Abstract
This study deals with the determination of germinability of black medick (Medicago lupulina L.) seeds in conditions of different drought intensity induced by different concentrations of PEG 8000 solutions (0.2; 0.4; 0.6 mol). Four batches of seed were tested (Ekola 2–5). At the same time, the influence of boron, some other elements (N, P, K, Ca, Mg, Zn, Cu, Mn) and compounds (starch, lipids and sugars) contents in black medick seeds on their germinability (percentages of germinated, dead and hard seeds) was evaluated. The effect of drought was manifested by germination which was reduced max. by 8% as compared with the control variant and was only partially significant. Statistically significant (P < 0.01) negative correlations (R = − 0.64) were recorded between germinability and the boron content in the seeds, and positive correlations were found between the percentages of hard seeds and the boron content in the seeds. The results show that the higher content of boron in the seeds of black medick increases the proportion of hard seeds at the expense of the germinated seeds percentages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Black medick is a species widely spread in Europe and Asia, which occurs on abandoned fields as well as on alpine plateaus at altitudes of 2900 and 3000 m above sea level. The plant has tiny, distinctly yellow flowers appearing from June to October. Seeds ripen from the end of August to mid-September (Zhang et al. 2010). Black medick grows on grasslands, fields, wastelands, pastures and roads. It often occurs as a garden weed on acidic and calcareous soils. It is used as a part of seed mixtures sown on meadows or pastures, suitable, namely for dry regions. Black medick is also an ideal honey crop (Baloch et al. 2013).
Plants can cope with drought by adapting metabolic processes leading to a better accumulation of substances, such as sugars. Soluble sugars play a great role in the regulation of osmotic pressure during germination (Gorham et al. 1981, Kaur Gill 2003; Gyuricza et al. 2015). Alternative sources of sugar during germination are starch and hemicellulose (Balasaraswathi and Sadasivam 1997). During the first 24 h, sugars are mobilized as an initial source of energy important for germination (Tonguç et al. 2012). Vandecasteele et al. (2011) point to a positive correlation of available sugar and germinability of Medicago truncatula Gaertn. The correlation between the percentage of germination capacity and the percentage of starch, protein or fat supplies was not demonstrated though (Alencar et al. 2012).
Germination depends on the presence of many different elements. An increased concentration of macronutrients in the surrounding environment often results in the production of seeds of higher weight and amount and with increased concentrations of nitrogen and phosphorus (Kołodziejek et al. 2017). Nitrogen contained in the seeds of plants, namely in their germ reserves, is important for the growth and development of plants (Naegle et al. 2005). The produce of grown plants is affected by the amount of phosphorus in seeds. It is assumed that the higher amount of phosphorus in the seeds is, the higher is the crop yield with no regard to whether the soil was or was not fertilized with phosphorus (Bolland et al. 1990). In terms of plant nutrition, also calcium is an important element in the nutrition of cell walls and for the correct functioning of plant membranes. Calcium demonstrably inhibits the uptake of nitrogen ions, hence reducing their adverse effect on the germination capacity of seeds (Zehra et al. 2012). Micro-elements that are necessary for the germination of seeds as well as for the growth of plants should be considered very important, too. They mostly include metals (zinc, iron) and semi-metals, such as boron and selenium (Rerkasem et al. 1997; Shaikh et al. 2013; Alamris et al. 2018).
Metals have generally a negative effect on the germination of seeds, reducing the uptake and transport of water or causing embryonic damage or seed dieback. Phytotoxicity in plants is also affected by the concentration of heavy metals with the high amount of metals increasing phytotoxicity to roots, whilst the low concentrations of heavy metals result in the decreased phytotoxicity to roots (Shaikh et al. 2013; El Rasafi et al. 2016). Zinc is, for example, an element necessary for the growth of plants as it participates in biochemical and physiological processes as well as in the process of germination (Caramete et al. 1974; Burzo et al. 1999; Rout et al. 2003; Tsonev et al. 2012, Stratu et al. 2015). According to Stratu et al. (2015), an unfavourable effect of high zinc concentration on the seed germination was not recorded; instead, an apparent impact of zinc on the slower growth of transplants was demonstrated.
An indispensable element enabling plants to accomplish their life cycle is boron (Alamris et al. 2018). Boron is a biogenic element the plants could not grow without or reproduce by seeds. Rerkasem et al. (1997) assessed the seeds of soybean (Glycine max L.) with the low concentration of boron and found out that the low concentration of boron causes permanent damage to germ embryos, which subsequently prevents the plant germination or the germinated plants are damaged. In seeds with a boron concentration slightly higher than in the other variants, the permanent damage did not occur but the plants required a higher concentration of boron in the surrounding environment to provide for normal development. Seeds which developed in plants with a sufficient supply of boron exhibited a high germination percent (Pandey and Gupta 2013).
Typical symptoms of boron insufficiency at a stage of reproduction include the drop of buds, flowers and developing fruits and changes in the quality of fruits and lower viability of seeds (Marschner 1995). Chen et al. (2020) inform that alfalfa accumulated large amounts of sugars when deficient of boron, which limited the transport of sugars to other organs and seeds, thus impacting the seed yield. It is assumed that the accumulation of sugars in cells protects plants from stress (Liu et al. 2015). Recent evidences show that a high concentration of soluble sugars can lead to the accumulation of phenolic substances in plants with the insufficient content of boron (Brown et al. 2002). Accumulation of phenolic substances is a typical indicator of stress due to boron deficiency in plants (Camacho-Cristobal et al. 2004) and increases endogenous levels of indole-3-acetic acid (IAA) (Archana and Pandey 2016). Boron regulates the IAA concentration to optimal level, which supports the germination of seeds. It is known that high IAA concentrations inhibit the seed germination (Brady et al. 2003; Jarvis et al. 1984). It was published that apparently normal seeds of plants with the lack of boron might have altered physiological and growth characteristics (Dell and Huang 1997).As mentioned above, the symptoms of boron deficiency for plants and its influence on their growth anf production of seeds, i.e. on the reproduction of individual seed plants were well described (Marschner 1995; Brown et al. 2002; Chen et al. 2020). However, it has not been sufficiently investigated how the micro-element affects the vitality of seeds during the process of their germination as in modern agriculture, the germination of seeds represents a decisive moment determining the success of the whole process of growing a specific crop (Norman 2002, Küchenmeister et al. 2012, 2013).
A significant factor affecting the germination capacity of seeds is the hardseededness of clovers. It is a type of dormancy whose mechanism is based on the impermeability of seed case for water which induces swelling and germination. In wild plants which stay on the same site for a longer period and regenerate the stand also by gradual germination of hard seeds, hardseededness can be considered a favourable feature important for their survival (Norman 2002). In a majority of cultural clovers, however, even germination and emergence are required. A high share of hard seeds in the seed stock of clovers prevents the formation of closed and equal stands; hardseededness is then considered an undesirable feature of seed stock (Smetham 2003). Methods of testing drought resistance in vitro and understanding mechanisms of dormancy facilitate an increased use of legumes in monocultures as well as in mixtures of legumes and cereals in the systems of mixed cultures. Such a testing could possibly also contribute to extend the supply of cover crops seeds and their use in improving the quality of arable land (Kintl et al. 2018; Handlirova et al. 2017; Kintl et al. 2020).
The main goal of this research was to determine the germination capacity of black medick seeds in conditions of different intensities of drought induced by various concentrations of PEG 8000 solution. At the same time, the influence of boron content in black medick seeds on their germinability and the number of hard seeds was studied.
Material and Methods
Drought resistance of seeds from five seed lots of black medick (Medicago lupulina L.) var. Ekola harvested in 2015–2017 was evaluated in laboratory conditions. All seed stocks used were registered by Central Institute for Supervising and Testing in Agriculture of the Czech Republic in line with EU seed marketing directives:
Ekola 02: 6-0280-60605/01 (Basic seed).
Ekola 03: 5-0280-60601/02 (Certified seed).
Ekola 04: 7-0280-60605/02 (Certified seed).
Ekola 05: 5-0280-60601/01 (Certified seed).
The mineralization or incineration of individual samples was followed by analyses of the contents of N, Ca, P, K, Mg, B, Cu, Zn, Fe, and Mn and the contents of organic matter, i.e. starch, fat and sugar in the seeds according to Browman (1989). The content of N was determined according to Lu (1999); K according to Nowosielski (1974) and determination of Ca and Mg was performed on the basis of Ryan et al. (2001). All analyses and all measurements were performed using atomic absorption spectrometry (AAS; Agilent 55B AA; Agilent Technologies, CA, USA). The content of P was determined using spectrophotometry (Spectrophotometer: Onda VIS V-10 Plus, Giorgio Bormac, ITA) according to Olsen and Sommers (1982). Available Cu, Zn, Fe and Mn contents were measured after extraction by diethylenetriaminepentaacetic acid (Linday et al. 1978) using AAS (Agilent 55B AA; Agilent Technologies, CA, USA) according to Jones (2001).
The content of starch in seeds was determined using the polarimetric method—(Polamat S, Carl Zeiss Jena GmbH, Jena, DEU) according to ISO 6493:2000. The content of fat was determined gravimetrically using the water-cooled Soxhlet’s extractor by direct sample extraction with petrolether (Garcı́a-Ayuso and Luque de Castro 1999). The content of sugars (reducing saccharides) was established using the Luff–Schoorl method (Pomeranz & Meloan 1994; Marrubini et al. 2017).
Drought stress was simulated by the application of polyethylene glycol solution (PEG 8000) in three different concentrations (0.2, 0.4, 0.6). Thus, there were altogether three experimental groups prepared with the simulation of drought (0.2, 0.4 and 0.6 mol of PEG) and one control group (0.0 mol of PEG) with distilled water. Each group was divided into four variants with the different ratio of seed stock: Ekola 2, Ekola 3, Ekola 4 and Ekola 5. The experimental variants were represented by three stages of different osmotic pressures induced by different concentrations of PEG solution (0.2, 0.4 and 0.6) according to Kintl (2019). Distilled water was used as a control sample.. A list of the experimental variants is presented in Table 1 and the laboratory experiment organization is illustrated in Annex A.
For the purposes of this research, we studied the effect of drought simulation (using PEG 8000 solution) and substances contained in seeds on their germinability and on the number of hard and dead seeds. Germinability was evaluated based on the methodology of International Seed Testing Association (ISTA 2017), according to which the percentage of seed germinability is determined by testing 100 seeds in four repetitions, i.e. 400 seeds in each variant. The procedure is shown in Annex B. It started with pre-cooling the seeds in order to interrupt dormancy. The seeds were spread onto a moistened filter paper (with or without the addition of PEG) in Petri dishes of 8 cm in diameter. The Petri dishes were then placed in the thermostat where they were kept for 4 days at a temperature of 5 °C. After the end of the pre-cooling process, the Petri dishes were maintained at a temperature of 20 °C. Germinated seeds were counted at specified intervals. The germinative capacity of seeds of the given species and treatment variant was determined after the counting had been accomplished in all repetitions.
Germination percentage is calculated according to the following formula (Faijunnahar et al. 2017):
In addition to the number of germinable seeds, numbers of dead and hard seeds were count in all repetitions.
The obtained data were analysed using the Statistica 12 programme (Dell Software, Round Rock, Texas, USA). The initial input data analysis was followed using the one- way ANOVA combined with the post-hoc Tukey’s HSD test. The correlation analysis was then made to establish the Spearman’s correlation coefficient R between germinability, number of hard seeds and boron content in the seeds. The relation between the contents of individual elements (P, K, Ca, Mg and N) and organic matter and germinability was analysed using PCA and factor analysis. All analyses were performed at a significance level of P < 0.05.
Results and Discussion
Comparison of the Numbers of Germinable, Hard and Dead Seeds in Dependence on Drought Simulation
Average germinative capacity of all four seed lots that were not exposed to drought simulation (without PEG treatment) ranged from 86.0 (Ekola 2) to 94.0% (Ekola 4). Statistically significant differences in the percentages of germinated seeds were found between the Ekola 2 and Ekola 5 seed lots and the Ekola 4 seed lot (Table 2). Similar results were observed also in the percentages of hard and dead seeds. Thus, it is possible to state that in ideal conditions without the drought stress, significant differences exist amongst the individual lots of seeds. The measured data show that the best germinative capacity in the given conditions was recorded in Ekola 4.
Further on, a general influence of drought simulation on the germinative capacity of seeds was studied (Table 3). The average germinability of all four seed lots during the treatment with the PEG solution (concentrations 0.0 – 0.6 mol) ranged from 85.9 (Ekola 2) to 93.6% (Ekola 4). Thus, a maximum difference in germinability between the Ekola 2 and Ekola 4 seed lots was 7.7%. Similarly as in the set of seeds without the PEG treatment (Table 2), statistically significant differences (P < 0.05) in the numbers of germinable, hard and dead seeds were found in variants with the PEG treatment (Table 3) between the Ekola 2 and Ekola 5 seed lots and the Ekola 4 seed lot. In addition, significant differences were found also as compared with the Ekola 3 seed lot. The measured values show again that Ekola 4 exhibited the best features in terms of germinative capacity both without the simulated drought (Table 2) and with the exposure to the action of PEG solution (Table 3). Statistical differences at a significance level P < 0.05 were found also in the germinable and hard seeds at a significance level of P < 0.01 (Table 2). In the dead seeds, only one significant difference was recorded at the more rigorous significance level of 1% (P < 0.01) between Ekola 3 and the other seed lots. In Ekola 3, it was the highest representation of dead seeds (8%).
Differences found in the germinated seeds and hard seeds were significant even at a more rigorous significance level of P < 0.01 (Table 3). On the other hand, in the case of dead seeds, a lower number of significant differences were recorded, similarly as in the preceding case of comparing only the control variants (Table 2). Differences (P < 0.01) were detected only between the Ekola 2–3 and Ekola 4–5 variants with the representation of dead seeds in the Ekola 2 and Ekola 3 variants was significantly the highest as compared with the Ekola 4 and Ekola 5 variants in which it was the lowest (less than 3% of dead seeds).
In order to determine the potential influence of drought as a significant factor on seed germinability, the analysis of average data from the respective seed lots (Table 3) was complemented with another comparison, this time of all seed lots (Table 4). We monitored and analysed the influence of PEG application (control = 0.0 PEG, i.e. only distilled water; drought simulation = 0.2; 0.4; and 0.6 mol PEG) without the distinction of individual seed lots. Statistically significant differences (P < 0.05) were found only in the parameter of the germinated percentages (Table 4). The measured values clearly show that differences in the germinative capacity are not too big, max. 4.4% between the control (0.0 mol) and the highest intensity of PEG treatment (0.6 mol), i.e. the most intensive drought simulation. The values are relatively interesting because they indicate that some seed lots exhibited a higher tolerance to drought, this concerning Ekola 3 and Ekola 4, the mean germinative capacity of which was by more than 5% higher as compared with Ekola 2 and Ekola 5. Reasons should be probably sought in the characteristics of plants whose seeds were used in the experiment and in the origin of the specific seed lots. Above all, in the case of more rigorous significance level (P < 0.01), no significant effect of PEG application was found (Table 4).
Apart from one exception (Ekola 5), the individual seed lots of Ekola (2; 3; 4 and 5) with and without the PEG treatments of diverse intensities (control; 0.2; 0.4; 0.6 mol) did not show any statistically significant differences in the numbers of germinable seeds (Table 5). Nevertheless, all seed lots exhibited a mild increase of germinative capacity at the first degree of drought simulation (PEG = 0.2 mol). Although the increase was non-significant, it might have indicated that a mild drought stress was desirable for the given species. Then we monitored differences between the respective seed lots. Ekola 4 exhibited at all times the highest germinative capacity (> 91%) at all degrees of drought simulation; high values were recorded also in the seed lot of Ekola 3 (> 89%). On the other hand, the seed lots of Ekola 2 and Ekola 5 showed the lowest values at all degrees of drought simulation. In case of degree 0.2, 0.6 and the control variant 0.0, the values of germinability were significantly lower than those of Ekola 4. Comparing the measured values also in respect of different significance levels, it is apparent that significant values were not found in any of the measured parameters within individual’s variants (seed lots) at a more rigorous level (P < 0.01). On the contrary, significant differences (P < 0.01) were found between the individual seed lots. Variants Ekola 3 and Ekola 4 achieved the highest germination in the control treatment and in the simulation of drought with PEG solution (0.4 mol).
In this study, drought was simulated using a non-penetrating osmotic of polyethylene glycol (PEG) 8000 which is standardly used to simulate drought in experiments with plants and seeds (Gill et al. 2003). PEG decreases availability of water to seeds, does not enter seed cells and does not interfere with their metabolism. Interpreting results of experiments with PEG, one has to be aware of differences existing as compared with the natural drought stress in the soil environment. The most significant difference is in the onset of drought which is mostly slow in the soil environment whilst being immediate with the use of PEG solution (Gill et al. 2003). Thus, a situation may occur that if high PEG concentrations are used, the seeds are exposed to unnatural intensive drought stress and their response (reduced germinability) is apparently different than it would be in the natural conditions (Gill et al. 2003; Muscolo et al. 2006). Based on Gill et al. (2003), Muscolo et al. (2006), Tang et al. (2019), Kintl et al. (2019) and Kintl et al. (2021), we decided to use the PEG solution in several concentrations in order to eliminate the possible adverse effect of rapid onset of drought. Looking on the measured values (Table 4 and Table 5), we can see that the onset of drought was slow and germinative capacity was decreasing continuously. Similar results were recorded by Tang et al. (2019) who studied germinative capacity and germination energy of kenaf seeds using various concentrations of PEG 6000 to simulate drought. Germinative capacity was gradually decreasing from the control variant to the variant with the highest PEG concentration. There, it can be assumed that in our study we succeeded in having had induced drought stress as similar as possible to the stress which the seeds could have been exposed to in the soil environment.
The seed lot (Ekola 2–5) was apparently a factor which had a greater influence on the percentage of germinated, dead and hard seeds than the treatment with various PEG concentrations in order to induce diverse intensities of drought. The measured values even indicate that a mild drought stress could have been potentially favourable for the tested seeds. Based on results of experiments with two Medicago species, Patanè (2008) found out that the ratio of germinable and hard seeds very strongly depended on the degree of stress caused by the lack of water during the development and ripening of seeds. Plants can bloom within six weeks from emergence and blossom during the whole growing period (Turkington and Cavers 1979). This is why a single plant can bear seeds in different phases of ripening. Seeds are formed 9 weeks after the beginning of bloom (Turkington and Cavers 1978).
The germinative capacity of black medick seeds is affected by a range of environmental factors (Kintl et al. 2021). The differences observed between the studied seed lots (Ekola 2–5) might be related to the seed origin and conditions in which the ripening of seeds took place. Boe et al. (2016) inform that seeds which ripened at the beginning of August were by 25% heavier but their germinability was by 20% lower than that of seeds which ripened in September.
According to Sidhu (1971), average weights of seeds for five studied populations ranged from 0.0019 g to 0.0023 g. Average seed weights for individual plants ranged from 0.00169 to 0.0025 g. Küchenmeister et al. (2012) and Küchenmeister et al. (2013) claim an average weight of seed for the Ekola variety to be 0.00169 g. These values are higher than an average weight of 0.00129 g claimed by Stevens (1932). Sidhu (1971) assumes that seed weight differences reflect differences in genotypes and/or moisture conditions.
It suggests that differences in the size, dormancy and vitality of seedlings are characteristic for the seeds of black medick, and may differ in dependence on changes in predominant conditions of the environment during the growing season. In their development, seeds of black medick have a non-dormant phase (Sidhu and Cavers 1977). During this phase which lasts approximately 10 days in field conditions, the seeds can immediately germinate if the conditions are favourable. Phase duration depends on the environment in which the seeds ripen and on the genotype of mother plant. Germinability of seeds increases within 16 days after the end of flowering, and then decreases again when the seeds start ripening and hardening (Sidhu and Cavers 1977). The variability of hardseededness makes it possible that some seeds can germinate instantly whilst in other seeds it may last several years before dormancy is discontinued (Sidhu and Cavers 1977). Norman et al. (2002) point to the fact that this genetic variability must exist if the population should maintain the capability of responding to environmental changes by means of hardseededness based on natural selection. In annual populations of legumes, dormancy has two important roles: it reduces the risk of dieback connected with the germination spread across a longer period of time, and increases the probability of its occurrence at an optimal time during the growing period (Philippi 1993).
Effect of Seed Chemical Composition on Germination
The tested seed lots (Ekola 2–5) were analysed for the contents of inorganic substances (Fig. 1 and 2) and organic matter (Fig. 3). Inorganic substances included the following elements: P, Ca, Mg, K, Fe, Mn, B, Cu and N, which are collectively referred to as biogenic and hence indispensable for the development of plants from the seed. The measured values show (Figs. 1 and 2) that differences existed amongst the seed lots in the contents of individual elements as well as in the concentrations of individual elements mutually which was also corroborated by the performed t-test (Annex C). The highest contents of elements in the seeds were those of N (> 4 wt.%) and K (> 1 wt.%). The other elements occurred in the seeds in markedly lower concentrations not exceeding 0.6 wt.% and the order of measured values was as follows: P > Ca > Mg > Fe > Zn > Mn > B > Cu. The increased content of Fe which was demonstrably higher than the contents of Zn, Mn, B and Cu (Fig. 2) was rather surprising. The lowest contents in the seeds (< 0.001 wt.%) were those of Cu, B and Mn. Comparing the concentrations of N, K, P, Ca and Mg (Fig. 1) with the concentrations of Fe, Zn, Mn, B and Cu (Fig. 2), we can see a clear and demonstrable difference. The first group of elements occurred in concentrations from 0.2 to 4.9 wt.% while the second group ranged from 0.05 to 0.7 wt.%.
Contents of Mg, Ca, and P and in the individual seed lots. Average values of the contents of selected elements (n = 4) ± SD in individual seed lots are shown. Different letters indicate a significant difference (P < 0.01; Anova post-hoc Tukey´s HSD test) in the contents of individual nutrients (Mg, Ca, P, K—specific colour for each nutrient) between the seed lots and do not indicate differences between the individual nutrients
Contents of Fe, Zn, Mn, B and Cu in the individual seed lots. Average values of the contents of selected elements (n = 4) ± SD in the individual seed lots are shown. Different letters inside columns indicate a significant difference (P < 0.01; Anova post-hoc Tukey´s HSD test) in the contents of individual nutrients (Cu, B, Mn, Zn, Fe—specific colour for each nutrient) between the seed lots, do not indicate differences between the individual nutrients
Contents of organic matter in the individual seed lots. Average values of the contents of selected elements (n = 4) ± SD in the individual seed lots are shown. Different letters inside columns indicate a significant difference (P < 0.01; one-way Anova post-hoc Tukey´s HSD test) in the contents of individual substances (Lipids, CAR, Starch—with a specific colour for each) between the seed lots and do not indicate differences between the individual nutrients. CAR carbohydrates
The content of all elements (Fig. 1 and 2) significantly fluctuated (P < 0.01) across the individual seed lots; only in the cases of K and Ca the same concentration was found, K in the Ekola 3 and Ekola 5 seed lots and Ca in the Ekola 4 and Ekola 5 seed lots. In the other cases, a demonstrable difference (Fig. 1 and 2) was always found between the individual lots. The greatest significant differences between the individual variants were recorded in the content of P (Fig. 1) and also in the contents of Cu, Fe and Mn (Fig. 2). The highest content of K was recorded in the Ekola 3 and Ekola 5 variants, the highest Fe content in the Ekola 2 variant, the highest P content in Ekola 3, the highest Zn content in Ekola 5, Ca in Ekola 5, Mg in Ekola 3, Mn and B in Ekola 2 and the highest content of Cu in Ekola 5. It follows from the measured values that none of the seed lots showed increased contents of all elements but at all times just increased contents of some of them. If we look for a tangible change in the content of nutrients, we can find it only in the Ekola 3 and Ekola 4 variants where very low values of Mn, B and Cu were recorded as compared with Ekola 2 and 5. The measured values show clearly that the individual seed lots differed in their inorganic composition.
In addition to the elements of inorganic nature, contents of organic matter were determined, too, namely the contents of starch, sugars and fat (Fig. 3). Similarly, as in the case of inorganic substances, a t-test was made of measured values, and based on its results, significant differences could be stated amongst the respective substances (Annex C). Their representation was in the following order: starch > sugars > lipids. Further on, the content of these substances was compared in the experimental seed lots. The statistically significantly highest contents of starch and sugars (P < 0.05) were found in Ekola 2 and Ekola 3 (starch only). Ekola 5 exhibited the generally highest content of fats. In the case of organic compounds, differences were less significant.
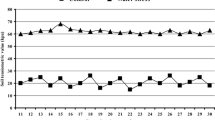
Based on the determined contents of N, Ca, P, K, Mg, B, Cu, Zn, Fe, Mn, (Fig. 1 and 2) and starch, fat and sugars (Fig. 3) in the seeds, the influence of these substances on germinability, number of hard and dead seeds was evaluated using the factor analysis (Annex D). The measured values show that the highest influence on germinability was that of boron content in the seeds in spite of the fact that its concentration in the plant seeds was low-up to 0.0005 wt% (Figs. 2 and 4). Boron, namely, negatively correlated with germinability and positively correlated with the number of hard seeds (Table 7 and Annex E), both in the case of simulated drought stress and in the control variant. In terms of statistical significance, all negative correlations between germinability and the boron content were detected at a significance level of P < 0.05 in all experimental variants. In the case of more rigorous significance level (P < 0.01), a significant dependence was recorded in the variant with a PEG concentration of 0.4; in the control variant and comparing all measured values across the variants. The results indicate a strong negative correlation between germinability and boron content in the seeds. Apart from this, a weak positive correlation of germinability and Mg content in the seeds was observed (R = 0.53; Annex E).
As already mentioned above, statistically significant differences were recorded in the seed content of boron amongst all four seed lots Ekola 2–5 (Fig. 2). Figure 4 shows the dependence of the germination and the boron content in the seeds as a result of the analysis of data from all experimental variants. The development of values shows that the lowest germinative capacity was found where the highest content of boron was recorded (P < 0.05 for all variants; P < 0.01 for control variants and when comparing all measured values). The relation between the germinability of seeds and the B content was further statistically analysed (Table 7) using the regression analysis which took into account both the germinative capacity at the individual PEG concentrations and the total average germinability from all PEG concentrations. The correlation matrix and Fig. 4 indicate that the increasing B content in the seeds had a moderately strong negative (R > − 0.64 and P < 0.00001, Fig. 4) influence on the seed germinative capacity. By contrast, in comparing the number of hard seeds, a positive dependence was found between the increasing B content and the increasing number of hard seeds. This effect of B apparently applies both under standard conditions (PEG = 0.0 mol), and in situations when the seed is exposed to drought stress (PEG > 0.0 mol). The measured values demonstrate (P < 0.05 and P < 0.01) that the Ekola 2 and Ekola 5 seed lots with the highest B contents exhibited the lowest average germinability (Table 5) and the highest average number of hard seeds (Table 3) taking into consideration all PEG concentrations. Thus, the data confirm the significance of boron action.
The relation between the seed composition (content of individual nutrients and organic matter) and germinability was further analysed using PCA (Fig. 5) and factor analysis (Annex E). Based on the scree plot (Annex F), two main factors (PC 1 and PC 2) were ascertained, which explained more than 90% of the variability of measured values. PC 1 strongly positively correlated with the values of B content in the seed and with the NHS value whilst PC 2 did not show any relation to these variables and weakly positively correlated with the value of NGS and with the contents of some elements in the seed (Zn, Cu, Mg and N). Thus, it can be assumed that PC 1 describes the variability of measured values related to the content of B in the seed, and PC 2 probably explains the variability of measured values with respect to the effect of seed lot. In general, the projection of variables into the factor plane indicates the already mentioned strong antagonism between germinability (NGS) and the B content in the seed as well as a weak negative influence of Mn content. On the contrary, a positive influence of Mg content can be observed on NGS.
In their experiments with soya, Bellaloui et al. (2017) recorded a positive correlation between the contents of boron and lignin in the seeds. Lignin is an important factor affecting the seed quality including germinability, hardseededness and seed case permeability for water. Cell wall which consists primarily of lignin and cellulose is a key factor affecting the incidence of hard seeds in the seed stock (Cosgrove 2005).
As to seeds and plants in relation to the action of boron, available literature dealt only with the action of boron occurring in the soil environment or boron applied into the soil, in the germination medium, on seeds or plants at diverse growth stages by various ways. Higher concentrations of boron in the environment can be toxic to plants and can lead to various physiological effects during their life cycle. Bañuelos et al. (1999) observed the inhibition of seed germination caused by surplus boron in Chilean and domestic (USA) gene sources, namely in maize (Zea mays L.), carrot (Daucus carota L.), tomato (Lycopersicum esculentum L.) and alfalfa (Medicago sativa L.).
Habtamu et al. (2014) conducted a laboratory experiment in which they studied the influence of boron on the germination of seeds and development of wheat (Triticum aestivum L.) plants. The seeds were sown into Petri dishes with different boron concentrations (0.00; 0.25; 0.50; 1.00; 2.00; 4.00; 8.00 and 16.00 mg/l). A significant decrease of germinative capacity was observed at boron concentrations higher than 0.50 mg/l. At concentrations of 8.00 and 16.00 mg/l, the wheat seeds did not germinate at all, which suggests that germination is totally suppressed at such high boron concentrations. The permanent decrease of germinability and seed germination rate at a concentration higher than 0.25 mg/l in their study is in line with the findings of Yau and Saxen (1997) and Muhammad et al. (2013) who informed that a high boron concentration decreased the percentage of germination in wheat and maize. This was also confirmed by results of experiments made by Alamris et al. (2018) with barley where low levels of boron significantly increased the germinative capacity of seeds while a higher boron supply inhibited their germinability. Ölçer and Kocaçaliskan (2007) stated too that a surplus of boron reduced the percentage of germinability as well as the activity of polyphenol oxidase in embryos and endosperm of maize seeds (Zea mays L. cv. „Arifiye “).
Conclusion
In the present experiment, the effect of drought and composition of black medick seeds on their germinability was studied. Drought was simulated using the PEG solution. Based on the measured results of drought stress simulation by applying PEG in various concentrations (0.2, 0.4 and 0.6 mol) on the seeds of black medick, it can be stated that all studied seed lots of Ekola variety exhibited a relatively high germinability and hence high drought resistance even at the highest PEG concentration of 0.6 mol. Statistically significant differences were found among the individual Ekola seed lots in the percentages of germinated, hard and dead seeds. The recorded differences were at all times significant at a level of P < 0.05 and in a majority of cases at a level of P < 0.01. Therefore, black medick can be considered a crop suitable for being sown at warm and sunny sites even in areas endangered with drought stress.
As to the influence of the composition of black medick seeds on their germinability, an important factor was the content of boron in them, which was in the negative correlation to their germinability. Results of the experiment suggest that a higher content of boron in the seeds of black medick increases the number of hard seeds at the expense of germinated seeds. The essence of this phenomenon, correlations with other substances contained in the seeds and metabolic processes amongst those substances have not been sufficiently clarified yet. It is a complex issue the solution of which requires a further research.
The impact of other substances (macro- and micro-elements) contained in the black medick seeds on their germinability was not recorded. The measured data show, however, that the analysis of seed stock for the contents of inorganic substances as well as organic matter should be an important part of seed quality evaluation. Such an analysis should then complement the data on seed germinability as, for example the B content in the seeds can apparently cause their delayed emergence. A practical contribution of our research can be seen in a possibility for farmers planning to sow black medick in areas potentially affected by moisture deficit to have their seed stock tested for the content of boron and resistance to the PEG solution effect (drought simulation) before sowing. Then it is up to them to decide according to results of the analysis of germinability whether to use the offered seed stock or to prefer a more suitable variety exhibiting higher drought resistance.
References
Alamris S, Siddiqui MH, Al-Khaishani M, Ali HM (2018) Boron induces seed germination and seedling growth of Hordeum vulgare L. under NaCl stress. J Adv Agric 8(1):1224–1234. https://doi.org/10.24297/jaa.v8i1.7116
Alencar NL, Innecco R, Gomes-Filho E, Galläo MI, Alvarez-Pizarro JC, Prisco JT, José T, De Oliveira A (2012) Seed reserve composition and mobilization during germination and early seedling establishment of Cereus jamacaru D.C. ssp. jamacaru (Cactaceae). An Acad Bras Cienc 84:823–832. https://doi.org/10.1590/S0001-37652012000300024
Archana PN (2016) Physiological and biochemical effects of boron toxicity in mustard during the seedling stage. J Plant Nutr 39:820–827. https://doi.org/10.1080/01904167.2015.1047523
Bäjesci I, Chiriac A (1984) Distribuţia microelementelor in solurile din România (implicaţii în agricultură). Ceres, Bucureşti
Balasaraswathi R, Sadasivam S (1997) Changes in oil, sugars and nitrogenous components during germination of sunflower seeds, Helianthus annuus. Plant Foods Hum Nutr 51:71–77. https://doi.org/10.1023/A:1007924026633
Baloch N, Kakar AM, Nabis S, Wajid Z, Kakar MA, Alkahraman D (2013) In vitro antimicrobial, insecticidal, antitumor activities and their phytochemical estimation of methanolic extract and its fractions of medickago lupulina leaves. World Appl Sci J 23(4):500–506
Banuelos GS, Ajwa HA, Cáceres L, Dyer D (1999) Germination responses and boron accumulation in germplasm from Chile and the United States grown with boron-enriched water. Ecotoxicol Environ Saf 43:62–67. https://doi.org/10.1006/eesa.1999.1765
Bellaloui N, Smith JR, Mengistu A (2017) Seed nutrition and quality, seed coat boron and lignin are influenced by delayed harvest in exotically-derived soybean breeding lines under high heat. Front Plant Sci 8:1–16. https://doi.org/10.3389/fpls.2017.01563
Bityutskii N, Magnitski S, Lapshina I, Lukina E, Soloviova A, Patsevith V (2001) Distribution of micronutrients in maize grain and their mobilisation during germination. Springer, Dordrecht
Boe A, Bortnem R, Johnson P (2017) Changes in weight and germinability of black medick seed over a growing season, with a new seed predator. Proc South Dakota Acad Sci 95:105
Bolland M, Riley M, Thomson B, Paynter B, Baker M (1990) Seed phosphorus - its effect on plant production. J Agric (western Australia) 31:20–22
Bowman RA (1989) A rapid plant digestion method for analysis of ρ and certain cations by inductively coupled plasma emission spektrometry. Commun Soil Sci Plant Anal 20(5–6):539–533
Brown PH, Bellaloui N, Wimmer MA, Bassil ES, Ruiz J, Hu H, Pfeffer H, Dannel F, Romheld V (2002) Boron in plant biology. Plant Biol 4:205–223. https://doi.org/10.1055/s-2002-25740
Brady SM, Sarkar SF, Bonetta D, Mccourt P (2003) The Abscisic Acid Insensitive 3 (ABI3) gene is modulated by farnesylation and is involved in auxin signaling and lateral root development in Arabidopsis. Plant J 34:67–75. https://doi.org/10.1046/j.1365-313X.2003.01707.x
Burzo I, Toma S, Craciun C, Voican V, Dobrescu A, Delian E (1999) Fiziologia plantelor de cultură. Poligrafică Ştiinţa, Chişinău
Camacho-Cristobal JJ, Lunar L, Lafont F, Baumert A, Gonzalez-Fontes A (2004) Boron deficiency causes accumulation of chlorogenic acid and caffeoyl polyamine conjugates in tobacco leaves. J Plant Physiol 161:879–881. https://doi.org/10.1016/j.jplph.2003.12.003
Caramete C, Caramete A, Corbean S, Dumitrescu F, Idriceanu A, Popescu S, Sändulache R, Stan S, Vineş I (1974) Nutriţia plantelor şi aplicarea îngrăşămintelor. Ceres.
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Bio 6(11):850–861. https://doi.org/10.1038/nrm1746
Dell B, Huang L (1997) Physiological response of plants to low boron. Plant Soil 193:103–120. https://doi.org/10.1023/A:1004264009230
Dordas C (2006) Foliar boron application improves seed set, seed yield, and seed quality of alfalfa. Agron J 98:907–913. https://doi.org/10.2134/agronj2005.0353
El Rasafi T, Nouri M, Bouda S, Haddioui A (2016) The effect of Cd, Zn and Fe on seed germination and early seedling growth of wheat and bean. Ekológia 35(3):213–223. https://doi.org/10.1515/eko-2016-0017
Faijunnahar M, Baque A, Habib MA, Hossain THMM (2017) Polyethylene glycol (PEG) induced changes in germination, seedling growth and water relation behavior of wheat (Triticum aestivum L.) genotypes. Univers J Plant Sci 5(4):49–57. https://doi.org/10.13189/ujps.2017.050402
Garcı́yuso LE, Luque de Castro M (1999) A multivariate study of the performance of a microwave-assisted Soxhlet extractor for olive seeds. Anal Chim Acta 382:309–316. https://doi.org/10.1016/S0003-2670(98)00795-8
Gill PK, Sharma AD, Singh P, Bhullar SS (2003) Changes in germination, growth and soluble sugar contents of Sorghum bicolor (L.) Moench seeds under various abiotic stresses. Plant Growth Regul 40:157–162. https://doi.org/10.1016/j.indcrop.2019.01.019
Gyuricza C, Smutný V, Percze A, Pósa B, Birkás M (2015) Soil condition threats in two seasons of extreme weather conditions. Plant Soil Environ 61(4):151–157. https://doi.org/10.17221/855/2014-PSE
Gorham J, Hughes L, Jones R (2006) Low-molecular-Weight carbohydrates in some salt-stressed plants. Physiol Plant 53:27–33. https://doi.org/10.1111/j.1399-3054.1981.tb05040.x
Habtamu A, Ibrahim H, Urgecha F, Worku N (2014) Influence of boron on seed germination and seedling growth of wheat (Triticum aestivum L.). Afr J Plant Sci 8(2):133–139. https://doi.org/10.5897/AJPS2014.1148
Handlirova M, Lukas V, Smutný V (2017) Yield and soil coverage of catch crops and their impact on the yield of spring barely. Plant Soil Environ 63(5):195–200. https://doi.org/10.17221/801/2016-PSE
Chen L, Xia F, Mingya W, Wanf W, Mao P (2020) Metabolomic analyses of alfalfa (Medickago sativa L. cv. ‘Aohan’) reproductive organs under boron deficiency and surplus conditions. Ecotoxicol Environ Saf. https://doi.org/10.1016/j.ecoenv.2020.111011
Chmielowska-Bąk J, Zinicovscaia I, Frontasyeva M, Milczarek A, Micheli S, Vysochanska M, Deckert J (2018) Soybean seedlings enriched with iron and magnesium—impact on germination, growth and antioxidant properties. Ecol Chem Eng S 25:631–641. https://doi.org/10.1515/eces-2018-0042
ISTA (2017) International Rules for Seed Testing, 2017
Jarvis BC, Yasmin S, Ali AHN, Hunt R (1984) The interaction between auxin and boron in adventitious root development. New Phytol 97:197–204. https://doi.org/10.1111/j.1469-8137.1984.tb04122.x
Jones JB Jr (2001) Laboratory guide for conducting soil tests and plant analysis. CRC Press, Boca Raton. https://doi.org/10.1201/9781420025293
Kaur Gill P, Sharma A, Singh P, Bhullar S (2003) Changes in germination, growth and soluble sugar contents of Sorghum bicolor (L.) Moench seeds under various abiotic stresses. Plant Growth Regul 40:157–162. https://doi.org/10.1023/A:1024252222376
Kintl A, Huňady I, Vymyslický T, Ondrisková V, Hammerschmiedt T, Brtnický M, Elbl J (2021) Effect of seed coating and PEG-induced drought on the germination capacity of five clover crops. Plants 10:724. https://doi.org/10.3390/plants10040724
Kintl A, Elbl J, Vitez T et al (2020) Possibilities of using white sweetclover grown in mixture with maize for biomethane production. Agronomy 10:1407. https://doi.org/10.3390/agronomy10091407
Kintl A, Elbl J, Kadankova P et al (2019) Drought Tolerance of the Seeds of White Sweet Clover (Melilotus albus Medik.). In: Conference: 14th Scientific and Technical Seminar on Seed and Seedlings Location, Prague, Czech Republic
Kintl A, Elbl J, Lošák T, Vaverková MD, Nedělník J (2018) Mixed intercropping of wheat and white clover to enhance the sustainability of the conventional cropping system: effects on biomass production and leaching of mineral nitrogen. Sustainability 10:3367. https://doi.org/10.3390/su10103367
Kolodziejek J (2017) Effect of seed position and soil nutrients on seed mass, germination and seedling growth in Peucedanum oreoselinum (Apiaceae). Sci Rep 7(1):1959. https://doi.org/10.1038/s41598-017-02035-1
Küchenmeister K, Küchenmeister F, Wrage N, Kayser M, Isselstein J (2012) Establishment and early yield development of five possible alternatives to Trifolium repens as a grassland legume. J Agric Sci 4(8):86–95. https://doi.org/10.5539/jas.v4n8p86
Küchenmeister K, Küchenmeister F, Kayser M, Wrage-Mönnig N, Isselstein J (2013) Influence of drought stress on nutritive value of perennial forage legumes. Int J Plant Prod 7(4):693–710. https://doi.org/10.22069/IJPP.2013.1265
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42:421–428
Liu GD, Dong XC, Liu LC, Wu LS, Peng SA, Jiang CC (2015) Metabolic profiling reveals altered pattern of central metabolism in navel orange plants as a result of boron deficiency. Physiol Plant 153:513–524. https://doi.org/10.1111/ppl.12279
Lu RK (1999) Analytical methods of soil agrochemistry. Chinese Agriculture Science and Technology Press, Beijing
Marschner H (1995) Mineral nutrition of higher plants. Academic Press, San Diego
Matsoukas IG, Massiah AJ, Thomas B (2013) Starch metabolism and antiflorigenic signals modulate the juvenile-to-adult phase transition in Arabidopsis. Plant Cell Environ. https://doi.org/10.1111/pce.12088
Marrubini G, Papetti A, Genorini E, Ulrici A (2017) Determination of the sugar content in commercial plant milks by near infrared spectroscopy and Luff-Schoorl total glucose titration. Food Anal Methods 10:1556–1567. https://doi.org/10.1007/s12161-016-0713-1
Muhammad HRS, Tasveer ZB, Uzma Y (2013) Boron irrigation effect on germination and morphological attributes of Zea mays cultivars (Cv.Afghoee & Cv Composite). Int J Sci Engi Res 4(8):1563–1569
Muscolo A, Sidari M, Anastasi U, Santonoceto C, Maggio A (2014) Effect of PEG-induced drought stress on seed germination of four lentil genotypes. J Plant Interact 9:354–363. https://doi.org/10.1080/17429145.2013.835880
Naegle ER, Burton JW, Carter TE, Rufty T (2005) Influence of seed nitrogen content on seedling growth and recovery from nitrogen stress. Plant Soil 271:329. https://doi.org/10.1007/s11104-004-3242-4
Norman H, Cocks P, Galwey N (2002) Hardseededness in annual clovers: Variation between populations from wet and dry environments. Crop Pasture Sci 53:821–829. https://doi.org/10.1071/AR01115
Nowosielski O (1968) Metody oznaczania potrzeb nawożenia. Państwowe Wydawnictwo Rolnicze i Leńne. Warszawa, 668
Nyomora AMS, Brown PH, Freeman M (1997) Fall foliar applied boron increases tissue boron concentration and nut set of almond. J Am Soc Hortic Sci 122:405–410. https://doi.org/10.21273/JASHS.122.3.405
Ölçer H, Kocaçaliskan I (2007) Excess boron reduces polyphenol oxidase activities in embryo and endosperm of maize seed during germination. J Biosci 62:111–115
Pandey N, Gupta B (2013) The impact of foliar boron sprays on reproductive biology and seed quality of black gram. J Trace Elem Med Biol 27:58–64. https://doi.org/10.1016/j.jtemb.2012.07.003
Patanè C, Cosentino S, Venera C (2008) Hardseededness in two medickago species as affected by water stress during seed development. Opt Méditérr 79:345–348
Philippi T (1993) Bet-hedging germination of desert annuals: beyond the fîrst year. Am Nat 142:474–487
Pomeranz Y, Meloan CE (1994) Carbohydrates. In: Pomeranz Y, Meloan CE (eds) Food analysis. Springer, Boston
Rerkasem B, Bell R, Lodkaew S, Loneragen JF (1997) Relationship of seed boron concentration to germination and growth of soybean (t Glycine max). Nut Cycl Agroecosys 48:217. https://doi.org/10.1023/A:1009725311624
Rout GJ, Das P (2003) Effect of metal toxicity on plant growth and metabolism. Springer, Dordrecht. https://doi.org/10.1007/978-1-4615-6998-5_36
Ryan J, Estefan G, Rashid A (2001) Soil and plant analysis lab manual. 2nd ed. ICARDA, Aleppo, Syria. National Agricultural Research Center, Islamabad, Pakistan.
Shaikh IR, Shaikh PR, Shaikh RA, Shaikh AA (2013) Phytotoxic effects of heavy metals (Cr, Cd, Mn and Zn) on wheat (Triticum aestivum L.) seed germination and seedlings growth in black cotton soil of Nanded. India. Res J Chem Sci 3(6):14–23
Sidhu SS, Cavers PB (1977) Maturity-dormancy relationships in attached and detached seeds of medickago lupulina L. (black medickk). Bot Gaz 138:174–182
Sidhu SS (1971) Some aspects of the ecology of black medickk (medickago lupulina L.). Ph.D. Thesis, University of Western Ontario, London, Ontorio, Canada
Smetham M (2003) A review of subterranean clover (Trifolium subterraneum L.): its ecology, and use as a pasture legume in Australasia. Adv Agron 79:303–350. https://doi.org/10.1016/S0065-2113(02)79006-8
Stevens OA (1932) The number and weight of seeds produced by weeds. Amer J Bot 19:784–794
Stratu A, Costica N (2015) The influence of zinc on seed germination and growth in the first ontogenetic stages in the species Cucumis melo L. Pres Environ Sustain Dev. https://doi.org/10.1515/pesd-2015-0038
Tang D, Wei F, Qin S, Khan A, Kashif MH, Zhou R (2019) Polyethylene glycol induced drought stress strongly influences seed germination, root morphology and ytoplasmo f different kenaf genotypes. Ind Crop Prod. 137:180–186. https://doi.org/10.1007/BF0002503810.1007/BF00025038
Tonguç M, Elkoyunu R, Erbaş S, Karakurt Y (2012) Changes in seed reserve composition during germination and initial seedling development of safflower (Carthamus tinctorius L.). Turk J Biol 36:107–112. https://doi.org/10.3906/biy-1012-164
Tsonev T, Lindon FJC (2012) Zinc in plants—an overview. Emir J Food Agric 24(4):322–333
Turkington R, Cavers P (1979) The biology of Canadian weeds. 33. medickago lupulina L. Can J Plant Sci 59:99–110. https://doi.org/10.4141/cjps79-015
Vandecasteele C, Teulat-Merah B, Morére-Le Paven MC, Leprince O, Ly VuB, Viau L, Ledroit L, Pelletier S, Payet N, Satour P, Lebkas C, Gallardo K, Huguet T, Limani AM, Prosperi JM, Buitink J (2011) QTL analysis reveals a correlation between the ratio of sucrose/raffinose family oligosaccharides and seed vigour in medickago truncatula. Plant Cell Environ 34:1473–1487. https://doi.org/10.1111/j.1365-3040.2011.02346.x
Yau SK, Saxena MC (1997) Variation in growth, development, and yield of durum wheat in response to high soil boron. I. Average effects. Aust J Agric Res 48:945–950. https://doi.org/10.1071/A96144
Zehra A, Gul B, Ansari R, Khan MA (2012) Role of calcium in alleviating effect of salinity on germination of Phragmites karka seeds. S Afr J Bot 78:122–128. https://doi.org/10.1016/j.sajb.2011.05.016
Zhang S, Zhao CH, Lamb E (2010) Cotyledon damage affects seed number through final plant size in the annual grassland species medickago lupulina. Ann Bot 107:437–442. https://doi.org/10.1093/aob/mcq259
Acknowledgements
The research was financially supported by TA CR, project: TH03030236 “Growing maize for grain in the controlled system of mixed culture with the use of clovers”and supported by the Ministry of Agriculture of the Czech Republic, institutional support MZE-RO1722.
Author information
Authors and Affiliations
Contributions
Conceptualization, JE and AK; methodology, IH and AK; validation, IH, JE and MB; formal analysis, JE, VO, KV and ZK; investigation, AK, IH, TH, and JE; data curation, JE and IH; writing—original draft preparation, IH, AK and JE; writing—review and editing, JE, IH and AK; visualization, JE, IH and AK; supervision, JE and AK; project administration, AK; funding acquisition, AK. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling Editor: Pramod kumar nagar.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Annex
Annex A: overview of experiment

Annex B: seed testing

Annex C: t-test for separate means

SD standard deviation, n number of measurements, SE standard error of mean, red colour represents a significant difference.
Annex D: t-test for separate means

SD standard deviation, n number of measurements, SE standard error of mean, red colour represents a significant difference.
Annex E: Correlation matrix—result of factor analysis

CAR carbohydrates, GS germinated seeds, HS hard seeds and DS dead seeds. Red colour Pearson´s coefficient shows a significant correlation between the selected parameters at a level of P < 0.05 and *P < 0.01.
Annex F: Scree plot

Legend: Eigenvalues of correlation matrix. Only active variables are presented
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kintl, A., Huňady, I., Ondrisková, V. et al. Influence of Boron and Drought Simulation on Germinability and Hardseededness of Black Medick Seeds (Medicago lupulina L.). J Plant Growth Regul 42, 1704–1719 (2023). https://doi.org/10.1007/s00344-022-10652-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-022-10652-y