Abstract
Previous studies have shown the great potential of using plasma-activated water (PAW) on improving agriculture seed germination, however, information on the influence of PAW on crop plantlet juice remains scanty. In this research, the effect of PAW generated by atmosphere pressure Ar–O2 plasma jet for 1–5 min on wheat seed germination, seedling growth and nutritional properties of wheat plantlet juice was investigated. Results revealed that all PAWs could enhance wheat seed germination and seedling growth in 7 days by improving the germination rate, germination index, fresh weight, dry weight and vigour index, and especially that PAW activated for 3 min (PAW-3) showed the best overall performance. In addition, the application of PAWs enhanced the nutritional properties of wheat plantlet juice from those grown for 14 days by improving total soluble solids, protein content, photosynthetic pigments, total phenolic content, antioxidant activity, enzyme activity, free amino acids and minerals content, and the best enhancement was also observed in PAW-3. It was concluded that PAWs would be an effective technique to enhance the growth and nutritional properties of crop sprouts, which could be served as functional foods in many forms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the rapid increase of the world population and public awareness, demands for quantity and quality of food have continuously been elevated. However, nearly 690 million people in the world still lack adequate food supply, and this number will exceed 840 million by 2030 even if the potential impact of the COVID-19 outbreak is set aside (FAO et al. 2020). Therefore, it is important to develop more efficient and sustainable crop production methods to address the basic food need of people. It is well known that wheat (Triticum aestivum L.) is one of the most important crop plants supplying staple food throughout the world. The nutritional value of wheat comes from its high content of carbohydrates, proteins and dietary fibres, as well as considerable proportions of vitamins, minerals and antioxidants such as carotenoids, phenolics and phytosterols (Abdel-Aal and Rabalski 2008). After germination, wheat sprouts are a homology of medicine and food, which are rich in bioactive compounds and could be consumed as raw juice, tablets and capsules (Akbas et al. 2017), and wheat plantlet juice is thus increasingly being studied (Ahmed et al. 2020; Qamar et al. 2019; Sharma et al. 2020). Therefore, improving wheat seed germination and growth is of great importance to the industry.
Traditional methods in improving wheat seed germination are mainly based on chemical treatments, which can cause environmental hazards; therefore, novel physical treatment techniques have been investigated for their potential enhancement effects on wheat seed germination and seedling growth, which includes treatments by ultrasound (Guimarães et al. 2020), pulsed electric field (Ahmed et al. 2020), irradiation (AlSalhi et al. 2019), magnetic field (Balakhnina et al. 2015) and cold plasma (Kučerová et al. 2019; Lotfy et al. 2019; Roy et al. 2018). Amongst these techniques, cold plasma is the most impressive and the most studied in the latest decades (Chen et al. 2020; Chizoba Ekezie et al. 2017; Jiang et al. 2020; Pan et al. 2019a).
Cold plasma generated by electrical discharges is known for its active species such as UV light, radicals, reactive oxygen species (ROS) and reactive nitrogen species (RNS), and environment-friendly properties, which lays the foundation for its applications in agriculture and food processing (Scholtz et al. 2019; Song et al. 2020; Ali et al. 2022; Esua et al. 2021; Han et al. 2019a; Pan et al. 2021; Wu et al. 2022; Zhu et al. 2022). There are two different ways of applying cold plasma for seed treatments in agriculture: direct treatments, which are frequently used in previous research and have shown positive impacts on crop growth (de Groot et al. 2018; Ling et al. 2014; Velichko et al. 2019; Xu et al. 2019), and indirect treatments by using plasma-activated water (PAW) (Esua et al. 2021; Esua at al. 2022a; Esua et al. 2022b).
Compared with the direct treatment, PAW is easier to realize the uniform treatment of crop seeds and the storage and transport of plasma active species. PAW is typically generated by applying cold plasma on the surface of water or underneath the water with various plasma sources, and the composition of PAW depends on the plasma-liquid interactions, which usually leads to changes in pH, electrical conductivity (EC) and especially the formation of reactive oxygen and nitrogen species (RONS) (Ndiffo Yemeli et al. 2020; Ali et al. 2021a; Esua et al. 2022c; Pan et al. 2020). Several studies have identified that the long-life species (H2O2, NO2− and NO3−) are the source of activity of PAW. Although short-life species have higher physiological activity, they disappear soon after being generated (Asim et al. 2020; Bradu et al. 2020; Zhou et al. 2020).
Previous studies have indicated the positive effects of PAW on seed germination and seedling growth. Adhikari et al. (2019) investigated the effect of PAW on tomato seedlings by applying distilled water exposed to cold atmospheric-air jet plasma for 15, 30 and 60 min and found that PAW irrigation could upregulate seedling growth, endogenous RONS, defence hormones and expression of pathogenesis-related genes, which depended on the RONS concentration in PAW. Gierczik et al. (2020) clarified that PAW could improve the tolerance of barley against combined low temperature and hypoxia stresses during germination for the effect on the antioxidant system by RONS. Similarly, Zhang et al. (2017) compared the effect of tap water, PAW and commercial fertilizer on lentil germination and stem growth and showed that PAW increased the germination rate to 80% as compared with 30% using tap water, and the stem lengths were better enhanced than using commercial fertilizer, which was strongly related to H2O2 and NO3− in PAW. In addition, Kučerová et al. (2019) studied the effects of PAW prepared by transient spark discharge on wheat in vitro and in vivo and revealed that PAW might effectively stimulate the growth of wheat seedlings and positively affect their metabolism, which was correlated with the concentration of RONS in the PAW. However, the research about the effect of PAW on the nutritional properties of crop plantlets or their juice remains scanty at present.
Therefore, the objective of the current study was to investigate the influence of PAW generated by Ar–O2 plasma for 1–5 min on wheat seed germination, seedling growth and nutritional properties of wheat plantlet juice. It is hoped that the current study should provide an effective method to accelerate the growth and nutrition enrichment of crop plants.
Materials and Methods
Materials and Chemicals
Fresh wheat (Triticum aestivum L.) seeds (Jimai 23) used in the current study were supplied by Shandong Luyan Agricultural Co., Ltd. (Shandong, China). Uniform wheat kernels based on shape, size and colour were selected and debris and damaged seeds were removed from the experimental samples. Ascorbic acid (ASA), 2,6-dichloroindophenol (DIP), 2,2’-azobis-amidinopropane (ABAP), Folin-Ciocalteu reagent and gallic acid (GA) were purchased from Sangon Biotech Co., Ltd. (Shanghai, China). 2,2-Diphenyl-1-picrylhydrazyl (DPPH), sodium carbonate (Na2CO3), acetone, hydrochloric acid (HCl), metaphosphoric acid and potassium nitrate (KNO3) were obtained from Aladdin Reagent Co., Ltd. (Shanghai, China). Enzyme activity assay kits were acquired from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). The hydrogen peroxide assay kit was bought from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Distilled water was procured from Watsons Co., Ltd. (Guangzhou, China).
Experimental Setup for PAW Production
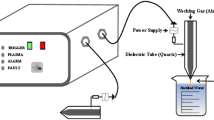
The plasma-generating device employed in the current study was an atmospheric pressure plasma jet (UPL-310B, Uniplasma Co., Ltd, Shenzhen, China), which has been previously described in detail by our lab members (Chizoba Ekezie et al. 2018; Han et al. 2019b; Chizoba Ekezie et al. 2019a; Chizoba Ekezie et al. 2019b; Pan et al. 2019b). The scheme of the plasma jet and the arrangement for the treatment of distilled water are shown in Fig. 1A. The plasma was powered by an alternating current single-phase power source at 220 V with an output voltage of 7 kV and the working power of the device was fixed to 600 W. Mixed gas (98% Ar and 2% O2) was used as the feed gas with a fixed gas flow rate of 40 L/min. For the production of PAWs, 75 mL of distilled water were poured into a 100 mL glass beaker (4.6 × 7.2 cm, diameter × height) and exposed to the plasma jet for a period of 1, 2, 3, 4 and 5 min, respectively. The length of the plasma plume was about 14 mm and the distance between the nozzle of the plasma jet and the water level in the beaker was set to 15 mm to increase gas–liquid interactions. The beaker was shaken constantly by a magnetic stirring apparatus (ZGCJ-3A, Zigui Co., Ltd., Shanghai, China) during plasma jet treatment to ensure a homogeneous distribution of active species. The temperature of the water in the beaker was monitored by a digital thermometer (ZG-8029S, Chigo Co., Ltd., Ningbo, China) before and after the treatment. After treatment, water samples treated by plasma for 1–5 min were designated as PAW-1, PAW-2, PAW-3, PAW-4 and PAW-5, respectively, and these PAWs were used for testing or irrigating wheat seeds within 30 min to prevent the changes of PAW and ensure repeatability. Untreated distilled water was used as control and designated as DW.
The plasma source was analysed by a computer-controlled optical emission spectrometer (OES) (HR2000+, Ocean Optics, Inc., FL, USA) equipped with a fibre optic (0.22 µm). The spectra were obtained in the range from 200 to 1100 nm. The axial and radial distances between the middle of the nozzle and the detector were both approximately 5 mm. Identification of the peaks was carried out by comparing experimental results with the National Institute of Standards and Technology (NIST) Atomic Spectra Database.
Characterization of PAWs
The characterization of PAW was carried out by measuring pH, electric conductivity (EC), NO2−, NO3− and H2O2 content. The pH and EC values were measured by a pH meter (SP-2300, Suntex Instrument Co., Ltd., Taiwan, China) and a conductivity meter (DDS-11A, Leici Chuangyi Instrument & Meter Co., Ltd., Shanghai, China), respectively. The contents of NO2− and NO3− were measured by methods reported previously (Shen et al. 2016). Nitrite concentration in PAWs was quantified with Griess reagent consisting of an equal volume of 10 g/L sulfanilic acid and 1.0 g/L naphthol. Briefly, 10 mL PAW was mixed with 0.5 mL Griess reagent and incubated at room temperature for 20 min. Then the absorbance of the mixture was determined by a spectrophotometer (UV-1800, Shimadzu Co., Kyoto, Japan) at 540 nm and nitrite solution (0.1–1.0 mg/L) was used as the standard for calculation. As for NO3−, 20 mL PAW was added with 1.0 mL of 1% sulfamic acid and diluted to 50 mL with distilled water. The absorbances at 220 nm (A220) and 275 nm (A275) of the mixture were determined, and nitrate concentration was calculated by the difference between A220 and A275 according to the standard curve of nitrate solution (0–8.0 mg/L). The H2O2 content was determined using a hydrogen peroxide assay kit (A064-1-1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China) based on the photometrical determination at 405 nm after the reaction of H2O2 with molybdic acid (Xiang et al. 2018). The results were calculated according to the standard curve of H2O2 solution (0–16.3 mmol/L).
Germination of seeds
To evaluate the effect of PAWs on wheat seed germination and seedling growth, germination tests for 7 days were performed. In total, there were 6 samples with 3 replications per sample and each replication contained 50 wheat seeds. Seeds were germinated in a germination box containing one layer of filter paper and watered with the same volume of DW, PAW-1, PAW-2, PAW-3, PAW-4 and PAW-5, respectively, to keep the moisture needed for seed germination. The germination boxes were placed in an illumination incubator with a temperature of 20 ± 1 ℃ and relative humidity of 70 ± 5% during the whole germination test. After 3 days, 2000 lx light for 12 h was added every day. The number of germinated seeds was observed and recorded daily. After germination for 7 days, 15 seedlings of each box were randomly selected for fresh and dry weight determination. Germination rate, germination index and vigour index were calculated according to the following equations:
Juice Preparation of Wheat Plantlets
To evaluate the effect of PAWs on the quality and nutritional characteristics of wheat plantlet juice, pot experiments for 14 days were conducted. For the growth of wheat plantlets, 24 pots were divided into 6 groups, each containing 180 g of vermiculite and 200 mL of DW, PAW-1, PAW-2, PAW-3, PAW-4 and PAW-5, respectively. In each group, 25 g of seeds were used, which were divided on average into 4 replications and the seeds were sowed uniformly 2 cm underneath the surface of the vermiculite. All the pots were placed inside the laboratory where sufficient light and airflow were present. During the growth period, the weight of each pot was recorded every day in order to add an appropriate amount of DW or PAWs in time. Wheat plantlets watered with DW or PAWs were designated as DW wheat plantlets, PAW-1 wheat plantlets, PAW-2 wheat plantlets and so on, and were cut with scissors after 14 days, 1 cm above the surface. Wheat plantlet juice was extracted with the cold pressing method by using a specialized manual juice extractor (MM-XP01, Linyi Maimiao Trading Co., Ltd., Linyi, Shandong), which could prevent oxidation and heat. After extraction, wheat plantlet juice was centrifuged immediately at 10,000 rpm for 5 min using a centrifuge (JW-3024HR, Anhui Jiaven Equipment Industry Co., Ltd., Hefei, China), which was then divided into 2 mL centrifuge tubes and stored at -20 ℃ for further determination. All the tests were accomplished within one week.
Physical and Chemical Analysis of Wheat Plantlet Juice
pH, Total Soluble Solids and Colour
The pH value of wheat plantlet juice was determined by the pH meter. The total soluble solids (TSS) were determined by dropping 300 μL of wheat plantlet juice into a digital refractometer (Atago Co., Ltd., Tokyo, Japan) at room temperature of 25 °C and the results were expressed as °Brix. The colour assessment was conducted at room temperature of 25 °C using a CR-400 colourimeter (Konica Minolta Inc., Osaka, Japan), and the hunter L* (lightness), a* (redness or greenness), and b* (yellowness or blueness) values of wheat plantlet juice were measured and the total colour difference (△E*) was calculated using the equation below:
Vitamin C Contents
The vitamin C content of wheat plantlet juice was determined using the 2,6-dichloroindophenol (DIP) titrimetric method as reported previously (Wang et al. 2017) with some modification. Briefly, 1.0 mL wheat plantlet juice and 9.0 mL chilled 2% (w/v) metaphosphoric acid buffer were added to a 50 mL conical flask for titration. Ascorbic acid (ASA) was used as the standard for calculation, and the content of vitamin C was expressed as milligram per 100 g of wheat plantlets in fresh weight (mg/100 g FW).
Soluble Protein Contents
Soluble protein was assayed according to the Bradford method (Ling et al. 2015). Briefly, 20 μL of appropriately diluted wheat plantlet juice was mixed well with 1.0 mL coomassie brilliant blue G-250 solution and the absorbance of the mixture was determined after 2 min using the spectrophotometer at 595 nm. Bovine serum albumin (BSA) was used as the standard for calculation and the results were expressed as grams per 100 g of wheat plantlets in fresh weight (g/100 g FW).
Pigments Contents
The values of chlorophyll a (\({\text{C}}_{\text{a}}\)), chlorophyll b (\({\text{C}}_{\text{b}}\)) and carotenoids (\({\text{C}}_{\text{x + c}}\)) were measured by the spectrophotometer at 646.8, 663.2 and 470 nm (Lichtenthaler & Buschmann, 2001). Concretely, 1.0 mL of properly diluted wheat plantlet juice was mixed with 4.0 mL of acetone and 50 mg of CaCO3. After standing for 5 min, the mixture was centrifuged at 10,000 rpm for 5 min and the absorbances at 646.8, 663.2 and 470 nm for the supernatant were measured, respectively. The amounts of pigments present in the juice samples were calculated according to the formula below:
The results were expressed as μg per 100 g of wheat plantlets in fresh weight (μg/100 g FW).
Total Phenolic Contents
The total phenolic content (TPC) of wheat plantlet juice was quantified by the Folin-Ciocalteu reagent method as modified by Xiang et al. (2017). Gallic acid (GA) was used as the standard for calculation. The results were calculated according to the standard curve of gallic acid concentrations (0–600 μg/mL) and expressed as milligram gallic acid equivalent (GAE) per 100 g of wheat plantlets in fresh weight (mg GAE/100 g FW).
Antioxidant Activities
Antioxidant activities of wheat plantlet juice were evaluated using the DPPH free radical scavenging assay (Ali et al. 2019) and oxygen radical absorbance capacity (ORAC) assay (Xiang et al. 2017).
For the DPPH assay, 100 μL of diluted wheat plantlet juice was mixed with 900 μL of 150 μmol/L DPPH and then incubated at room temperature of 25 °C for 30 min in the dark. Distilled water and vitamin C were used as the blank control and Trolox standard, respectively. The absorbance at 519.5 nm was measured and used to calculate the percentage of quenched DPPH. The results were calculated according to the standard curve of vitamin C concentrations (0–400 μg/mL).
For ORAC assay, 20 μL of appropriately diluted wheat plantlet juice was added with 200 μL of 0.96 μM fluorescein and incubated at 37 ℃ for 20 min with intermittent shaking. After adding 20 μL of 119.4 mM ABAP reagent, the fluorescence intensity was monitored every 4.5 min for 35 cycles at excitation of 485 nm and emission of 535 nm by a Tecan Infinite™ M200 Multimode Microplate Reader (Tecan Inc., Männedorf, Switzerland).
The results of antioxidant activities were expressed as μmol Trolox equivalent (TE) per gram of wheat plantlets in fresh weight (μmol TE/g FW).
Enzyme Activities
The activities of superoxide dismutase (SOD), peroxidase (POD) and polyphenol oxidase (PPO) in wheat plantlet juice were analysed by assay kits R22261, R30312 and R30314 (Shanghai Yuanye Bio-Technology Co., Ltd., Shanghai, China), respectively.
For the SOD assay, the nitroblue tetrazolium (NBT) method was used. The absorbance was determined at 560 nm after 20 min light reaction and the light-avoiding tube was used as control. The enzyme activity unit was defined as inhibiting 50% of NBT reduction.
For the POD assay, the guaiacol method was used. The absorbance was recorded continuously at 470 nm for 3 min and the sample boiled for 5 min was used as the control. Enzyme activity unit was defined as the amount of enzyme required to change absorbance by 0.01 per minute.
For the PPO assay, the catechol method was used. The absorbance was recorded continuously at 420 nm for 1 min and the boiled samples were used for the blank control. Similarly, the enzyme activity unit was defined as the amount of enzyme required to change absorbance by 0.01 per minute.
The measured values were expressed as enzyme activity unit per gram of wheat plantlets in fresh weight (U/g FW).
Free Amino Acids
The free amino acids of wheat plantlet juice were measured by an L-8900 amino acid analyzer (Hitachi Inc., Tokyo, Japan). Briefly, 4.0 mL of 15% (w/v) sulfosalicylic acid was added to 1.0 mL of wheat plantlet juice and mixed well to precipitate protein. Then, the mixture was centrifuged at 10,000 rpm for 5 min and the supernatant was collected and diluted properly for detection. Free amino acids were separated by liquid chromatography with a column (855–4507, Hitachi Inc., Tokyo, Japan) and detected through ninhydrin reaction at 570 nm and 440 nm. External standards (Sigma-Aldrich Trading Co., Ltd., Shanghai, China) were used for concentration calculation. All values were described in milligram per 100 g wheat plantlets in fresh weight (mg/100 g FW).
Mineral Elements
Mineral contents of wheat plantlet juice were estimated using an inductively coupled plasma-optical emission spectrometer (iCAP 7200, Thermo Scientific™, MA, USA). Briefly, 1.0 mL of juice sample was poured into a Teflon vessel with the addition of 4.0 mL of 65% HNO3 and 1.0 mL of 30% H2O2 for digestion on a microwave work station (Mars 6, CEM Co., Matthews, NC, USA). After that, digested samples were diluted with ultrapure water to 50 mL for measurement. For each mineral compound, the standards were prepared within the range of the concentration of mineral elements contained in the sample. The results were described in micrograms per gram of wheat plantlets in fresh weight (μg/g FW).
Statistical Analysis
Data were presented as mean ± standard deviation (SD) for at least three independent experiments. Statistical analysis was performed using SPSS software for Windows (V 22.0, IBM Co., New York, USA). Significance tests of data were performed by applying one-way analysis of variance (ANOVA) and Duncan’s multiple comparison post-test with a confidence level of 95%. A p value less than 0.05 was regarded as statistically significant. Correlation tests between the PAW characteristics and the observed effects in wheat were performed using Pearson’s correlation. Significant differences were represented by *p < 0.05 and **p < 0.01.
Results
Emission Characteristics of Plasma Discharge
To examine the emission spectrum generated by the atmospheric pressure plasma jet, OES was used. As shown in Fig. 1B, the optical emission spectrum of the discharge for the Ar–O2 plasma jet was mainly comprised of the emission lines of argon and its excited states as Ar was the main constituent of the feed gas. In the meantime, the emission lines of atomic oxygen at 777.1 and 844.5 nm were also observed, suggesting that argon and oxygen reactive species were responsible for the activation of the treated water.
Physicochemical Properties of PAWs
To clarify the properties of PAWs, the changes in the pH, EC, temperature, and NO2−, NO3− and H2O2 concentrations of PAWs after plasma treatment were monitored and presented in Fig. 2. In general, with an increase in plasma application time (activation time), the pH of the PAWs decreased, whilst the EC, temperature, and NO2− and NO3− concentrations increased and the H2O2 concentration remained zero. For the pH, with applying plasma, the pH of PAWs started to drop and after activation for 5 min, the value decreased to 4.15 ± 0.04 from an initial value of 5.50 ± 0.02. For EC, the change tendency was totally opposite to that of the pH, and the highest EC value of 30.00 ± 2.32 μS/cm occurred in PAW-5 as compared with an initial value of 1.61 ± 0.09 μS/cm for DW. Similarly, the temperature of PAWs increased until the plasma treatment was finished, and the highest temperature rise appeared in PAW-5 with 13.45 ± 0.39 ℃, indicating the nature of cold plasma treatment. For NO2− and NO3− concentrations, their changing patterns were the same as that of temperature or EC, and the values increased linearly from an initial zero to 0.75 ± 0.00 and 3.34 ± 0.19 mg/L for PAW-5, respectively. These results suggested that the composition of PAWs was strongly dependent on activation time.
Germination and Growth Parameters
To access the effects of PAWs on the germination and early growth of wheat, wheat seeds were cultivated with different PAW for 7 days with DW used for the control. The germination and growth parameters of wheat seeds irrigated by DW and PAWs are presented in Fig. 3. The results showed that the germination rates of all samples were higher than 97% and the seeds watered with PAW-3 showed significantly higher germination rates than those watered with DW. Particularly, seeds irrigated with PAW-3 exhibited a 100% germination rate. Besides, using PAWs to water wheat seeds could increase the germination index as compared with using DW, but only PAW-2 showed a significant difference. In addition, after growth for 7 days, the mean fresh weight and vigour index A of wheat seedlings watered with PAW-1, PAW-3 and PAW-4 were significantly higher than with DW and that of wheat seedlings watered with PAW-2 was higher with no significant difference. Similarly, wheat seedlings watered with PAWs accumulated more dry matter and showed enhanced vigour index B. More specifically, the mean dry weight of wheat seedlings was increased by 6.64%-8.74% in 7 days of growth with PAWs. These results indicated that the current PAWs were able to stimulate wheat seed germination and seedling growth.
Quality Attributes of Wheat Plantlet Juice
To further access the effects of PAWs on wheat plantlet growth and nutrition accumulation, wheat plantlets were cultivated and prepared as juice after 14 days. The quality attributes of juice derived from wheat plantlets watered with DW or PAWs for 14 days are shown in Table 1. Compared with DW, PAW-3 significantly increased the TSS of the wheat plantlet juice by 13.82%, whilst other PAWs increased the TSS below 4%. The measured protein concentrations slightly decreased in PAW-1 wheat plantlet juice, PAW-2 wheat plantlet juice and PAW-5 wheat plantlet juice with no significant difference. In comparison, PAW-3 and PAW-4 significantly increased the soluble protein contents by 19.48% and 7.79%, respectively. However, none of the PAWs caused increases in vitamin C levels in wheat plantlet juice, and in fact, a significant decrease was observed in all wheat plantlets juice, especially PAW-5 wheat plantlet juice showed reduced vitamin C contents by as much as 19.19%. In contrast, all the samples treated by PAW contained more chlorophyll a, chlorophyll b and carotenoids. Amongst them, PAW-3 led to the largest increase in chlorophyll a, chlorophyll b and carotenoids by 89.46%, 112.46% and 91.58%, respectively, and PAW-5 resulted in the smallest increase by 8.94%, 31.96% and 6.35%, respectively in comparison with that of DW.
The TPC was significantly increased only in PAW-3 wheat plantlet juice by 10.46% whilst other PAW wheat plantlet juice showed no significant enhancement when compared with the control. The changes in antioxidant capacity of wheat plantlet juice by PAW treatments were evaluated by DPPH and ORAC assay. In the case of the DPPH assay, PAWs (PAW-1 to PAW-5) resulted in different increases in antioxidant activity of wheat plantlet juice by 5.51%, 7.87%, 14.96%, 8.66% and 15.75%, respectively, whilst PAW-1 exhibited no significant difference from DW. With regard to the ORAC assay, ORAC values of PAW wheat plantlet juice (PAW-1 to PAW-5) were increased by 10.52%, 13.15%, 25.29%, 9.28% and 35.34%, respectively, but PAW-1 wheat plantlet juice and PAW-4 wheat plantlet juice showed no significant difference from DW wheat plantlet juice. As for enzyme activity, a 24.66% enhancement in POD activity of PAW-1 wheat plantlet juice was observed, whereas other PAWs decreased the POD activity of wheat plantlet juice at different degrees. In the case of PPO activity, treatments with PAW-2, PAW-3 and PAW-5 exhibited enhancement effects, however, PAW-1 and PAW-4 showed a debilitating effect. The greatest enhancement of 44.60% was achieved with PAW-2 treatment as compared with the control. In addition, PAWs (PAW-1 to PAW-5) resulted in 27.97%, 34.08%, 47.12%, 31.52% and 40.04% increase, respectively, in SOD activity compared with the control. Moreover, the most notable colour change was found in PAW-3 wheat plantlet juice, which became darker, less yellow and less green, as indicated by the changes in L*, a*, b* and ∆E* values, and PAW-1 was able to get the juice colour lighter and showed an obvious colour difference. In contrast, PAW-2, PAW-4 and PAW-5 exhibited less ability to change the colour of wheat plantlet juice.
Free Amino Acids Profile
The contents of the free amino acids in wheat plantlet juice derived from DW or PAW treating plantlets are shown in Table 2. PAW treatments could significantly increase the total amount of free amino acids in the juice, and nearly all of the free amino acids increased in PAW wheat plantlet juice except that Cys decreased. Specifically, PAWs (PAW-1 to PAW-5) increased the total amount of free amino acids by 19.28%, 9.12%, 28.23%, 18.86% and 15.50%, respectively, when compared with the control. Individually, the levels of Thr, Val, Met, Leu, Phe, Trp, Ser, Ala, Tyr, Arg and γ-aminobutyric acid (GABA) were significantly increased in all PAW wheat plantlet juice, whilst Gly and Pro contents of PAW-1 wheat plantlet juice, Ile, Lys, His, Glu, Gly and Pro contents of PAW-2 wheat plantlet juice, Pro content of PAW-3 wheat plantlet juice, Gly content of PAW-4 wheat plantlet juice and His, Asp, Gly contents of PAW-5 wheat plantlet juice showed no significant difference from DW wheat plantlet juice. In particular, amongst 19 determined free amino acids, 18 free amino acids were significantly increased and 14 free amino acids levels were the highest in PAW-3 wheat plantlet juice, which suggested that PAW-3 treatment was the best for the accumulation of free amino acids in wheat seedlings.
Mineral Compositions
The results of elemental analysis of the juice derived from wheat plantlets treated by DW or PAWs are shown in Table 3. The data showed that Ca, P, K, S, Mg and Mn contents of all PAW wheat plantlet juice, Na content of PAW-2 wheat plantlet juice, PAW-3 wheat plantlet juice and PAW-4 plantlet juice, Fe content of PAW-3 wheat plantlet juice and Zn content of PAW-1 wheat plantlet juice, PAW-2 wheat plantlet juice, PAW-3 wheat plantlet juice and PAW-5 wheat plantlet juice were significantly increased when compared with that of DW wheat plantlet juice. Remarkably, applying PAW-3 significantly promoted the accumulation of all the mineral elements in wheat seedlings and PAW-3 wheat plantlet juice contained the highest content of all kinds of determined minerals amongst the samples. Specifically, the levels of Ca, P, Na, K, S, Mg, Mn, Fe, Zn of PAW-3 wheat plantlet juice were increased by 17.19%, 24.98%, 4.43%, 19.51%, 22.43%, 17.68%, 22.15%, 31.14% and 20.54%, respectively. However, Na content of PAW-5 wheat plantlet juice was significantly decreased. The results suggested that PAW treatments could enhance the accumulation of macroelements (Ca, P, Na, K, S and Mg) and microelements (Mn, Fe and Zn) during wheat seedling growth, and PAW-3 was the most suitable treatment.
Pearson Correlation Analysis of PAW Characteristics and Effects in Wheat
Pearson’s correlations between physicochemical properties of PAWs and wheat germination parameters, quality attributes of wheat plantlet juice, free amino acids and minerals were evaluated, respectively. As shown in Table S1, Pearson correlation coefficients in mean dry weight and vigour index B in association with pH were -0.860 and -0.801, those between the above germination parameters and EC were 0.659 and 0.543. With regard to NO2− and NO3−, Pearson correlation coefficients were 0.681, 0.564 and 0.684, 0.561, respectively. However, there were nearly no correlations between mean fresh weight, vigour index A and EC, NO2− and NO3−, and other germination parameters showed weak correlations with PAW characteristics. The extremely significant positive correlations were obtained amongst EC, NO2− and NO3−, and the extremely significant negative correlations were obtained between pH and EC, NO2− and NO3−, respectively. In general, the promoting effect of PAWs on wheat seed germination and seedling growth depended on NO2− and NO3−.
The results of Pearson correlation analysis of PAW characteristics and quality attributes, free amino acids and minerals of wheat plantlet juice were shown in Tables S2, S3 and S4, respectively. The data showed that there were negative correlations between pH and most of the determined indexes except for vitamin C, POD, Cys and Na, whilst the opposite results were obtained of the correlations between other determined PAW compositions and indexes of wheat plantlet juice. However, Pearson correlation coefficients of NO2− and NO3− in association with TSS, carotenoids, PPO, Asp, Cys, Fe and Zn were below 0.2. Remarkably, NO2− or NO3− exhibited significant positive correlations with DPPH, Met, Ala, Pro, GABA and P. In summary, the enhancements of nutritional materials of wheat plantlet juice and antioxidant activities could be attributed to the nitrite and nitrate of PAWs.
Discussion
With the growing population and the outbreak of COVID-19 throughout the world, food shortages are becoming more and more serious (FAO et al. 2020), which challenges people to find more efficient and sustainable crop production methods to address the basic food need. In fact, researchers have tried a variety of novel physical treatment techniques including ultrasound, pulsed electric field, irradiation, magnetic field and cold plasma, to solve this worldwide problem and cold plasma is the most impressive amongst these techniques (Ahmed et al. 2020; AlSalhi et al. 2019; Balakhnina et al. 2015; Guimarães et al. 2020; Ndiffo Yemeli et al. 2020). In the meantime, wheat (Triticum aestivum L.) is consumed in huge quantities as one of the most important crop plants throughout the world. In this case, the current study chose wheat to demonstrate the effects of indirect cold plasma (PAW) treatments on its germination, early growth and nutrition accumulation, so as to provide an effective method to improve crop production.
Generation of PAWs
Numerous studies clarified that the characteristics of PAW depended on different plasma parameters such as types of discharge, discharge pressure, discharge distance, electrode configuration, power supply, types of the feed gas, gas flow, treatment time, and water volume (Kim and Kim 2021; Thirumdas et al. 2018; Zhou et al. 2020). In our study, argon and oxygen reactive species were generated by an atmosphere pressure Ar–O2 plasma discharge to activate the water and nitrate and nitrite were formed in PAWs, which was in line with those reported previously (Ali et al. 2021; Chizoba Ekezie et al. 2018). However, other studies (Karmakar et al. 2021; Vlad and Anghel 2017) found that there were weak emission lines of OH radicals and nitrogen reactive species in open atmosphere Ar or Ar–O2 cold plasma, which was not shown in the current research. The reason might be that argon and oxygen active species generated in plasma would motivate the emission of ambient air and the distance between the OES probe and plasma plume, which was just 5 mm in the current research, affected the results. In addition, at the same distance, a slight change in the orientation of the OES probe would also influence the result. Vlad and Anghel (2017) reported that when treatment time varied from 10 to 50 min, argon-discharged PAW contained a higher concentration of H2O2, whilst air-discharged PAW contained a higher concentration of RNS. Whereas, in the current study, H2O2 could not be detected in all PAWs, which was reasonable as a higher water volume of 75 mL was used in the current study than most other studies, and only 3.34 ppm NO3− was thus generated for treatment for 5 min. As a result, the effects of NO2− and NO3− rather than the combined effects of NO2−, NO3− and H2O2 on wheat seeds could be analysed in the current study. In the meantime, our results showed that the formation of NO2− and NO3− resulted in reduced pH and increased EC, which was consistent with other studies. For example, Sergeichev et al. (2021) investigated the physicochemical properties of pure water treated by an Ar microwave plasma jet and showed that EC values and NO2− and NO3− concentrations increased linearly with plasma exposure times. Similarly, Zhou et al. (2019) showed the same change tendency with lower pH and higher RONS in PAW treated with N2, He, Air and O2 plasma. Moreover, the formation process of RNS in PAW was well summarized (Bradu et al. 2020) and not repeated in the current study.
Effects of PAWs on Wheat Seed Germination
It is widely acknowledged that the biological activity of PAW was attributed to NO2−, NO3− and H2O2. Previous studies showed that H2O2 could diffuse through the plant cell membrane and act as a signalling molecule mediating in the early growth during imbibition and germination (Ranieri et al. 2020), yet the positive effects of PAW in the current study could not be associated with it. In PAWs, NO3− and NO2− could serve as the source of N and be metabolized by wheat seed, which was the key effector, on the other hand, they could be reduced to NO, which modulated various hormones controlling seed dormancy and germination such as abscisic acid, gibberellins and indoleacetic acid (Chen et al. 2020; Mu et al. 2018). And the content of plant nitrogen was closely related to photosynthesis and further affected the accumulation of dry matter in crops (Verkroost and Wassen 2005), which explained why wheat seeds exhibited higher mean dry weight and seed vigour when irrigated with PAWs as reported in the current study. However, there was a limited range of beneficial RNS concentrations, and concentrations too high or too low were detrimental to crop growth. For example, Fan et al. (2020) investigated the effect of PAW on mung beans and showed that NO3− concentrations higher than 20 mg/mL could not promote the growth of stem and root, and for NO3− concentrations reaching 118.39 mg/mL, the germination rate decreased. In contrast, Lo Porto et al. (2018) found that PAW containing 1.24 or 10.54 mg/L of NO3− exhibited a positive effect on soybean seed germination and growth. Similarly, in the current study, the total concentrations of NO3− and NO2− in PAWs were below 5 mg/L and all the PAWs exhibited enhanced effects on wheat seed germination and growth at different degrees, especially for PAW-3, the best overall improvement was achieved. Notably, pH < 4.5 and EC > 3.0 dS/m were not recommended for crop growth because they would affect enzyme activity and water absorption of seeds (Ranieri et al. 2020). Research on the effects of pH on the uptake of 0.5 mM NO2− and NO3− of 7-day-old wheat seedlings demonstrated that the nitrate uptake was not affected by pH, and the nitrite uptake at pH 4 hardly increased with time, whilst its amount was comparable to nitrate at pH 6.5 suggesting that NO2− at low pH was strong oxidant and toxic substance as HNO2 (Zsoldos et al. 1999). A similar report showed that the dry matter of shoot of the common wheat seedlings was increased linearly with 0–0.5 mM nitrite at pH 4, whilst NO2− content beyond 0.5 mM could cause stress for the root growth (Bona et al. 2001). Amongst the PAWs generated in our study, the nitrite concentrations of all PAWs were below 0.1 mM, but only PAW-1 achieve the recommended pH value, suggesting that abiotic stress might occur in long-term cultivation. Whereas PAWs showed limited enhancement on germination rate because the germination rate of all samples was higher than 97%, which is the normal rate for fresh seeds (Tian et al. 2019). Moreover, extremely weak correlations between germination index and NO2− and NO3− were obtained in our study which suggested that germination velocity did not depend on the RNS of PAWs. Similarly, Kučerová et al. (2019) quantified the NO2−, NO3− and H2O2 of plasma-activated tap water with and without the presence of wheat seeds for 7 days. Without seeds, the concentration of H2O2 decreased slowly and the residue could still be determined after 6 days. In the meantime, the concentrations of NO2− and NO3− remained stable. With the presence of seeds, the concentration of H2O2 decreased rapidly in several minutes and could not be detected after 18 h, whilst the concentration of NO2− and NO3− started to decrease after 24 h when seeds started to germinate. The authors, therefore, concluded that seeds interacted with H2O2 mainly in the early stage of imbibition and germination, whereas NO2− and NO3− were metabolized after germination. Another explanation was that the transport of NO3− was relatively slower than that of H2O2 as prior was regulated by specialized membrane transport proteins (Forde 2000). Mean fresh weight was the reflection of water absorption during wheat growth and Pearson correlation coefficients showed that NO2− and NO3− in PAWs were not the cause of the enhancements in wheat seedling fresh weight. Scanning electron micrograph images of mung bean seed treated by PAW showed that the seed coat was eroded and chapped which resulted in the enhancement in water absorption (Zhou et al. 2019). Whereas the salinity of PAWs caused the increase in osmotic pressure and high osmotic pressure would decrease the water uptake of the seeds by regulating the transcellular transport path (Knipfer et al. 2021; Yue et al. 2018). As shown in Fig. 3, taken as a whole, the mean fresh weight of wheat seeds treated by PAWs showed first increasing and then decreasing tendency, but only PAW-5 did not increase it as compared with the control, which might result from the combined effect of PAWs on the improvement of seed coat wettability and increasing of osmotic potential. Similar results were obtained in wheat with 0.5 min/mL to 3 min/mL PAW (Kučerová et al. 2019). As germination index and mean dry weight of it showed a significant difference from the control, it was supposed that water loss during sampling of wheat seeds treated by PAW-2 resulted in no significant increase in mean fresh weight. Nevertheless, it did not affect our conclusion that PAWs exhibited promotion effects on wheat seed germination and seedling growth for 7 days.
Effects of PAWs on Quality Attributes of Wheat Plantlets Juice
Wheat plantlets grown for 8–15 days are normally acknowledged for high nutritional value and are commonly consumed as raw juice (Bianchi et al. 2019). In the current study, PAWs especially PAW-3 could greatly improve the nutritional value of juice derived from wheat plantlet grown for 14 days by increasing TSS, protein content, pigments, TPC, free amino acids and mineral elements, which was consistent with the performance of PAWs in the germination test. Nutrient status reflected the condition of plant growth and development. Carbohydrates were strongly required in dividing and differentiating cells and their metabolism was important for plant growth and previous study selected soluble sugar content as the evaluation index for wheat seedling growth (Eveland and Jackson 2012; Zhu et al. 2021). Although PAW-3 increased 13.82% of TSS, the correlation coefficients indicated that it was not related to the determined parameters of PAW. A previous report found that 0.1 mM NO3− treatment could significantly increase the plant dry weight, leaf soluble protein and plant N content of wheat plantlet grown for 24–37 days and result in a significant positive correlation between plant N content and plant dry weight, but plant N content and leaf soluble protein content were not significantly correlated which could be attributed to the root to shoot dry weight ratio (Andrews et al. 1999). A similar result was obtained in the current study that NO2− and NO3− of PAWs showed weak correlations with the soluble protein content of wheat plantlet juice. In addition, another study demonstrated that applying nitrate-nitrogen increased the NO3− and reduced the vitamin C content of spinach cultivated for 9 days, and the transfer of the spinach to N-free media would recover the vitamin C and reduce the NO3− level as vitamin C was recognized as the inhibiter of N-nitroso compounds formed from nitrite (Mozafar 1996). In the same way, NO2− and NO3− of PAWs coexisted and significantly decreased the vitamin C content of wheat plantlet juice. It is reported that RNS stimulated the formation of pigments by upregulating the amounts of stromal and thylakoid proteins of leaves and increasing the chloroplasts during leaf growth (Than et al. 2021). Chlorophyll a and chlorophyll b are essential in photosynthesis influencing the synthesis of organic matter in land plants and their contents reflect the plant nitrogen status (Tanaka and Tanaka 2011). Our results showed that PAWs could improve the utilization of nitrogen in wheat plantlets and PAW-3 performed best, indicating that the levels of NO2− and NO3− did not dominate the nitrogen transformation which was in line with the Pearson correlation coefficients. In addition, chlorophyll was found to increase with increasing stress levels in stress-tolerant plants which suggested that it might be a potential indicator of stress resistance (Wang et al. 2022). Thus, the downward trend of chlorophyll from PAW-3 wheat plantlet juice to PAW-5 plantlet juice was supposed to result from the abiotic stress caused by low pH and increasing NO2− content after 14 days of growth. Carotenoids and TPC are widely acknowledged for their biological functions such as antioxidation, anticancer action and immunological enhancement (Maoka 2020). The increased carotenoids, TPC and antioxidant activities of PAW wheat plantlet juice suggested that PAW treatment could improve the physiological functions of wheat plantlet juice. PPO and POD belong to spoilage enzymes that can result in the browning of juices, and the inactivation of these two enzymes has positive effects on browning reduction (Jimenez-Sanchez et al. 2017). However, in the case of raw juice, the retention of PPO and POD activities are beneficial for human health (Skoczylas et al. 2018). In this aspect, PAW-1 and PAW-3 showed advantages in the preservation of POD and PPO activities. Moreover, POD and SOD were enzyme antioxidant systems activated for ROS scavenging and metabolism to alleviate oxidative damage. Tari and Csiszár (2003) found that 1 mM nitrite at pH = 4 led to nitrosative stress and reduction of POD activity in wheat. Kučerová et al. (2021) compared the effects of PAW, H2O2 and NO3− on Lettuce growth and found that only 0.85 mM NO3− increased the SOD activity. In the current study, SOD activity of PAW wheat plantlet juice was increased which could be related to NO3− in PAWs. However, only POD activity of PAW-1 wheat plantlet juice was increased suggesting that PAW-2, PAW-3, PAW-4 and PAW-5 might cause the nitrosative stress in wheat plantlets cultivated for 14 days.
Effects of PAWs on Free Amino Acids Profile
Nitrogen is utilized directly for synthesizing amino acids, with an initial process involving the synthesis of glutamine and glutamate via the 2-oxoglutarate-glutamate synthase pathway (Rossi et al. 2021), and the enhancement of free amino acids could thus be attributed to the NO2− and NO3− occurring in PAWs. GABA and proline are able to improve abiotic stress tolerance in various plants through free radical-scavenging, osmotic adjustment and chlorophyll metabolism (Abd El-Gawad et al. 2021; Li et al. 2016; Rossi et al. 2020). Al-Quraan et al. (2013) reported that salt stress (100 mM NaCl) and osmotic stress (200 mM sorbitol or 200 mM mannitol) could result in a drastic increase of GABA and chrolophy b metabolites in wheat. Guo et al. (2018) found that drought stress induced by water deficiency would increase the proline metabolites and decrease the photosynthetic pigment metabolites of wheat plantlets grown for 4 weeks. On this basis, the increase of GABA and proline contents of PAW wheat plantlet juice in the current study was supposed to be associated with the osmotic stress and oxidative stress caused by low pH, NO2− and NO3− of PAWs, and the variation of enhancements in GABA and proline amongst PAWs might result from the different levels of stresses. Moreover, plant responses to combined stresses were complex and involved many changes in gene expression and metabolites, which was different from the responses to individual stress (Carillo 2018). For example, it was found that GABA accumulation under salinity in durum wheat plants only appeared with high nitrate and high light, and in these conditions, plants exhibited lower ROS levels and higher photosynthetic efficiency than plants under salinity at low light (Woodrow et al. 2017). As increased free amino acid in PAW wheat plantlets, therefore, wheat plantlets irrigated by PAWs might exhibit higher abiotic stress tolerance and alleviate the deleterious effects caused by worse climate and environment.
Effects of PAWs on Mineral Compositions
Mineral composition analysis indicated the variations of mineral concentrations in wheat plantlets as affected by PAW treatments. Macroelements (Ca, P, Na, K, S and Mg) and microelements (Mn, Fe and Zn) are essential for plant growth and human health (Kopriva 2015; Martínez-Ballesta et al. 2011). Therefore, the application of PAWs especially PAW-3 could enhance wheat plantlet growth by promoting minerals absorption. Albornoz and Lieth (2015) found that nitrate absorption had a positive correlation with the absorption of H2PO4−, K+, Ca2+, Mg2+ and SO42− for lettuce roots. Li et al. (2007) investigated the influence of nitrate on metal sorption and bioaccumulation in marine phytoplankton and clarified that 6–55 μmol/L of nitrate greatly affected the absolute adsorption of Fe, Zn and Mn. However, Pearson correlation analysis showed that the enhancements of Na, Fe and Zn contents were not related to the levels of NO2− and NO3− of PAWs. Furthermore, an acidic condition created by NO2− and NO3− in PAWs would reduce the absorption of minerals. As zinc was a constituent of enzyme carbonic anhydrase, alcoholic dehydrogenase and superoxide dismutase and iron was a component of porphyrin compounds, it was speculated that abiotic stresses induced by low pH, NO2− and NO3− affected the absorption of Zn and Fe. In agreement with our proposal, (Chen et al. 2011) reported that the levels of Fe and Zn of wheat seedlings were decreased under salt stress and supplementation with antioxidant (ascorbic acid or N-acetyl-l-cysteine) increased the accumulation of Fe and Zn. Moreover, Xu et al. (2014) evaluated the effect of Zn on salt tolerance of wheat and elucidated that sufficient Zn nutrition maintained antioxidant enzyme activities and reduced ROS over-accumulation in 20-day-old wheat seedlings. In the meantime, they also revealed that sufficient Zn nutrition decreased the Na+ accumulation by upregulating the expression of Na+/H+ antiporter genes, TaSOS1 and TaNHX1 to improve salt tolerance in wheat seedlings. Therefore, the enhancement of minerals absorption of wheat plantlets in the current study was due to the increased nitrogen sources and beneficial low abiotic stresses induced by pH, NO2− and NO3− of PAWs.
Altogether, it was concluded that plasma-activated water generated by atmosphere pressure Ar–O2 plasma jet for 1–5 min could improve wheat seed germination, seedling growth and nutrient accumulation of wheat plantlets which was attributed to the increased nitrogen supply and induced beneficial abiotic stresses of PAWs. Remarkably, PAW-3 was the best choice for the stimulation of wheat growth. However, the mechanism of PAW on wheat seed could not be fully explained by the current limited research and further research was needed to elucidate it.
Conclusions
In the current study, it was demonstrated that PAWs generated by atmosphere pressure Ar–O2 plasma jet possessed the ability to enhance wheat seed germination, seedling growth and nutritional properties of wheat plantlet juice, which could be attributed to the increased nitrogen supply and induced beneficial abiotic stresses of PAWs. Results indicated that PAW-3 could not only accelerate the germination and growth of wheat seeds in 7 days but could also significantly enhance the nutrients in the juice extracted from wheat plantlets grown for 14 days such as TSS, protein content, pigments, TPC, free amino acids and mineral elements. Besides, PAW-3 exhibited the possibility to improve stress resistance of wheat plantlets by increasing the SOD activity, GABA and proline content. Moreover, PAW-3 showed the highest ability in changing the colour of wheat plantlet juice, although this influence could not be simply judged as beneficial or harmful. The current results suggested that PAWs could be a potential green technology for agriculture production.
Change history
23 August 2022
Missing Open Access funding information has been added in the Funding Note.
References
Abd El-Gawad HG, Mukherjee S, Farag R, Abd Elbar OH, Hikal M, Abou El-Yazied A, Abd Elhady SA, Helal N, ElKelish A, El Nahhas N, Azab E, Ismail IA, Mbarki S, Ibrahim MFM (2021) Exogenous gamma-aminobutyric acid (GABA)-induced signaling events and field performance associated with mitigation of drought stress in Phaseolus vulgaris L. Plant Signal Behav 16(2):1–15
Abdel-Aal E-S, Rabalski I (2008) Bioactive compounds and their antioxidant capacity in selected primitive and modern wheat species. Open Agric J 2(1):7–14
Adhikari B, Adhikari M, Ghimire B, Park G, Choi EH (2019) Cold atmospheric plasma-activated water irrigation induces defense hormone and gene expression in tomato seedlings. Sci Rep 9(1):16080
Ahmed Z, Manzoor MF, Ahmad N, Zeng XA, Din ZU, Roobab U, Qayum A, Siddique R, Siddeeg A, Rahaman A (2020) Impact of pulsed electric field treatments on the growth parameters of wheat seeds and nutritional properties of their wheat plantlet juice. Food Sci Nutr 8(5):2490–2500
Akbas E, Kilercioglu M, Onder ON, Koker A, Soyler B, Oztop MH (2017) Wheat plantlet juice to wheat grass powder: Encapsulation, physical and chemical characterization. J Funct Foods 28:19–27
Albornoz F, Lieth JH (2015) Diurnal macronutrients uptake patterns by lettuce roots under various light and temperature levels. J Plant Nutr 38(13):2028–2043
Ali N, Popović V, Koutchma T, Warriner K, Zhu Y (2019) Effect of thermal, high hydrostatic pressure, and ultraviolet-C processing on the microbial inactivation, vitamin, chlorophyll, antioxidants, enzyme activity, and color of wheat plantlet juice. J Food Process Eng 43(1):13036
Ali M, Cheng JH, Sun D-W (2021a) Effects of dielectric barrier discharge cold plasma treatments on degradation of anilazine fungicide and quality of tomato (Lycopersicon esculentum Mill) juice. Int J Food Sci Technol 56:69–75
Ali M, Cheng JH, Sun DW (2021b) Effect of plasma activated water and buffer solution on fungicide degradation from tomato (Solanum lycopersicum) fruit. Food Chem 350:129195
Ali M, Sun D-W, Cheng JH, Esua OJ (2022) Effects of combined treatment of plasma activated liquid and ultrasound for degradation of chlorothalonil fungicide residues in tomato. Food Chem 371:131162
Al-Quraan NA, Sartawe FA, Qaryouti MM (2013) Characterization of gamma-aminobutyric acid metabolism and oxidative damage in wheat (Triticum aestivum L.) seedlings under salt and osmotic stress. J Plant Physiol 170(11):1003–1009
AlSalhi MS, Tashish W, Al-Osaif SS, Atif M (2019) Effects of He–Ne laser and argon laser irradiation on growth, germination, and physico-biochemical characteristics of wheat seeds (Triticum aestivum L.). Laser Phys 29(1):1–8
Andrews M, Sprent JI, Raven JA, Eady PE (1999) Relationships between shoot to root ratio, growth and leaf soluble protein concentration of Pisum sativum, Phaseolus vulgaris and Triticum aestivum under different nutrient deficiencies. Plant Cell Environ 22:949–958
Asim M, Ullah Z, Xu F, An L, Aluko OO, Wang Q, Liu H (2020) Nitrate signaling, functions, and regulation of root system architecture: insights from arabidopsis thaliana. Genes 11(6):633
Balakhnina T, Bulak P, Nosalewicz M, Pietruszewski S, Włodarczyk T (2015) The influence of wheat Triticum aestivum L. seed pre-sowing treatment with magnetic fields on germination, seedling growth, and antioxidant potential under optimal soil watering and flooding. Acta Physiol Plant 37(3):1–10
Bianchi G, Falcinelli B, Tosti G, Bocci L, Benincasa P (2019) Taste quality traits and volatile profiles of sprouts and wheatgrass from hulled and non-hulled Triticum species. J Food Biochem 43(7):1–11
Bona L, Zsoldos F, Pécsváradi A, Vashegyi Á (2001) Root growth and enzyme activity studies for indication of low pH stress in common and durum wheat. In: Bedö Z, Lang L (eds) Wheat in a global environment. Developments in plant breeding, vol 8. Springer, Dordrecht
Bradu C, Kutasi K, Magureanu M, Puač N, Živković S (2020) Reactive nitrogen species in plasma-activated water: generation, chemistry and application in agriculture. J Phys D Appl Phys 53(22):1–21
Carillo P (2018) GABA shunt in durum wheat. Front Plant Sci 9:100
Chen L, Yin HX, Xu J, Liu XJ (2011) Enhanced antioxidative responses of a salt-resistant wheat cultivar facilitate its adaptation to salt stress. Afr J Biotechnol 10(74):16887–16896
Chen J, Liu S, Zhang S, Ge C, Shen Q, Ma H, Zhang X, Dong H, Zhao X, Pang C (2020) Nitrogen modulates cotton root morphology by affecting abscisic acid (ABA) and salicylic acid (SA) content. Arch Agron Soil Sci 1:1–17
Chen YQ, Cheng JH, Sun D-W (2020) Chemical, physical and physiological quality attributes of fruit and vegetables induced by cold plasma treatment: mechanisms and application advances. Crit Rev Food Sci Nutr 60:2676–2690
Chizoba Ekezie FG, Cheng JH, Sun D-W (2017) A review on recent advances in cold plasma technology for the food industry: current applications and future trends. Trends Food Sci Tech 69:46–58
Chizoba Ekezie FG, Cheng JH, Sun D-W (2018) Effects of mild oxidative and structural modifications induced by argon plasma on physicochemical properties of actomyosin from king prawn (Litopenaeus vannamei). J Agric Food Chem 66(50):13285–13294
Chizoba Ekezie FG, Sun D-W, Cheng JH (2019a) Altering the IgE binding capacity of king prawn (Litopenaeus Vannamei) tropomyosin through conformational changes induced by cold argon-plasma jet. Food Chem 300:125143
Chizoba Ekezie FG, Cheng JH, Sun D-W (2019b) Effects of atmospheric pressure plasma jet on the conformation and physicochemical properties of myofibrillar proteins from king prawn (Litopenaeus vannamei). Food Chem 276:147–156
de Groot GJJB, Hundt A, Murphy AB, Bange MP, Mai-Prochnow A (2018) Cold plasma treatment for cotton seed germination improvement. Sci Rep 8:14372–14381
Esua OJ, Cheng JH, Sun D-W (2021a) Novel technique for treating grass carp (Ctenopharyngodon idella) by combining plasma functionalized liquids and ultrasound: effects on bacterial inactivation and quality attributes. Ultrason Sonochem 76:105660
Esua OJ, Cheng JH, Sun D-W (2021b) Optimization of treatment conditions for reducing shewanella putrefaciens and salmonella typhimurium on grass carp treated by thermoultrasound-assisted plasma functionalized buffer. Ultrason Sonochem 76:105609
Esua OJ, Sun D-W, Cheng JH, Wang H, Chen C (2022a) Hybridising plasma functionalized water and ultrasound pretreatment for enzymatic protein hydrolysis of larimichthys polyactis: parametric screening and optimization. Food Chem 385:132667
Esua OJ, Sun D-W, Cheng JH, Wang H, Lv M (2022b) Functional and bioactive properties of larimichthys polyactis protein hydrolysates as influenced by plasma functionalized water-ultrasound hybrid treatments and enzyme types. Ultrason Sonochem 86:106023
Esua OJ, Sun D-W, Cheng JH, Li JL (2022c) Evaluation of storage quality of vacuum-packaged silver pomfret (Pampus argenteus) treated with combined ultrasound and plasma functionalized liquids hurdle technology. Food Chem 391:133237
Eveland AL, Jackson DP (2012) Sugars, signalling, and plant development. J Exp Bot 63(9):3367–3377
Fan L, Liu X, Ma Y, Xiang Q (2020) Effects of plasma-activated water treatment on seed germination and growth of mung bean sprouts. J Taibah Univ Sci 14(1):823–830
FAO, IFAD, UNICEF, WFP, WHO (2020) The state of food security and nutrition in the world. Roma
Forde BG (2000) Nitrate transporters in plants: structure, function and regulation. Biochem Biophys Acta 14(65):219–235
Gierczik K, Vukušić T, Kovács L, Székely A, Szalai G, Milošević S, Kocsy G, Kutasi K, Galiba G (2020) Plasma-activated water to improve the stress tolerance of barley. Plasma Processes Polym 17(3):1–16
Guimarães B, Polachini TC, Augusto PED, Telis-Romero J (2020) Ultrasound-assisted hydration of wheat grains at different temperatures and power applied: effect on acoustic field, water absorption and germination. Chem Eng Process 155:1–11
Guo R, Shi L, Jiao Y, Li M, Zhong X, Gu F, Liu Q, Xia X, Li H (2018) Metabolic responses to drought stress in the tissues of drought-tolerant and drought-sensitive wheat genotype seedlings. AoB Plants 10(2):1–13
Han YX, Cheng JH, Sun D-W (2019a) Activities and conformation changes of food enzymes induced by cold plasma: a review. Crit Rev Food Sci Nutr 59: 794–811
Han YX, Cheng JH, Sun D-W (2019b) Changes in activity, structure and morphology of horseradish peroxidase induced by cold plasma. Food Chem 301:125240
Jiang YH, Cheng JH, Sun D-W (2020) Effects of plasma chemistry on the interfacial performance of protein and polysaccharide in emulsion. Trends Food Sci Tech 98:129–139
Jimenez-Sanchez C, Lozano-Sanchez J, Segura-Carretero A, Fernandez-Gutierrez A (2017) Alternatives to conventional thermal treatments in fruit-juice processing. Part 2: Effect on composition, phytochemical content, and physicochemical, rheological, and organoleptic properties of fruit juices. Crit Rev Food Sci Nutr 57(3):637–652
Karmakar S, Billah M, Hasan M, Sohan SR, Hossain MF, Faisal Hoque KM, Kabir AH, Rashid MM, Talukder MR, Reza MA (2021) Impact of LFGD (Ar+O2) plasma on seed surface, germination, plant growth, productivity and nutritional composition of maize (Zea mays L.). Heliyon 7(3):1–12
Kim S, Kim CH (2021) Applications of plasma-activated liquid in the medical field. Biomedicines 9(11):1–14
Knipfer T, Danjou M, Vionne C, Fricke W (2021) Salt stress reduces root water uptake in barley (Hordeum vulgare L.) through modification of the transcellular transport path. Plant Cell Environ 44(2):458–475
Kopriva S (2015) Plant sulfur nutrition: from sachs to big data. Plant Signal Behav 10(9):1–5
Kučerová K, Henselová M, Slováková Ľ, Hensel K (2019) Effects of plasma activated water on wheat: germination, growth parameters, photosynthetic pigments, soluble protein content, and antioxidant enzymes activity. Plasma Processes Polym 16(3):1–14
Kučerová K, Henselová M, Slováková Ľ, Bačovčinová M, Hensel K (2021) Effect of plasma activated water, hydrogen peroxide, and nitrates on lettuce growth and its physiological parameters. Appl Sci 11(5):1985
Li S-X, Hong H-X, Zheng F-Y, Deng N-S, Lin F (2007) Influence of nitrate on metal sorption and bioaccumulation in marine phytoplankton, Dunaliella Salina. Environ Toxicol 22(6):582–586
Li Z, Yu J, Peng Y, Huang B (2016) Metabolic pathways regulated by gamma-aminobutyric acid (GABA) contributing to heat tolerance in creeping bentgrass (Agrostis stolonifera). Sci Rep 6:1–16
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. Curr Protoc Food Anal Chem 1:431–438
Ling L, Jiafeng J, Jiangang L, Minchong S, Xin H, Hanliang S, Yuanhua D (2014) Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci Rep 4:5859–5866
Ling L, Jiangang L, Minchong S, Chunlei Z, Yuanhua D (2015) Cold plasma treatment enhances oilseed rape seed germination under drought stress. Sci Rep 5:13033–13042
Lo Porto C, Ziuzina D, Los A, Boehm D, Palumbo F, Favia P, Tiwari B, Bourke P, Cullen PJ (2018) Plasma activated water and airborne ultrasound treatments for enhanced germination and growth of soybean. Innov Food Sci Emerg Technol 49:13–19
Lotfy K, Al-Harbi NA, Abd El-Raheem H (2019) Cold atmospheric pressure nitrogen plasma jet for enhancement germination of wheat seeds. Plasma Chem Plasma Process 39(4):897–912
Maoka T (2020) Carotenoids as natural functional pigments. J Nat Med 74(1):1–16
Martínez-Ballesta MC, Dominguez-Perles R, Moreno DA, Muries B, Alcaraz-López C, Bastías E, García-Viguera C, Carvajal M (2011) Minerals in plant food: effect of agricultural practices and role in human health. Agron Sustain Dev 30(2):295–309
Mozafar A (1996) Decreasing the NO3− and increasing the vitamin C contents in spinach by a nitrogen deprivation method. Plant Foods Hum Nutr 49:155–162
Mu X, Chen Q, Wu X, Chen F, Yuan L, Mi G (2018) Gibberellins synthesis is involved in the reduction of cell flux and elemental growth rate in maize leaf under low nitrogen supply. Environ Exp Bot 150:198–208
Ndiffo Yemeli GB, Švubová R, Kostolani D, Kyzek S, Machala Z (2020) The effect of water activated by nonthermal air plasma on the growth of farm plants: case of maize and barley. Plasma Processes Polym 18(1):1–16
Pan Y, Cheng JH, Sun D-W (2019a) Cold plasma-mediated treatments for shelf life extension of fresh produce: a review of recent research developments. Compr Rev Food Sci F 18(5):1312–1326
Pan Y, Cheng JH, Lv X, Sun D-W (2019b) Assessing the inactivation efficiency of Ar/O2 plasma treatment against listeria monocytogenes cells: sublethal injury and inactivation kinetics. LWT-Food Sci Technol 111:318–327
Pan Y, Zhang Y, Cheng JH, Sun D-W (2020) Inactivation of listeria monocytogenes at various growth temperatures by ultrasound pretreatment and cold plasma. LWT-Food Sci Technol 118:108635
Pan YW, Cheng JH, Sun D-W (2021) Inhibition of fruit softening by cold plasma treatments: affecting factors and applications. Crit Rev Food Sci Nutr 61(12):1935–1946
Qamar A, Saeed F, Nadeem MT, Hussain AI, Khan MA, Niaz B (2019) Probing the storage stability and sensorial characteristics of wheat and barley grasses juice. Food Sci Nutr 7(2):554–562
Ranieri P, Sponsel N, Kizer J, Rojas-Pierce M, Hernández R, Gatiboni L, Grunden A, Stapelmann K (2020) Plasma agriculture: review from the perspective of the plant and its ecosystem. Plasma Processes Polym 18(1):1–24
Rossi S, Chapman C, Huang B (2020) Suppression of heat-induced leaf senescence by γ-aminobutyric acid, proline, and ammonium nitrate through regulation of chlorophyll degradation in creeping bentgrass. Environ Exp Bot 177:1–9
Rossi S, Chapman C, Yuan B, Huang B (2021) Improved heat tolerance in creeping bentgrass by γ-aminobutyric acid, proline, and inorganic nitrogen associated with differential regulation of amino acid metabolism. Plant Growth Regul 93(2):231–242
Roy NC, Hasan MM, Kabir AH, Reza MA, Talukder MR, Chowdhury AN (2018) Atmospheric pressure gliding arc discharge plasma treatments for improving germination, growth and yield of wheat. Plasma Sci Technol 20(11):1–11
Scholtz V, Sera B, Khun J, Sery M, Julak J (2019) Effects of nonthermal plasma on wheat grains and products. J Food Qual 2019:1–11
Sergeichev KF, Lukina NA, Sarimov RM, Smirnov IG, Simakin AV, Dorokhov AS, Gudkov SV (2021) Physicochemical properties of pure water treated by pure argon plasma jet generated by microwave discharge in opened atmosphere. Front Phys 8:1–11
Sharma N, Tiwari V, Vats S, Kumari A, Chunduri V, Kaur S, Kapoor P, Garg M (2020) Evaluation of anthocyanin content, antioxidant potential and antimicrobial activity of black, purple and blue colored wheat flour and wheat-grass juice against common human pathogens. Molecules 25(24):1–19
Shen J, Tian Y, Li Y, Ma R, Zhang Q, Zhang J, Fang J (2016) Bactericidal effects against S. aureus and physicochemical properties of plasma activated water stored at different temperatures. Sci Rep 6:28505
Skoczylas U, Korus A, Tabaszewska M, Gedos K, Szczepanska E (2018) Evaluation of the quality of fresh and frozen wheat plantlet juices depending on the time of grass harvest. J Food Process Preserv 42(1):1–8
Song JS, Kim SB, Ryu S, Oh J, Kim DS (2020) Emerging plasma technology that alleviates crop stress during the early growth stages of plants: a review. Front Plant Sci 11:988–1002
Tanaka R, Tanaka A (2011) Chlorophyll cycle regulates the construction and destruction of the light-harvesting complexes. Biochem Biophys Acta 1807(8):968–976
Tari I, Csiszár J (2003) Effects of NO2− or NO3− supply on polyamine accumulation and ethylene production of wheat roots at acidic and neutral pH: implications for root growth. Plant Growth Regul 40:121–128
Than HAQ, Pham TH, Nguyen DKV, Pham TH, Khacef A (2021) Non-thermal plasma activated water for increasing germination and plant growth of Lactuca sativa L. Plasma Chem Plasma Process 42(1):73–89
Thirumdas R, Kothakota A, Annapure U, Siliveru K, Blundell R, Gatt R, Valdramidis VP (2018) Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci Technol 77:21–31
Tian P-P, Lv Y-Y, Yuan W-J, Zhang S-B, Hu Y-S (2019) Effect of artificial aging on wheat quality deterioration during storage. J Stored Prod Res 80:50–56
Velichko I, Gordeev I, Shelemin A, Nikitin D, Brinar J, Pleskunov P, Choukourov A, Pazderů K, Pulkrábek J (2019) Plasma jet and dielectric barrier discharge treatment of wheat seeds. Plasma Chem Plasma Process 39(4):913–928
Verkroost AW, Wassen MJ (2005) A simple model for nitrogen-limited plant growth and nitrogen allocation. Ann Bot 96(5):871–876
Vlad I-E, Anghel SD (2017) Time stability of water activated by different on-liquid atmospheric pressure plasmas. J Electrostat 87:284–292
Wang J, Ye Y, Li Q, Abbasi AM, Guo X (2017) Assessment of phytochemicals, enzymatic and antioxidant activities in germinated mung bean (Vigna radiata L. Wilezek). Int J Food Sci Technol 52(5):1276–1282
Wang C, Wei L, Zhang J, Hu D, Gao R, Liu Y, Feng L, Gong W, Liao W (2022) Nitric oxide enhances salt tolerance in tomato seedlings by regulating endogenous S-nitrosylation levels. J Plant Growth Regul. https://doi.org/10.1007/s00344-021-10546-5
Woodrow P, Ciarmiello LF, Annunziata MG, Pacifico S, Iannuzzi F, Mirto A, D’Amelia L, Dell’Aversana E, Piccolella S, Fuggi A, Carillo P (2017) Durum wheat seedling responses to simultaneous high light and salinity involve a fine reconfiguration of amino acids and carbohydrate metabolism. Physiol Plant 159(3):290–312
Wu Y, Cheng JH, Sun D-W (2022) Subcellular damages of colletotrichum asianum and inhibition of mango anthracnose by dielectric barrier discharge plasma. Food Chem 381:132197
Xiang N, Guo X, Liu F, Li Q, Hu J, Brennan CS (2017) Effect of light- and dark-germination on the phenolic biosynthesis, phytochemical profiles, and antioxidant activities in sweet corn (Zea mays L.) sprouts. Int J Mol Sci 18(6):1246–1259
Xiang Q, Kang C, Niu L, Zhao D, Li K, Bai Y (2018) Antibacterial activity and a membrane damage mechanism of plasma-activated water against Pseudomonas deceptionensis CM2. LWT Food Sci Technol 96:395–401
Xu LH, Wang WY, Guo JJ, Qin J, Shi DQ, Li YL, Xu J (2014) Zinc improves salt tolerance by increasing reactive oxygen species scavenging and reducing Na+ accumulation in wheat seedlings. Biol Plant 58(4):751–757
Xu W, Song Z, Luan X, Ding C, Cao Z, Ma X (2019) Biological effects of high-voltage electric field treatment of naked oat seeds. Appl Sci 9(18):3829–3843
Yue J, You Y, Zhang L, Fu Z, Wang J, Zhang J, Guy RD (2018) Exogenous 24-epibrassinolide alleviates effects of salt stress on chloroplasts and photosynthesis in Robinia pseudoacacia L. seedlings. J Plant Growth Regul 38(2):669–682
Zhang S, Rousseau A, Dufour T (2017) Promoting lentil germination and stem growth by plasma activated tap water, demineralized water and liquid fertilizer. R Soc Chem Adv 7(50):31244–31251
Zhou R, Li J, Zhou R, Zhang X, Yang S (2019) Atmospheric-pressure plasma treated water for seed germination and seedling growth of mung bean and its sterilization effect on mung bean sprouts. Innov Food Sci Emerg Technol 53:36–44
Zhou R, Zhou R, Wang P, Xian Y, Mai-Prochnow A, Lu X, Cullen PJ, Ostrikov K, Bazaka K (2020) Plasma-activated water: generation, origin of reactive species and biological applications. J Phys D Appl Phys 53(30):1–27
Zhu H, Han Z, Cheng JH, Sun D-W (2022) Modification of cellulose from sugarcane (Saccharum Officinarum) bagasse pulp by cold plasma: dissolution, structure and surface chemistry analysis. Food Chem 374:131675
Zhu M, Wang Q, Sun Y, Zhang J (2021) Effects of oxygenated brackish water on germination and growth characteristics of wheat. Agric Water Manag 245:1–9
Zsoldos F, Vashegyi A, Pecsvaradi A, Haunold E, Herger P (1999) Nitrate and nitrite transport into cereals affected by low pH and different temperatures. Cereal Res Commun 27:4
Funding
Open Access funding provided by the IReL Consortium. The authors are grateful to the National Natural Science Foundation of China (31972205) for its support. This research was also supported by the International S&T Cooperation Projects of Guangdong Province (2020A0505100007), Guangdong Basic and Applied Basic Research Foundation (2021A1515010644), the Contemporary International Collaborative Research Centre of Guangdong Province on Food Innovative Processing and Intelligent Control (2019A050519001), the Guangzhou Key Laboratory for Intelligent Sensing and Quality Control of Agricultural Products (202102100009) and the Common Technical Innovation Team of Guangdong Province on Preservation and Logistics of Agricultural Products (2021KJ145).
Author information
Authors and Affiliations
Contributions
JW: Writing—original draft, Formal analysis, Investigation. J-HC: Validation, Funding acquisition, Resources. D-WS: Supervision, Funding acquisition, Resources, Writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Additional information
Handling Editor: Stefan de Folter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, J., Cheng, JH. & Sun, DW. Enhancement of Wheat Seed Germination, Seedling Growth and Nutritional Properties of Wheat Plantlet Juice by Plasma Activated Water. J Plant Growth Regul 42, 2006–2022 (2023). https://doi.org/10.1007/s00344-022-10677-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-022-10677-3