Management of tannery wastewater for improving growth attributes and reducing chromium uptake in spinach through citric acid application. Environ Sci Pollut Res 25:10848–10856
Marino KD (2003) Lead screening initiatives in Hillsborough County, Florida: a detailed analysis of lead poisoning in children under six. University of South Florida, Florida
Marzuki I, Nisaa K, Asaf R, Armus R, Kamaruddin M, Sapar A, Emelda A (2021) Biodegradation mechanism of naphthalene using marine sponge symbiotic bacteria. IOP Publishing, Bristol, pp 12020–12020
Matrosova EA, Bobrov AV, Bindi L, Pushcharovsky DY (2021) Titanium minerals and their assemblages in the earth’s mantle: a review of natural and experimental data. Geochem Int 59:725–742
Mehta V, Kansara R, Srivashtav V, Savaliya P (2021) A novel insight into phytoremediation of heavy metals through genetic engineering and phytohormones. 1–8
Merrington G (2018) The good, the bad and the ugly: copper and arsenic in soils. Soil Health
Mohajerani A, Karabatak B (2020) Microplastics and pollutants in biosolids have contaminated agricultural soils: an analytical study and a proposal to cease the use of biosolids in farmlands and utilise them in sustainable bricks. Waste Manag 107:252–265
Muhammad D, Chen F, Zhao J, Zhang G, Wu F (2009) Comparison of EDTA-and citric acid-enhanced phytoextraction of heavy metals in artificially metal contaminated soil by Typha angustifolia. Int J Phytorem 11:558–574
Mukherjee A, Pokhrel S, Bandyopadhyay S, Mädler L, Peralta-Videa JR, Gardea-Torresdey JL (2014) A soil mediated phyto-toxicological study of iron doped zinc oxide nanoparticles (Fe@ ZnO) in green peas (Pisum sativum L.). Chem Eng J 258:394–401
Murtaza G, Riaz U, Aziz H, Shaheen N, Sohail MI, Saleem MH, Abualreesh MH, Alatawi A, Ali S (2021) Health risk assessment, pore water chemistry, and assessment of trace metals transfer from two untreated sewage sludge types to tomato crop (Lycopersicon esculentum) at different application levels. Sustainability 13:12394
Nagajyoti PC, Lee KD, Sreekanth T (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216
Naha A, Nath DC, Nath S (2020) Impact of pesticides on the growth of Coriandrum sativum. Int J Plant Physiol Biochem 12:17–25
Najeeb U, Xu L, Ali S, Jilani G, Gong H, Shen W, Zhou W (2009) Citric acid enhances the phytoextraction of manganese and plant growth by alleviating the ultrastructural damages in Juncus effusus L. J Hazard Mater 170:1156–1163
Naqhiyah Farhan A (2018) Piperazine functionalized magnetic sporopollenin for solid phase extraction of lead (II)/Naqhiyah Farhan Ahmad. University of Malaya, Kuala Lumpur
Parveen A, Saleem MH, Kamran M, Haider MZ, Chen J-T, Malik Z, Rana MS, Hassan A, Hur G, Javed MT (2020) Effect of citric acid on growth, ecophysiology, chloroplast ultrastructure, and phytoremediation potential of jute (Corchorus capsularis L.) seedlings exposed to copper stress. Biomolecules 10:592
Paul T, Saha NC (2022) Bioremediation of heavy metals. Biotechnol Zero Waste 67–81
Pescatore A, Grassi C, Rizzo AM, Orlandini S, Napoli M (2022) Effects of biochar on berseem clover (Trifolium alexandrinum L.) growth and heavy metal (Cd, Cr, Cu, Ni, Pb, and Zn) accumulation. Chemosphere 287:131986
Pour FH, Makkawi YT (2021) A review of post-consumption food waste management and its potentials for biofuel production. Energy Rep 7:7759–7784
Pradhan D, Sukla LB, Sawyer M, Rahman PKSM (2017) Recent bioreduction of hexavalent chromium in wastewater treatment: a review. J Ind Eng Chem 55:1–20
Pramanick P, Chakraborty A, Raychaudhuri SS (2022) Partial alleviation of zinc induced oxidative stress by polyamines in Plantago ovata Forsk. Plant Cell Tissue Organ Culture (PCTOC) 1–11
Prasad S, Yadav KK, Kumar S, Gupta N, Cabral-Pinto MMS, Rezania S, Radwan N, Alam J (2021) Chromium contamination and effect on environmental health and its remediation: a sustainable approaches. J Environ Manag 285:112174–112174
Priyadarshanee M, Chatterjee S, Rath S, Dash HR, Das S (2022a) Cellular and genetic mechanism of bacterial mercury resistance and their role in biogeochemistry and bioremediation. J Hazard Mater 423:126985–126985
Priyadarshanee M, Mahto U, Das S (2022b) Mechanism of toxicity and adverse health effects of environmental pollutants. Elsevier, New York, pp 33–53
Quarshie SD-G, Xiao X, Zhang L (2021) Enhanced phytoremediation of soil heavy metal pollution and commercial utilization of harvested plant biomass: a review. Water Air Soil Pollut 232:1–28
Raj SN, Anooj E, Rajendran K, Vallinayagam S (2021) A comprehensive review on regulatory invention of nano pesticides in agricultural nano formulation and food system. J Mol Struct 1239:130517
Rajput VD, Minkina TM, Behal A, Sushkova SN, Mandzhieva S, Singh R, Gorovtsov A, Tsitsuashvili VS, Purvis WO, Ghazaryan KA (2018) Effects of zinc-oxide nanoparticles on soil, plants, animals and soil organisms: a review. Environ Nanotechnol Monitor Manag 9:76–84
Rana M, Bhantana P, Imran M, Saleem M (2020) Molybdenum potential vital role in plants metabolism for optimizing the growth and development. Ann Environ Sci Toxicol 4:032–044
Raza A, Hussain S, Javed R, Hafeez MB, Hasanuzzaman M (2021) Antioxidant defense systems and remediation of metal toxicity in plants. Springer, New York, pp 91–124
Rehman MZ-U, Rizwan M, Ali S, Fatima N, Yousaf B, Naeem A, Sabir M, Ahmad HR, Ok YS (2016) Contrasting effects of biochar, compost and farm manure on alleviation of nickel toxicity in maize (Zea mays L.) in relation to plant growth, photosynthesis and metal uptake. Ecotoxicol Environ Saf 133:218–225
Rehman M, Liu L, Bashir S, Saleem MH, Chen C, Peng D, Siddique KH (2019a) Influence of rice straw biochar on growth, antioxidant capacity and copper uptake in ramie (Boehmeria nivea L.) grown as forage in aged copper-contaminated soil. Plant Physiol Biochem 138:121–129
Rehman M, Liu L, Wang Q, Saleem MH, Bashir S, Ullah S, Peng D (2019b) Copper environmental toxicology, recent advances, and future outlook: a review. Environ Sci Pollut Res 1–14
Rehman M, Saleem MH, Fahad S, Maqbool Z, Peng D, Deng G, Liu L (2020) Medium nitrogen optimized Boehmeria nivea L. growth in copper contaminated soil. Chemosphere 128972
Riaz M, Kamran M, Fang Y, Wang Q, Cao H, Yang G, Deng L, Wang Y, Zhou Y, Anastopoulos I (2020) Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: a critical review. J Hazard Mater 123919
Rizwan M, Ali S, ur Rehman MZ, Maqbool A (2019) A critical review on the effects of zinc at toxic levels of cadmium in plants. Environ Sci Pollut Res 26:6279–6289
Roopnarain A, Ndaba B, Rama H, Obi L, Bello-Akinosho M, Akindolire M (2022) Liquid gold: harnessing the potential of digestate to enhance smallholder farmer food security and livelihood. Springer, New York, pp 313–341
Roy M, Giri AK, Dutta S, Mukherjee P (2015) Integrated phytobial remediation for sustainable management of arsenic in soil and water. Environ Int 75:180–198
Saeedi Saravi SS, Dehpour AR (2016) Potential role of organochlorine pesticides in the pathogenesis of neurodevelopmental, neurodegenerative, and neurobehavioral disorders: a review. Life Sci 145:255–264
Saini S, Kaur N, Pati PK (2021) Phytohormones: key players in the modulation of heavy metal stress tolerance in plants. Ecotoxicol Environ Saf 223:112578
Sajid M, Nazal MK, Ihsanullah BN, Osman AM (2018) Removal of heavy metals and organic pollutants from water using dendritic polymers based adsorbents: a critical review. Sep Purif Technol 191:400–423
Saleem MH, Ali S, Seleiman MF, Rizwan M, Rehman M, Akram NA, Liu L, Alotaibi M, Al-Ashkar I, Mubushar M (2019) Assessing the correlations between different traits in copper-sensitive and copper-resistant varieties of jute (Corchorus capsularis L.). Plants 8:545
Saleem MH, Ali S, Hussain S, Kamran M, Chattha MS, Ahmad S, Aqeel M, Rizwan M, Aljarba NH, Alkahtani S (2020a) Flax (Linum usitatissimum L.) a potential candidate for phytoremediation? Biological and economical points of view. Plants 9:496
Saleem MH, Ali S, Irshad S, Hussaan M, Rizwan M, Rana MS, Hashem A, Abd_Allah EF, Ahmad P (2020b) Copper uptake and accumulation, ultra-structural alteration, and bast fibre yield and quality of fibrous jute (Corchorus capsularis L.) plants grown under two different soils of China. Plants 9:404
Saleem MH, Ali S, Kamran M, Iqbal N, Azeem M, Tariq Javed M, Ali Q, Zulqurnain Haider M, Irshad S, Rizwan M (2020c) Ethylenediaminetetraacetic acid (EDTA) mitigates the toxic effect of excessive copper concentrations on growth, gaseous exchange and chloroplast ultrastructure of Corchorus capsularis L. and improves copper accumulation capabilities. Plants 9:756
Saleem MH, Ali S, Rehman M, Hasanuzzaman M, Rizwan M, Irshad S, Shafiq F, Iqbal M, Alharbi BM, Alnusaire TS (2020d) Jute: a potential candidate for phytoremediation of metals—a review. Plants 9:258
Saleem MH, Ali S, Rehman M, Rana MS, Rizwan M, Kamran M, Imran M, Riaz M, Soliman MH, Elkelish A (2020e) Influence of phosphorus on copper phytoextraction via modulating cellular organelles in two jute (Corchorus capsularis L.) varieties grown in a copper mining soil of Hubei Province, China. Chemosphere 248:126032
Saleem MH, Ali S, Rehman M, Rizwan M, Kamran M, Mohamed IA, Bamagoos AA, Alharby HF, Hakeem KR, Liu L (2020f) Individual and combined application of EDTA and citric acid assisted phytoextraction of copper using jute (Corchorus capsularis L.) seedlings. Environ Technol Innov 100895
Saleem MH, Fahad S, Adnan M, Ali M, Rana MS, Kamran M, Ali Q, Hashem IA, Bhantana P, Ali M, Hussain RM (2020g) Foliar application of gibberellic acid endorsed phytoextraction of copper and alleviates oxidative stress in jute (Corchorus capsularis L.) plant grown in highly copper-contaminated soil of China. Environ Sci Pollut Res
Saleem MH, Fahad S, Khan SU, Ahmar S, Khan MHU, Rehman M, Maqbool Z, Liu L (2020h) Morpho-physiological traits, gaseous exchange attributes, and phytoremediation potential of jute (Corchorus capsularis L.) grown in different concentrations of copper-contaminated soil. Ecotoxicol Environ Saf 189:109915
Saleem MH, Fahad S, Khan SU, Din M, Ullah A, Sabagh AEL, Hossain A, Llanes A, Liu L (2020i) Copper-induced oxidative stress, initiation of antioxidants and phytoremediation potential of flax (Linum usitatissimum L.) seedlings grown under the mixing of two different soils of China. Environ Sci Pollut Res 27:5211–5221
Saleem MH, Fahad S, Rehman M, Saud S, Jamal Y, Khan S, Liu L (2020j) Morpho-physiological traits, biochemical response and phytoextraction potential of short-term copper stress on kenaf (Hibiscus cannabinus L.) seedlings. PeerJ 8:e8321
Saleem MH, Kamran M, Zhou Y, Parveen A, Rehman M, Ahmar S, Malik Z, Mustafa A, Anjum RMA, Wang B (2020k) Appraising growth, oxidative stress and copper phytoextraction potential of flax (Linum usitatissimum L.) grown in soil differentially spiked with copper. J Environ Manag 257:109994
Saleem MH, Rehman M, Kamran M, Afzal J, Noushahi HA, Liu L (2020l) Investigating the potential of different jute varieties for phytoremediation of copper-contaminated soil. Environmental Science and Pollution Research
Saleem MH, Wang X, Ali S, Zafar S, Nawaz M, Adnan M, Fahad S, Shah A, Alyemeni MN, Hefft DI, Ali S (2021) Interactive effects of gibberellic acid and NPK on morpho-physio-biochemical traits and organic acid exudation pattern in coriander (Coriandrum sativum L.) grown in soil artificially spiked with boron. Plant Physiology and Biochemistry
Saleem MH, Mfarrej MFB, Alatawi A, Mumtaz S, Imran M, Ashraf MA, Rizwan M, Usman K, Ahmad P, Ali S (2022a) Silicon enhances morpho–physio–biochemical responses in arsenic stressed Spinach (Spinacia oleracea L.) by minimizing its uptake. J Plant Growth Regul
Saleem MH, Parveen A, Khan SU, Hussain I, Wang X, Alshaya H, El-Sheikh MA, Ali S (2022b) Silicon fertigation regimes attenuates cadmium toxicity and phytoremediation potential in two maize (Zea mays L.) cultivars by minimizing its uptake and oxidative stress. Sustainability 14:1462
Saleem MH, Zhu H, Liu L (2022c) Synergistic and sustainable impact of reducing nitrogen fertilizer on growth, yield, and quality of ramie (Boehmeria nivea L.). Plant Prod Sci
Saleem MH, Rizwan M, Zia-ul-hassan Shah ND, Usman K (2022d) Chromium toxicity in plants: consequences on growth, chromosomal behaviour and mineral nutrient status.
Sandeep G, Vijayalatha KR, Anitha T (2019) Heavy metals and its impact in vegetable crops. Int J Chem Stud 7:1612–1621
Sandil S, Kumar R (2022) Soil contamination from construction projects. Springer, New York, pp 205–244
Sarwar N, Bibi S, Ahmad M, Ok YS (2014) Effectiveness of zinc application to minimize cadmium toxicity and accumulation in wheat (Triticum aestivum L.). Environ Earth Sci 71:1663–1672
Sarwar S, Akram NA, Saleem MH, Zafar S, Alghanem SM, Abualreesh MH, Alatawi A, Ali S (2022) Spatial variations in the biochemical potential of okra [Abelmoschus esculentus L. (Moench)] leaf and fruit under field conditions. PLOS ONE 17:e0259520
Schattschneider C, Kettenmann SD, Hinojosa S, Heinrich J, Kulak N (2019) Biological activity of amphiphilic metal complexes. Coord Chem Rev 385:191–207
Selvi A, Rajasekar A, Theerthagiri J, Ananthaselvam A, Sathishkumar K, Madhavan J, Rahman PKSM (2019) Integrated remediation processes toward heavy metal removal/recovery from various environments-a review. Front Environ Sci 7:66–66
Şenkal BC, Uskutoğlu T, Cesur C, Özavci V, Doğan H (2019) Determination of essential oil components, mineral matter, and heavy metal content of Salvia virgata Jacq. grown in culture conditions. Turk J Agric for 43:395–404
Shahid M, Khalid S, Abbas G, Shahid N, Nadeem M, Sabir M, Aslam M, Dumat C (2015) Heavy metal stress and crop productivity. Crop production and global environmental issues. Springer, New York, pp 1–25
Shahid M, Rafiq M, Niazi NK, Dumat C, Shamshad S, Khalid S, Bibi I (2017) Arsenic accumulation and physiological attributes of spinach in the presence of amendments: an implication to reduce health risk. Environ Sci Pollut Res 24:16097–16106
Shahid M, Javed MT, Tanwir K, Akram MS, Tazeen SK, Saleem MH, Masood S, Mujtaba S, Chaudhary HJ (2020) Plant growth-promoting Bacillus sp. strain SDA-4 confers Cd tolerance by physio-biochemical improvements, better nutrient acquisition and diminished Cd uptake in Spinacia oleracea L. Physiol Mol Biol Plants 1–17
Shahzad B, Tanveer M, Che Z, Rehman A, Cheema SA, Sharma A, Song H, ur Rehman S, Zhaorong D (2018) Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: a review. Ecotoxicol Environ Saf 147:935–944
Sharma P, Sharma A (2022) Heavy metals in ground water affect the human health global challenge. IGI Global pp. 139–158
Shi WG, Li H, Liu TX, Polle A, Peng CH, Luo ZB (2015) Exogenous abscisic acid alleviates zinc uptake and accumulation in P opulus× canescens exposed to excess zinc. Plant Cell Environ 38:207–223
Singh DV, Bhat RA, Upadhyay AK, Singh R, Singh DP (2021) Microalgae in aquatic environs: a sustainable approach for remediation of heavy metals and emerging contaminants. Environ Technol Innov 21:101340–101340
Soliman N, Moustafa A (2020) Industrial solid waste for heavy metals adsorption features and challenges; a review. J Market Res 9:10235–10253
Sperdouli I, Adamakis I-DS, Dobrikova A, Apostolova E, Hanć A, Moustakas M (2022) Excess zinc supply reduces cadmium uptake and mitigates cadmium toxicity effects on chloroplast structure, oxidative stress, and photosystem II photochemical efficiency in Salvia sclarea plants. Toxics 10:36–36
Spinosa L, Vesilind PA (2001) Sludge into biosolids. IWA publishing, London
Sreejith KP, Sharma AK, Basu PK, Kottantharayil A (2022) Etching methods for texturing industrial multi-crystalline silicon wafers: a comprehensive review. Sol Energy Mater Sol Cells 238:111531–111531
Sterckeman T, Thomine S (2020) Mechanisms of cadmium accumulation in plants. Crit Rev Plant Sci 39:322–359
Sufyan M, Ashfaq UA, Ahmad S, Noor F, Saleem MH, Aslam MF, El-Serehy HA, Aslam S (2021) Identifying key genes and screening therapeutic agents associated with diabetes mellitus and HCV-related hepatocellular carcinoma by bioinformatics analysis. Saudi J Biol Sci
Sun X-H, Yu G, Li J-T, Jia P, Zhang J-C, Jia C-G, Zhang Y-H, Pan H-Y (2014) A heavy metal-associated protein (AcHMA1) from the halophyte, Atriplex canescens (Pursh) Nutt., confers tolerance to iron and other abiotic stresses when expressed in Saccharomyces cerevisiae. Int J Mol Sci 15:14891–14906
Sun Q, Li Y, Shi L, Hussain R, Mehmood K, Tang Z, Zhang H (2022) Heavy metals induced mitochondrial dysfunction in animals: Molecular mechanism of toxicity. Toxicology, 153136–153136
Tang L, Hamid Y, Liu D, Shohag MJI, Zehra A, He Z, Feng Y, Yang X (2020) Foliar application of zinc and selenium alleviates cadmium and lead toxicity of water spinach–bioavailability/cytotoxicity study with human cell lines. Environ Int 145:106122
Tang R, Zhu J, Liu Y, Wu N, Han J (2021) Formulation comprising arsenic trioxide and dimercaprol enhances radiosensitivity of pancreatic cancer xenografts. Technol Cancer Res Treat 20:15330338211036324–15330338211036324
Tang RH, Erskine PD, Nkrumah PN, Echevarria G, van der Ent A (2022) Soil-plant relationships of metallophytes of the zinc-lead-copper Dugald River gossan, Queensland, Australia. Plant Soil 471:227–245
Tangahu BV, Abdullah S, Rozaimah S, Basri H, Idris M, Anuar N, Mukhlisin M (2011) A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int J Chem Eng
Tanveer Y, Yasmin H, Nosheen A, Ali S, Ahmad A (2022) Ameliorative effects of plant growth promoting bacteria, zinc oxide nanoparticles and oxalic acid on Luffa acutangula grown on arsenic enriched soil. Environ Pollut 300:118889
Tariq MI, Afzal S, Hussain I, Sultana N (2007) Pesticides exposure in Pakistan: a review. Environ Int 33:1107–1122
Tariq F, Wang X, Saleem MH, Khan ZI, Ahmad K, Saleem Malik I, Munir M, Mahpara S, Mehmood N, Ahmad T, Memona H, Ugulu I, Fiaz S, Ali S (2021) Risk assessment of heavy metals in basmati rice: implications for public health. Sustainability 13:8513
Tela S, Sunday A, Manji H, Tsaware J (2012) Ethylenediaminetetraacetate (EDTA)-assisted phytoremediation of heavy metal contaminated soil by Eleusine indica L. Gearth. J Environ Chem Ecotoxicol 4:103–109
Tombarkiewicz B, Antonkiewicz J, Lis MW, Pawlak K, Trela M, Witkowicz R, Gorczyca O (2022) Chemical properties of the coffee grounds and poultry eggshells mixture in terms of soil improver. Sci Rep 12:1–10
Treviño S, Díaz A, Sánchez-Lara E, Sanchez-Gaytan BL, Perez-Aguilar JM, González-Vergara E (2019) Vanadium in biological action: chemical, pharmacological aspects, and metabolic implications in diabetes mellitus. Biol Trace Elem Res 188:68–98
Ubando AT, Africa ADM, Maniquiz-Redillas MC, Culaba AB, Chen W-H, Chang J-S (2020) Microalgal biosorption of heavy metals: a comprehensive bibliometric review. J Hazard Mater 402:123431
Uddin Nizam M, Mokhlesur Rahman M, Kim J-E (2016) Phytoremediation potential of Kenaf (Hibiscus cannabinus L.), Mesta (Hibiscus sabdariffa L.), and Jute (Corchorus capsularis L.) in arsenic-contaminated soil. Korean J Environ Agric 35:111–120
Ullah A, Farooq M, Rehman A, Hussain M, Siddique KH (2020) Zinc nutrition in chickpea (Cicer arietinum) a review. Crop Pasture Sci 71:199–218
Ullah I et al (2022) Comparative effects of biochar and NPK on wheat crops under different management systems. Crop and Pasture Science, Clayton
Umair Hassan M, Aamer M, Umer Chattha M, Haiying T, Shahzad B, Barbanti L, Nawaz M, Rasheed A, Afzal A, Liu Y (2020) The critical role of zinc in plants facing the drought stress. Agriculture 10:396
Vardhan KH, Kumar PS, Panda RC (2019) A review on heavy metal pollution, toxicity and remedial measures: current trends and future perspectives. J Mol Liq 111197
Waigi MG, Sun K, Gao Y (2017) Sphingomonads in microbe-assisted phytoremediation: tackling soil pollution. Trends Biotechnol 35:883–899
Wan X, Lei M, Chen T (2016) Cost–benefit calculation of phytoremediation technology for heavy-metal-contaminated soil. Sci Total Environ 563:796–802
Wang J, Shi L, Zhai L, Zhang H, Wang S, Zou J, Shen Z, Lian C, Chen Y (2020) Analysis of the long-term effectiveness of biochar immobilization remediation on heavy metal contaminated soil and the potential environmental factors weakening the remediation effect: a review. Ecotoxicol Environ Saf 207:111261
Wibowo H, Susanto H, Grisdanurak N, Hantoko D, Yoshikawa K, Qun H, Yan M (2021) Recent developments of deep eutectic solvent as absorbent for CO2 removal from syngas produced from gasification: current status, challenges, and further research. J Environ Chem Eng 9:105439–105439
Widyastuti S, Jupri A, Nikmatullah A, Kurniawan NSH, Kirana IAP, Abidin AS, Hernawan A, Sunarpi H, Prasedya ES (2021) Analyses of organic matter and heavy metal composition in formulated macroalgae-based organic fertilizer. IOP Publishing, Bristol, pp 12024–12024
Wu C, Li F, Yi S, Ge F (2021) Genetically engineered microbial remediation of soils co-contaminated by heavy metals and polycyclic aromatic hydrocarbons: advances and ecological risk assessment. J Environ Manag 296:113185–113185
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. Isrn Ecology
Yang Q-w, Xu Y, Liu S-j, He J-f, Long F-y (2011) Concentration and potential health risk of heavy metals in market vegetables in Chongqing, China. Ecotoxicol Environ Saf 74:1664–1669
Yang Q, Tu S, Wang G, Liao X, Yan X (2012) Effectiveness of applying arsenate reducing bacteria to enhance arsenic removal from polluted soils by Pteris vittata L. Int J Phytorem 14:89–99
Yang Z, Shi W, Yang W, Liang L, Yao W, Chai L, Gao S, Liao Q (2018) Combination of bioleaching by gross bacterial biosurfactants and flocculation: a potential remediation for the heavy metal contaminated soils. Chemosphere 206:83–91
Zaheer IE, Ali S, Rizwan M, Farid M, Shakoor MB, Gill RA, Najeeb U, Iqbal N, Ahmad R (2015) Citric acid assisted phytoremediation of copper by Brassica napus L. Ecotoxicol Environ Saf 120:310–317
Zaheer IE, Ali S, Saleem MH, Ali M, Riaz M, Javed S, Sehar A, Abbas Z, Rizwan M, El-Sheikh MA, Alyemeni MN (2020a) Interactive role of zinc and iron lysine on Spinacia oleracea L. growth, photosynthesis and antioxidant capacity irrigated with tannery wastewater. Physiol Mol Biol Plants
Zaheer IE, Ali S, Saleem MH, Noor I, El-Esawi MA, Hayat K, Rizwan M, Abbas Z, El-Sheikh MA, Alyemeni MN (2020b) Iron–lysine mediated alleviation of chromium toxicity in Spinach (Spinacia oleracea L.) plants in relation to morpho-physiological traits and iron uptake when irrigated with tannery wastewater. Sustainability 12:6690
Zaheer IE, Ali S, Saleem MH, Yousaf HS, Malik A, Abbas Z, Rizwan M, Abualreesh MH, Alatawi A, Wang X (2022) Combined application of zinc and iron-lysine and its effects on morpho-physiological traits, antioxidant capacity and chromium uptake in rapeseed (Brassica napus L.). PLoS ONE 17:e0262140
Zamora-Ledezma C, Negrete-Bolagay D, Figueroa F, Zamora-Ledezma E, Ni M, Alexis F, Guerrero VH (2021) Heavy metal water pollution: a fresh look about hazards, novel and conventional remediation methods. Environ Technol Innov 22:101504–101504
Zhang Y, Zhou M (2019) A critical review of the application of chelating agents to enable Fenton and Fenton-like reactions at high pH values. J Hazard Mater 362:436–450
Zhang T, Liu J-M, Huang X-F, Xia B, Su C-Y, Luo G-F, Xu Y-W, Wu Y-X, Mao Z-W, Qiu R-L (2013) Chelant extraction of heavy metals from contaminated soils using new selective EDTA derivatives. J Hazard Mater 262:464–471
Zhang YY, Stockmann R, Ng K, Ajlouni S (2021) Opportunities for plant-derived enhancers for iron, zinc, and calcium bioavailability: a review. Compr Rev Food Sci Food Saf 20:652–685
Zhu F, Zheng Y-M, Zhang B-G, Dai Y-R (2020) A critical review on the electrospun nanofibrous membranes for the adsorption of heavy metals in water treatment. J Hazard Mater 401:123608
Zubrod JP, Bundschuh M, Arts G, Brühl CA, Imfeld G, Knäbel A, Payraudeau S, Rasmussen JJ, Rohr J, Scharmüller A (2019) Fungicides: an overlooked pesticide class? Environ Sci Technol 53:3347–3365
Zvezdanović J, Marković D, Nikolić G (2007) Different possibilities for the formation of complexes of copper and zinc with chlorophyll inside photosynthetic organelles: chloroplasts and thylakoids. J Serbian Chem Soc 72:1053–1062
Acknowledgements
This work was supported by the Qatar University vegetable factory project QUEX-CAS-MJF-VF-18/19.
Funding
Open Access funding provided by the Qatar National Library.
Author information
Authors and Affiliations
Contributions
MHS, KU, and MA conceived and designed the article and HAJ critically revised the manuscript and approved the final version. MHS and KU, MA wrote the manuscript. MR and HAJ critically edited and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no competing interest in the publication of this manuscript.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Written consent was sought from each author to publish the manuscript.
Data Availability
Data and material is available for research purpose and for reference.
Additional information
Handling Editor: Saddam Hussain.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alsafran, M., Saleem, M.H., Al Jabri, H. et al. Principles and Applicability of Integrated Remediation Strategies for Heavy Metal Removal/Recovery from Contaminated Environments. J Plant Growth Regul 42, 3419–3440 (2023). https://doi.org/10.1007/s00344-022-10803-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-022-10803-1