Abstract
In this study, we investigated the physiological responses of maize with different amylose content at seedlings to drought stress. For waxy maize (WMS) and normal maize (NMS), the decline of photosynthesis under drought stress (DS) was due to the stomatal limitation. DS increased the non-photochemical quenching coefficient (NPQ), whereas decreased the activities of peroxidase (POD) and the plant height (PH), compared with the plants under normal irrigation. The content of starch increased and decreased significantly upon moderate and severe drought stress, respectively. For high amylose maize (HAMSs), they showed stomatal limitation upon moderate stress, while non-stomatal limitation upon severe stress. The NPQ and POD showed contrary trend compared with WMS and NMS. DS significantly decreased the starch content and PH of them. The principal component analysis (PCA) showed HAMSs were more sensitive to drought than WMS and NMS. The GBSSIIa level of HAMSs was also lower than that of WMS and NMS. Therefore, we conclude that HAMSs respond to DS through redox regulation to avoid oxidative damage, whereas WMS and NMS by increasing starch biosynthesis, and the higher GBSSIIa level may produce more amylose, which could promote the growth of maize under drought effectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maize, a main crop over the world, has a great economic value. It can be widely applied into different areas (e.g., food, industrial materials, feed) (Huang et al. 2021). Nevertheless, it is also affected by different abiotic stresses during the growth, including drought, salt, and high temperature (Li et al. 2017). Drought stress plays a key role in these abiotic stresses (Zhang et al. 2020). It had reported that drought stress could reduce the content of chlorophyll, resulting in the destruction of photosynthesis (Naeem et al. 2018). Meanwhile, oxidative stress would also happen along with drought stress. It generates a lot of reactive oxygen species (ROS), which could result in membrane lipid peroxidation, protein degradation, DNA fragmentation, and cells death (Cui et al. 2017). All of those could affect the division, elongation, and differentiation of cells, which limits the growth of plants and reduce the yields (Kamran et al. 2018).
Maize also could respond to drought by a series of mechanisms, including redox regulation and osmotic regulation (Farooq et al. 2009). Redox regulation mainly clears ROS under the action of enzymes, such as superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT), to mitigate the oxidative damage on cells, and ensure the normal function of cells upon drought stress (Horváth et al. 2007). The previous researches showed the activities of SOD, POD, and CAT increased and decreased upon moderate drought and severe drought, respectively (Zhang et al. 2011). Meanwhile, plants also can avoid the death of cells because of water-shortage, through the accumulation of cytoplasmic solutes (e.g., proline, sucrose, soluble carbohydrate) (Anjum et al. 2011b). Among these cytoplasmic solutes, the content of proline was usually used to assess drought-resistance. The increase of proline can keep the balance of osmotic potential between protoplasm and environment (Szabados and Savouré 2010). It had been demonstrated that the content of proline increased significantly along with the duration of drought, and the peak was obtained at 10 days after treatment (Anjum et al. 2011a). In most species, plants also can release energy, sugar, and metabolite derivatives by the starch metabolism, all of which can be used as osmotic protectants and compatible solutes to weaken the damage of abiotic stresses (Thalmann and Santelia 2017). This is a basic adaptation process for plants and meaningful for the productivity of plants upon unfavorable environments (Krasensky and Jonak 2012). It had reported that the starch biosynthesis of leaf is helpful to maintain the leaf growth and photosynthesis under drought stress in maize (AbdElgawad et al. 2020).
Maize could be divided into waxy maize, normal maize, and high amylose maize based on the different amylose content in grains (Zhong et al. 2022). Waxy maize and high amylose maize are more widely used than normal maize. For example, waxy maize can not only be used as fresh-eating maize (Ketthaisong et al. 2014), but also can be applied into industry (adhesive) (Onusseit 1992). As for high amylose maize, it can be applied as functional food because of its digestion-resistance (Li et al. 2019). The physiological responses of maize seedlings to drought stress can affect the following growth, and then affect the yield (Aslam et al. 2015). However, the present researches mainly focus on normal maize (Ahmad et al. 2019; Anjum et al. 2011a; Moharramnejad et al. 2019). There are little researches about waxy maize and high amylose maize, which limits the processes of breeding and the determination of suitable cultivable measures, and also affected the application of these maize varieties. Therefore, in this study, we select four maize varieties (a waxy maize variety, a normal maize variety, and two high amylose maize varieties) to explore the physiological responses of maize with different amylose content upon drought stress in seedlings, thus providing theoretical bases for the determination of cultivable measures of maize upon drought stress.
Materials and Methods
Materials
Waxy maize variety (Shan Cainuo1911), normal maize variety (Xin Yu108), and high amylose maize (NAFU40 and NAFU50) were provided by the Maize Genetic Breeding Laboratory from Northwest A&F University in China.
The abbreviations of waxy maize, normal maize, and high amylose maize variety are WMS, NMS, and HAMSs, respectively.
Maize seeds were grown in pots (28 cm*30 cm), which contained 4.5 kg soil (soil:sand = 1:1). The determination of maximum water holding capacity (WHC) of the soil was according to the method described before (Ogbaga et al. 2014). Those four maize varieties were treated with three different drought stress (DS) levels: normal irrigation treatment (70% WHC), moderate DS treatment (50% WHC), and severe DS treatment (30% WHC). Four biological replicates were set for per maize variety under each treatment, and only one robust maize plant was retained per pot when the third leaf was fully expanded. The soils were fertilized with 1.17 g N, 2.96 g P, 1.17 g K, 0.52 g Mg, 2.02 g S, 0.345 g Ga, 0.01 g Fe, 1.08 × 10–5 g Cu, 0.75 × 10–4 g Mn, 0.27 × 10–5 g Mo, 0.75 × 10–5 g Zn, and 2.04 × 10–5 g B during the whole growth period. The pots were placed in a greenhouse at 28 ℃/23 ℃ (day/night) with a 16 h/8 h (light/dark) cycle, 60% humidity and 400 μmol photons m–2 s–1 light intensity. The pots were rearranged randomly once a week to avoid positional influences (Kränzlein et al. 2021).
Drought Treatment
The drought stress (DS) treatment was initiated when the ninth leaves of all varieties were expanded completely (Fig. 1). DS treatment was performed through natural drying and the loss of water was calculated by weighing each pot every day (Chen et al. 2016; Ogbaga et al. 2014). The normal irrigation treatment maintained the WHC of 70%. For the severe DS treatment, no watering was performed after drought treatment, while for the moderate DS treatment, pots were kept under normal watering conditions until 4 days after treatment (DAT). At the 8th DAT, the moderate and severe DS treatment samples reached the target degree of drought, and maintained the corresponding WHC for the next 5 days by weighing the pots and irrigating the required amount of water to maintain the soil water levels (Fig. 1). The ninth leaves of different biological replicates from three treatments were sampled at the 12th DAT. They were frozen immediately in liquid nitrogen and stored at −80 ℃ for future research.
Measurement of Gas Exchange and Chlorophyll Fluorescence Parameters
During the period of maintaining the target drought level, gas change parameters of the ninth leaves were determined by plant photosynthetic analyzer (Li-Cor 6400, USA) on a sunny day from 8:00 to 12:00am. The photosynthetic rate (Pn), transpiration rate (Tr), Stomatal conductance (Cond), and intercellular carbon dioxide concentration (Ci) were obtained.
On the same day, chlorophyll fluorescence parameters of samples were also determined by chlorophyll fluorometer (Dual-PAM 100, Germany). The primary light energy conversion efficiency (Fv/Fm), non-photochemical quenching coefficient (NPQ), photochemical quenching coefficient (PQ), and photochemical quantum yield (YII) were obtained.
Measurement of Plant Growth
The plant heights of samples were determined by the distance from the base to the youngest elongating leaf of plants using a ruler at harvest (Kränzlein et al. 2021). The fresh-weight of aboveground samples were determined immediately at harvest and then dried to constant weight in oven at 105 °C, which is the dry-weight of the samples (Kränzlein et al. 2021).
Measurement of Antioxidant Enzyme Activity and the Content of Malondialdehyde (MDA)
The measurement of SOD activity was according to the method of nitroblue tetrazole (NBT) photoreduction (Giannopolitis and Ries 1977). The POD activity was determined by following the method of guaiacol (Tewari et al. 2005) and the content of MDA was determined by the method of thiobarbituric acid (Cakmak and Marschner 1992).
Measurement of the Content of Sucrose, Starch, and Proline
The contents of sucrose and starch were determined according to the previous research (Leach and Braun 2016). 100 mg leaves (avoid vein) were ground into powder using mortar and pestle, then the powder was homogenized with 1 mL starch extraction buffer (MCW). The homogenate was transferred into centrifugal tube and centrifuged at 14000 rpm for 5 min, and the supernatant was transferred into a new tube. The sediment was added into 1 mL MCW and centrifuged at 14000 rpm for 5 min, and the supernatant was also transferred into the new tube, this process was repeated three times. The content of sucrose was determined using the supernatant and the sediment was used to determine the content of starch. Both of them were determined by K-SUFRG kit (Megazyme, Ireland) according to manufacturer’s instructions.
The content of proline was determined by the method of sulfosalicylic acid (Tiwari et al. 2010).
RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
Total RNA of maize leaves from each treatment was extracted using an RNAprep Pure Plant Plus Kit (Tiangen, Beijing, China) according to the manufacturer’s instructions. The quantity and quality of RNA were assessed with an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA), a NanoDrop spectrophotometer (Thermo Fisher Scientific Inc., Shanghai, China) and 1% agarose gel electrophoresis. Total RNA (10 μg per sample) was reverse transcribed using a Fastking RT Kit (Tiangen, Beijing, China) following the manufacturer’s instructions (10 μL of reaction mix, containing 2 μL of 10 × Fast RT Buffer, 1 μL of RT Enzyme Mix, 2 μL of FQ-RT Primer Mix, and 5 μL of RNase-Free ddH2O). Gene-specific primers were designed using Primer Premier 5.0 software (Premier, CAN). Primers are listed in Table S1.
The SuperReal PreMix Color (SYBR Green) kit (Tiangen, Beijing, China) was used for qRT-PCR with a total volume of 20 μL, including 2 × SuperReal Color Premix (10 μL), 50 × ROX Reference Dye (0.4 μL), forward primer (0.6 μL), reverse primer (0.6 μL), cDNA (2 μL), and RNase-free ddH2O (6.4 μL). The thermal cycling conditions were as follows: 95 ℃ for 15 min, then 40 cycles at 95 ℃ for 10 s, and 66 ℃ for 32 s. The housekeeping gene actin (Zm00001d032480) was used as an endogenous reference and an internal control to normalize the CT values of the target genes in the same run. The relative expression level was calculated by the 2−ΔΔCT method (Livak and Schmittgen 2001).
Statistical Analysis
All experiments were conducted with technical replicates (n = 3). Differences were analyzed using one-way analyses of variance (ANOVAs) followed by LSD test (p < 0.05) in SPSS 22.0 (SPSS Inc., Chicago, IL, USA).
Results
Effects of Drought Stress on the Photosynthesis of Maize
Drought stress decreased the photosynthetic rate (Pn) of maize with different amylose content (expect for WMS under moderate stress), and the decrease of both WMS and NAFU40 upon severe stress were significant (Fig. 2a). The transpiration rate (Tr) (Fig. 2b) and stomatal conductance (Cond) (Fig. 2c) showed a similar trend with Pn under drought stress, and only the Tr of NMS decreased significantly under severe stress, whereas the Cond of all maize varieties were decreased prominently upon severe stress. As for the intercellular carbon dioxide concentration (Ci), the drought stress had negligible effects on Ci of the four maize varieties (except for WMS under moderate stress) (Fig. 2d).
Effects of Drought Stress on the Chlorophyll Fluorescence of Maize
The primary light energy conversion efficiency (Fv/Fm) of the four maize varieties decreased upon drought stress, and only NAFU40 showed significant decrease under severe stress (Fig. 3a). The non-photochemical quenching coefficient (NPQ) of WMS and NMS increased, whereas HAMSs showed an opposite trend upon drought stress (only NAFU40 decreased prominently under moderate stress) (Fig. 3b). As for the photochemical quenching coefficient (QP), WMS and NAFU40 increased (only NAFU40 increased significantly under drought stress), whereas NMS and NAFU50 decreased under drought stress (Fig. 3c). The photochemical quantum yield (YII) showed a similar trend with QP, and drought stress had negligible effects on it (Fig. 3d).
The chlorophyll fluorescence parameters of different maize varieties under drought stress a primary light energy conversion efficiency (Fv/Fm); b non-photochemical quenching coefficient (NPQ); c photochemical quenching coefficient (QP); d photochemical quantum yield (YII). WMS waxy maize; NMS normal maize; NAFU40 High amylose I; NAFU50 High amylose II
Effects of Drought Stress on the Antioxidant Enzyme Activity and the MDA Content of Maize
The change trends of SOD activity of the four maize varieties were same upon drought, which increased under moderate stress and decreased under severe stress, and WMS and NAFU50 decreased significantly upon severe stress (Fig. 4a). The POD activity decreased in WMS and NMS, whereas it increased in HAMSs upon drought stress (drought stress had significant effects on NMS and NAFU50) (Fig. 4b). The content of MDA was almost not affected by drought stress in WMS and NAFU40, while it decreased prominently in NAFU50. It is worth to note that the content of MDA decreased significantly upon moderate stress and increased significantly upon severe stress in NMS (Fig. 4c).
Effects of Drought Stress on the Content of Proline, Sucrose and Starch of Maize
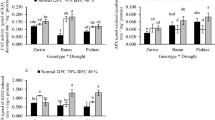
Under moderate drought stress, the content of proline was not significantly altered in WMS and NAFU40, whereas it increased significantly in NMS and NAFU50. The content of proline of the four maize varieties decreased prominently upon severe drought stress (Fig. 5c). The content of starch increased significantly in WMS and NMS, and HAMSs showed a contrary trend upon moderate stress, and it decreased significantly in the four maize varieties under severe stress (Fig. 5a). The content of sucrose increased prominently in WMS and NAFU40 under drought stress, whereas NMS showed a contrary trend. Drought stress had negligible effects on the content of sucrose of NAFU50 (Fig. 5b).
Effects of Drought Stress on the Growth of Maize
The fresh-weight (FW) of WMS and NAFU50 decreased significantly under drought stress, whereas NMS and NAFU40 were almost not affected (Fig. 6a). The changes of dry-weight (DW) in the four maize varieties showed the same trend under drought stress (Fig. 6b). The changes (compared to the plants with normal irrigation) of biomass (FW and DW) followed the order: NMS < NAFU40 < WMS < NAFU50 (Table 1). Except for NMS (under moderate drought stress), drought stress decreased the plant height (PH) of WMS, NMS, and NAFU40, whereas it had negligible effects on NAFU50 (Fig. 6c). The changes (compared to the plants with normal irrigation) of PH of WMS and NMS were less than HAMSs under drought stress (Table 1).
Effects of Drought Stress on the Relative Expression of GBSSIIa in Maize
The relative expression of GBSSIIa of WMS and NMS was affected significantly under drought stress (DS). DS decreased the GBSSIIa expression level significantly under moderate stress, whereas it recovered to normal level under severe stress in NMS. For NMS, the GBSSIIa expression level increased significantly with the increasing of drought degree. However, DS almost did not change the GBSSIIa level of HAMSs (except for NAFU40 under severe stress). It is worth noting that the GBSSIIa expression level of HAMSs is consistently lower than WMS and NMS (Fig. 7). We suggest that the different changes of the four maize varieties mainly due to the different genetic background (Zhong et al. 2021).
Principal Component Analysis (PCA)
The results of PCA (Fig. 7) based on all the physiological indices showed that (1) PC1and PC2 explained 34.3% and 21.1% of the total observed variance, respectively. (2) There are two groups, group 1 contained the four maize varieties under normal irrigation and both WMS and NMS under moderate drought stress, and group 2 contained the four maize varieties under severe drought stress and HAMSs under moderate drought stress. Therefore, we suggest that PC1 grouped the samples based on the degree of drought stress. It is worth noting that both WMS and NMS under moderate drought were located in the normal irrigation group, and HAMSs under moderate drought were located in the severe drought group. This indicated that HAMSs could be more sensitive to drought stress than WMS and NMS.
Discussion
HAMSs Could be More Sensitive to Drought Stress than WMS and NMS
The effects of drought stress on the photosynthesis of plants include stomatal limitation and non-stomatal limitation (Chaves et al. 2009). It had reported that the decrease of photosynthesis of plants is mainly due to the stomatal limitation upon moderate drought stress, and non-stomatal limitation upon severe drought stress (Efeoğlu et al. 2009). Our data showed that the Pn, Cond, and Ci of the four maize varieties (expect for WMS) decreased upon moderate drought stress (Fig. 2), which demonstrated that the stomatal limitation here was responsible for the decline of photosynthesis (Kazakov et al. 1986). It is worth noting that both the Cond and Ci increased significantly upon moderate drought stress in WMS. We suggest that this is the main reason for the increase of photosynthesis of WMS under moderate stress, because it could provide more CO2 as a substrate for the photosynthesis of plants (Campbell et al. 1988). However, for HMASs under severe drought stress, both the Pn and Cond decreased while Ci increased (Fig. 2), which suggests that the main limitation of photosynthesis had shifted from stomatal limitation to non-stomatal limitation (Kazakov et al. 1986). Nevertheless, both WMS and NMS still kept stomatal limitation under severe drought stress (Fig. 2), which indirectly demonstrated that HAMSs were more sensitive to drought stress than WMS and NMS. It is also in accordance with the results of PCA (Fig. 8).
The non-photochemical quenching coefficient (NPQ) is the luminous energy captured by antenna pigment, while it cannot be applied to the transport of photosynthetic electron, and lost as heat energy. This is a protection mechanism of plants to avoid the damage of photosynthesis (Niyogi and Truong 2013). Our data showed the NPQ increased in both WMS and NMS upon drought stress (Fig. 3), which means that their photosynthesis was protected. However, HAMSs showed a contrary trend under drought stress (Fig. 3), which indicated that their photosynthesis may be destroyed upon drought stress, as observed in the results of photosynthesis. The photosynthesis of WMS and NMS were protected under drought stress, which lead to that they still showed stomatal limitation upon severe drought stress. As for HAMSs, it showed stomatal limitation upon moderate drought stress, whereas non-stomatal limitation upon severe drought stress.
Different Strategies of HAMSs, WMS and NMS in Response to Drought Stress
In this study, the SOD activity of the four maize varieties increased and decreased upon moderate and severe drought stress, respectively (Fig. 4a), which was according with the previous research (Zhang et al. 2011). Our data showed the POD activity of HAMSs increased under drought stress, whereas WMS and NMS showed a contrary trend (Fig. 4b). Therefore, we suggest that HAMSs mainly respond to drought stress by redox regulation. To demonstrate this possibility, we analyzed the results of MDA, because the accumulation of ROS in plants will result in membrane lipid peroxidation, thus producing a large amount of MDA (Anjum et al. 2011b). The MDA content decreased in HAMSs under drought stress, which demonstrated our speculation (Fig. 4c). The results of correlation analysis between POD and MDA (− 0.69) also demonstrated this (Table S2). It is worth noting that the POD activity of NMS decreased (Fig. 4b), whereas its MDA content also decreased significantly upon moderate drought stress (Fig. 4c). We suggest that it was due to the increase of the proline content (Fig. 5). It had reported that the water loss of cells will lead to the break of vacuolar membrane and the damage of lipid membrane structure, which will also increase the content of MDA (Yao et al. 1993). Nevertheless, the proline is an important osmoregulation substance, which could avoid the death of cells because of water-deficiency (Szabados and Savouré 2010). Therefore, the significant increase of proline in NMS upon moderate stress can avoid the water-deficiency of cells, thus decreasing the content of MDA. Meanwhile, the decrease of proline content and increase of MDA content in NMS upon severe stress also demonstrated this indirectly.
The content of leaf starch increased in WMS and NMS upon moderate drought stress, whereas HAMSs showed an opposite trend (Fig. 5a). We suggest that the increase of starch content was the main reason that why WMS and NMS were more drought-resistance compared with HAMSs, because the starch can not only promote the growth of plants at night, but also can as a kind of osmoregulation substance to avoid the death of cells due to water-deficiency (Krasensky and Jonak 2012). Our data showed the plant height (PH) of WMS decreased 0.8% and NMS increased 2.01% compared with the plants with normal irrigation under moderate drought stress, whereas NAFU40 and NAFU50 decreased 4.54% and 4.11%, respectively (Table 1). We suggest that the increase of starch promote the growth of WMS and NMS, which results in the less PH loss under drought stress. The previous researches also demonstrated that the starch biosynthesis is helpful to maintain photosynthesis and growth of leaves in maize upon drought stress (AbdElgawad et al. 2020). However, the changes of biomass showed the following trend: NMS < NAFU40 < WMS < NAFU50 (Table 1). This may be due to that the accumulation of biomass of plants was controlled by multiple parameters, and it cannot be used to evaluate drought-resistance alone (Kränzlein et al. 2021).
The Role of Amylose in Drought-Resistance of Maize
Starch is composed of amylose and amylopectin (Bertoft 2017), and amylopectin is larger than amylose, the molecular weight of amylopectin is about 107–109 Daltons, whereas amylose only has 106 Daltons (Gilbert et al. 2013). The amylose plays a key role in starch, which determines the properties of starch (Zhou et al. 2015). However, in this study, due to the limitation of greenhouse, it is hard to isolate starch and determine the content of amylose (AC) in leaves. Therefore, we use the relative expression level of GBSSIIa, which is mainly responsible for the biosynthesis of amylose in leaves of plants (Vrinten and Nakamura 2000), to reflect the AC in leaves in our study. It is worth noting that the HAMSs expression level is lower than WMS and NMS significantly (Fig. 7), which means that HAMSs may produce less amylose. This is contrary with the amylose in seeds (Zhong et al. 2022). It is an interesting finding and the reason for this we are exploring in an individual study. Based on this result, we suggest that the higher AC in leaves may contribute to the drought-resistance in maize. To verify this possibility, we analyzed the correlation between the relative expression level of GBSSIIa and the physiological indices. The results showed that GBSSIIa showed a positive correlation with plant height (0.88), dry weight (0.79), fresh weight (0.66), and MDA (0.73), whereas negative correlation with Pn (− 0.74), Co (− 0.60), Tr (− 0.69), and Pro (− 0.77) (Table S3). A possible explanation is that the amylose could be hydrolyzed easily because of the smaller molecular weight, and also could provide energy to plants quickly. It is helpful for plants to grow and accumulate biomass under drought stress. Meanwhile, the degradation of amylose also contributes to osmotic regulation of plant cells by increasing intracelluar soultes (Thalmann and Santelia 2017), which could instead the function of proline to some extent. Starch is the production of photosynthesis (Nakano et al., 2000), and we also suggest that the amylose could be biosynthesized under weaker photosynthesis because of its lower molecular weight. However, due to the limitation of this study, it needs to be demonstrated in the future study. As discussed above, the plants with higher GBSSIIa had lower POD activity, which could not clear the MDA efficiently.
Therefore, HAMSs mainly respond to drought stress through redox regulation, while both WMS and NMS upon drought stress by increasing starch biosynthesis. Meanwhile, it had the higher GBSSIIa expression level in them, which contributes to produce more amylose in leaves, and it is helpful for maize to resist drought efficiently (Fig. 9).
Conclusion
This study mainly explored the physiological responses of maize with different amylose content to drought stress in seedlings. HAMSs were more sensitive to drought than WMS and NMS. Both WMS and NMS showed stomatal limitation under two drought levels, whereas HAMSs showed stomatal limitation and non-stomatal limitation under moderate and severe stress, respectively. HAMSs respond to DS by increasing the POD activity to avoid oxidative damage. Both WMS and NMS respond to drought stress by the increase of starch content, and the higher GBSSIIa expression level may lead to the production of more amylose, which could promote the growth of maize under drought effectively.
References
AbdElgawad H, Avramova V, Baggerman G, Van Raemdonck G, Valkenborg D, Van Ostade X, Guisez Y, Prinsen E, Asard H, Van den Ende WGTS, Beemster (2020) Starch biosynthesis contributes to the maintenance of photosynthesis and leaf growth under drought stress in maize. Plant Cell Environ 43(9):2254–2271
Ahmad S, Kamran M, Ding R, Meng X, Wang H, Ahmad I, Fahad S, Han Q (2019) Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. PeerJ 7:e7793
Anjum SA, Wang L, Farooq M, Khan I, Xue L (2011a) Methyl jasmonate-induced alteration in lipid peroxidation, antioxidative defence system and yield in soybean under drought. J Agron Crop Sci 197(4):296–301
Anjum SA, Xie X, Wang L, Saleem MF, Man C, Lei W (2011b) Morphological, physiological and biochemical responses of plants to drought stress. Afr J Agric Res 6(9):2026–2032
Aslam M, Maqbool MA, Cengiz R (2015) Drought stress in maize (zea maysl.) Effects, resistance mechanisms, global achievements and biological strategies for improvement. Springer, Berlin
Bertoft E (2017) Understanding starch structure: recent progress. Agronomy 7(3):56
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98(4):1222–1227
Campbell WJ, Allen L Jr, Bowes G (1988) Effects of CO2 concentration on rubisco activity, amount, and photosynthesis in soybean leaves. Plant Physiol 88(4):1310–1316
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103(4):551–560
Chen D, Wang S, Cao B, Cao D, Leng G, Li H, Yin L, Shan L, Deng X (2016) Genotypic variation in growth and physiological response to drought stress and re-watering reveals the critical role of recovery in drought adaptation in maize seedlings. Front Plant Sci 6:1241
Cui G, Zhao X, Liu S, Sun F, Zhang C, Xi Y (2017) Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol Biochem 118:138–149
Efeoğlu B, Ekmekçi Y, Çiçek N (2009) Physiological responses of three maize cultivars to drought stress and recovery. S Afr J Bot 75(1):34–42
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra S (2009) Plant drought stress: effects, mechanisms and management. Sustainable agriculture. Springer, Berlin, pp 153–188
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: i occurrence in higher plants. Plant Physiol 59(2):309–314
Gilbert RG, Witt T, Hasjim J (2013) What is being learned about starch properties from multiple-level characterization. Cereal Chem 90(4):312–325
Horváth E, Pál M, Szalai G, Páldi E, Janda T (2007) Exogenous 4-hydroxybenzoic acid and salicylic acid modulate the effect of short-term drought and freezing stress on wheat plants. Biol Plant 51(3):480–487
Huang L, Tan H, Zhang C, Li Q, Liu Q (2021) Starch biosynthesis in cereal endosperms: an updated review over the last decade. Plant Commun 2(5):100237
Kamran M, Wennan S, Ahmad I, Xiangping M, Wenwen C, Xudong Z, Siwei M, Khan A, Qingfang H, Tiening L (2018) Application of paclobutrazol affect maize grain yield by regulating root morphological and physiological characteristics under a semi-arid region. Sci Rep 8(1):1–15
Kazakov E, Kazakova S, Guliaev B (1986) Effect and aftereffect of drought on photosynthesis of leaves in sugar beet ontogenesis. Fiziologiia i Biokhimiia Kul’turnykh Rastenii 18(5):459–467
Ketthaisong D, Suriharn B, Tangwongchai R, Lertrat K (2014) Changes in physicochemical properties of waxy corn starches after harvest, and in mechanical properties of fresh cooked kernels during storage. Food Chem 151:561–567
Kränzlein M, Geilfus CM, Franzisky BL, Zhang X, Wimmer MA, Zörb C (2021) Physiological responses of contrasting maize (Zea mays L.) hybrids to repeated drought. J Plant Growth Regul. https://doi.org/10.1007/s00344-021-10468-2
Krasensky J, Jonak C (2012) Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot 63(4):1593–1608
Leach KA, Braun DM (2016) Soluble sugar and starch extraction and quantification from maize (Zea mays) leaves. Curr Protoc Plant Biol 1(1):139–161
Li P, Cao W, Fang H, Xu S, Yin S, Zhang Y, Lin D, Wang J, Chen Y, Xu C, Yang Z (2017) Transcriptomic profiling of the maize (Zea mays L.) leaf response to abiotic stresses at the seedling stage. Frontiers Plant Sci 8:290. https://doi.org/10.3389/fpls.2017.00290
Li H, Gidley MJ, Dhital S (2019) High-amylose starches to bridge the “Fiber Gap”: development, structure, and nutritional functionality. Compr Rev Food Sci Food Saf 18(2):362–379
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4):402–408
Moharramnejad S, Sofalian O, Valizadeh M, Asghari A, Shiri M, Ashraf M (2019) Response of maize to field drought stress: oxidative defense system, osmolytes’ accumulation and photosynthetic pigments. Pak J Bot 51(3):799–807
Naeem M, Naeem MS, Ahmad R, Ahmad R, Ashraf MY, Ihsan MZ, Nawaz F, H-u-R A, Ashraf M, Abbas HT, Abdullah M (2018) Improving drought tolerance in maize by foliar application of boron: water status, antioxidative defense and photosynthetic capacity. Arch Agron Soil Sci 64(5):626–639
Nakano H, Muramatsu S, Makino A, Mae T (2000) Relationship between the suppression of photosynthesis and starch accumulation in the pod-removed bean. Funct Plant Biol 27(2):167–173
Niyogi KK, Truong TB (2013) Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr Opin Plant Biol 16(3):307–314
Ogbaga CC, Stepien P, Johnson GN (2014) Sorghum (Sorghum bicolor) varieties adopt strongly contrasting strategies in response to drought. Physiol Plant 152(2):389–401
Onusseit H (1992) Starch in industrial adhesives: new developments. Ind Crops Prod 1(2–4):141–146
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15(2):89–97
Tewari RK, Kumar P, Sharma PN (2005) Signs of oxidative stress in the chlorotic leaves of iron starved plants. Plant Sci 169(6):1037–1045
Thalmann M, Santelia D (2017) Starch as a determinant of plant fitness under abiotic stress. New Phytol 214(3):943–951
Tiwari JK, Munshi AD, Kumar R, Pandey RN, Arora A, Bhat JS, Sureja AK (2010) Effect of salt stress on cucumber: Na+–K+ ratio, osmolyte concentration, phenols and chlorophyll content. Acta Physiol Plant 32(1):103–114
Vrinten PL, Nakamura T (2000) Wheat granule-bound starch synthase I and II are encoded by separate genes that are expressed in different tissues. Plant Physiol 122(1):255–264
Yao Y, Wang H, Hu D, Wang Z, Jiang X (1993) Relationship between ultrastructrue of wheat mesophyll cell under water stress and drought-resistance. Acta Botanica Boreall-Occidentalla Sinica 13(1):16–20
Zhang R, Zheng Y, Ma G, Zhang X, Lu H, Shi J, Xue J (2011) Effects of drought stress on photosynthetic traits and protective enzyme activty in maize seeding. Acta Ecol Sin 31(5):1303–1311
Zhang X, Mi Y, Mao H, Liu S, Chen L, Qin F (2020) Genetic variation in ZmTIP1 contributes to root hair elongation and drought tolerance in maize. Plant Biotechnol J 18(5):1271–1283
Zhong Y, Qu J, Blennow A, Liu X, Guo D (2021) Expression pattern of starch biosynthesis genes in relation to the starch molecular structure in high-amylose maize. J Agric Food Chem 69(9):2805–2815
Zhong Y, Qu J, Liu X, Ding L, Liu Y, Bertoft E, Petersen BL, Hamaker BR, Hebelstrup KH, Blennow A (2022) Different genetic strategies to generate high amylose starch mutants by engineering the starch biosynthetic pathways. Carbohydr Polym 287:119327. https://doi.org/10.1016/j.carbpol.2022.119327
Zhou W, Yang J, Hong Y, Liu G, Zheng J, Gu Z, Zhang P (2015) Impact of amylose content on starch physicochemical properties in transgenic sweet potato. Carbohyd Polym 122:417–427
Acknowledgements
This work was financially supported by 2021 Agricultural Technology and Innovation Program (NYKJ-2021-YL(XN)13) of Department of Agriculture and Rural Affairs of Shaanxi Province, China, Special corn germplasm creation, Variety Breeding and Industrialization Demonstration (2021ZDLNY01-08) and Construction of Agricultural Science and Technology Demonstration Park in Kazakhstan (Z2220121005).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
WW contributed to conceptualization, visualization, investigation, writing original draft, preparation, writing review, and editing. RX contributed to the investigation. NL contributed to writing review and editing. MZ, YS, and YD contributed to the Investigation. JX contributed to writing review and editing. XZ contributed to the conceptualization. DG contributed to conceptualization, funding acquisition, and writing review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
Handling Editor: Serena Varotto.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, W., Xu, R., Liu, N. et al. The Physiological Responses of Maize Seedlings with Different Amylose Content to Drought Stress. J Plant Growth Regul 42, 3291–3301 (2023). https://doi.org/10.1007/s00344-022-10790-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-022-10790-3