Abstract
The wide use of copper (Cu)-based fungicide has caused a stepwise accumulation of Cu in the environment increasing the occurrence of phytotoxicity in crops. To understand and alleviate this abiotic stress, maize seedlings were grown in hydroponic solution with different combinations of Cu and iron (Fe) forms. Results showed that maize Cu sensitivity is related to the nature of the form supplied and to the chelate-exchange processes that might involve other elements, such as Fe. The use of CuSO4 excess (100 µM) caused severe reduction of plant growth, over accumulation of Cu, high activity of antioxidant enzymes, and impairment of the acquisition of other nutrients. In presence of chelating agents (citrate and ethylenediaminetetraacetic acid, EDTA) the ability of plants to tolerate high Cu-levels depends on the Fe nutritional status. Copper phytotoxicity symptoms do not occur when Cu was supplied chelated by EDTA. The use of synthetic agent EDTA (as Cu-EDTA and Fe-EDTA) prevented the accumulation of toxic Cu-level in plants and allowed a better homeostasis among nutrients. In presence of citrate, high concentration of Cu occurred in plants but its phytotoxicity was limited when even EDTA was available in solution. Results suggest that maize plants can operate a good control of nutritional status when Cu-excess is present concomitantly with a synthetic chelator (as EDTA) even when supplied as a Fe-fertilizer. These results pave the way to provide guidelines for the fertilization managements on Cu-contaminated soils to alleviate phytotoxicity in crops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intense anthropogenic activities (e.g., mining, smelting, industrial, and agricultural activities) have led to an excessive released of copper (Cu) into the environment causing severe ecological risks for living organisms, such as plants, animals, and humans (Jung and Thornton 1996; Chopin and Alloway 2007; Rehman et al. 2019). The widespread use of Cu in agriculture has caused contamination of soils that may lead crops to bioaccumulate this metal and thus enters in the food chain. Especially in vineyards, the extensive use over decades of Cu-containing agrochemicals to prevent and control plant diseases (Cu-based fungicide treatments, e.g., Cu sulfate, Cu oxychloride) has determined Cu accumulation in soils leading to nutritional disorders and phytotoxicity in crops (Schramel et al. 2000; Brun et al. 2001; Chaignon et al. 2003; Chopin et al. 2008; Mackie et al. 2012).
Copper is also a micronutrient in plants and it is involved in numerous physiological processes (redox and detoxification reactions). It is required to assure healthy growth and reproduction success of plants, therefore, when its availability is not adequate to sustain plant needs, severe alterations of photosynthesis, respiration, cell membrane integrity, and enzyme activity can occur (Rehman et al. 2019).
In soils, Cu solubility and bioavailability is highly dependent on organic matter content and pH value (Bravin et al. 2012). Due to its low solubility at high pH, Cu bioavailability in calcareous soil is limited (Thiel and Finck 1973; Adriano 2001), and therefore this condition may lead to Cu-deficiency in plants. However, this nutritional disorder is a very rare condition that may appear when Cu concentration in plants is below 3–5 mg Cu kg−1dry weight (Marschner 1995). Symptoms of Cu-deficiency include stunted growth, necrosis of apical roots and shoot meristems, leaf deformation, an increase of susceptibility to biotic stresses and chlorosis of young leaves (Rahimi and Bussler 1973; Alloway and Tills 1984; Adrees et al. 2015). On the other hand, Cu-solubility in the soil solution increases in acidic soils, as low pH limits the Cu adsorption on the colloids and on the organic-matter surfaces (Sauvé et al. 1997; Adriano 2001; Brun et al. 2001). In plants, a toxic nutritional status is evaluated when Cu content is above 20–50 mg Cu kg−1 dry weight (Marschner 2011). Typical symptoms of Cu toxicity on plants include leaf chlorosis, altered photosynthesis, senescence of leaves, stunted growth, root length reduction, and peroxidative damages of cell membranes (Vinit-Dunand et al. 2002; Kopittke and Menzies 2006; Michaud et al. 2008; Lequeux et al. 2010; Feigl et al. 2013; Brunetto et al. 2016). For both nutritional disorders (Cu-deficiency and -toxicity), the severity and the occurrence of the symptoms are highly dependent on plant species, developmental stages, and bioavailability of other nutrients (Thiel and Finck 1973; Adrees et al. 2015).
In plants, Cu shares some common features with other nutrients, especially with iron (Fe): both are transition metals that are involved in redox reactions. Both metals are micronutrients that become toxic at high concentration; therefore, their uptake, utilization, and storage need to be tightly regulated to maintain an appropriate metal homeostasis (Grotz and Guerinot 2006; Waters and Armbrust 2013). Some works provide evidence that an excess of Cu can affect Fe uptake, Fe activity, and Fe content in plants, and vice versa (Welch et al. 1993; Pätsikkä et al. 2002; Chen et al. 2004; Burkhead et al. 2009). Thus, some typical symptoms of Cu-excess in plants are linked to Fe deficiency, such as an intensification of oxidative stress and a decrease of leaf chlorophyll content (Pätsikkä et al. 2002; Schaaf et al. 2003; Grotz and Guerinot 2006). Moreover, the acquisition mechanisms in roots for the uptake of Fe and Cu shared some features (Ryan et al. 2013; Adrees et al. 2015; Brunetto et al. 2016). The Fe(III)-chelate reductase (FRO) operates the reduction of Fe(III)-chelates to Fe2+ but other transition metals can be also substrates of this enzyme (i.e., Cu; Uren 1982; Marschner et al. 1986; Bernal et al. 2012). Up to date, the Cu uptake system in plants is not yet completely elucidated, nevertheless several plasma membrane proteins have been identified to mediate Cu transport, such as COPper Transporters (COPT, Sancenón et al. 2003), some Heavy Metal ATPases (HMA; Pilon and Tapken 2013), and Fe transporters (IRT/ZIP proteins mediate the uptake of Fe as well as of Cu, Cd, Co, and Zn; Korshunova et al., 1999). The involvement of FRO and IRT/ZIP proteins for Cu acquisition confirms the occurrence of a strong overlap between Cu and Fe nutritional pathways in plants (Grotz and Guerinot 2006). In dicots and non-grasses, Cu acquisition seems to preferentially occur in form of Cu+ based on a reductive mechanism (Bernal et al. 2012); in other species (as in grasses), this nutrient might be mainly acquired by a non-reductive strategy (as Cu2+) or by a complexation strategy (through the acquisition of Cu-chelates, Wintz et al. 2003; Printz et al. 2016; Brunetto et al. 2016). Similar strategies are used by plants to mediate Fe acquisition and in the past decades they have been deeply investigated: reductive based mechanism is mainly used by non-grasses (Strategy I plants), whereas the complexation mechanism is mainly operated by grasses (Strategy II plants; Marschner and Römheld 1994; Marschner 2011). The release of phytosiderophores (PS) by grasses is functional to promote the solubility of Fe and the chelated form Fe(III)-PS complexes are subsequently taken up into root cells by an Fe-PS transporter (ZmYS1, yellow stripe 1 in maize; Bashir et al. 2006). It is interesting note that Cu was found to be quantitatively the most important element competing with Fe for complexation by PS (Schenkeveld et al. 2014).
Once inside the cell, Cu can follow different routes. The metal can be stored into vacuoles or translocated into the shoots in different forms. In plants, Cu can be transported in the form of free Cu+ or Cu2+, however, in the xylem sap, the main translocated form is likely to be Cu(II)-chelate (for review, see Printz et al. 2016). Analyses on xylem sap revealed that several amino acids (including non-proteinogenic ones, such as the PS-precursor: nicotianamine) are able to chelate Cu (Kochian 1991; Liao et al. 2000; Irtelli et al. 2009; Curie et al. 2009; Hofmann 2012). Under excess condition, limiting Cu reactivity inside cells through complexation may represent a valid strategy to counteract its toxicity (Welch and Shuman 1995).
Aim of this work is to improve understanding on Cu nutrition in maize and evaluate antagonisms or synergisms between Fe- and Cu-nutritional pathways in maize. Physiological and biochemical responses of maize plants were evaluated when different Cu-levels and Cu-forms were applied to nutrient solution. Moreover, the interplay between Cu and Fe in maize was evaluated depending on the metal forms available in the root external media. Present work aims to improve the knowledge on Cu nutrition in plants and provides useful information to alleviate Cu phytotoxicity in contaminated soil.
Materials and Methods
Plant Material and Growth Conditions
Maize seeds (Zea mays L., inbred line P0423, Pioneer Hybrid Italia S.p.A.) were grown as described by Zanin et al. (2017). Briefly, germinated seeds were transferred for 15 days in a continuously aerated Cu and iron (Fe)-free nutrient solution (containing, μM: Ca(NO3)2 1000; CaSO4 500; K2SO4 200; MgSO4 100; KH2PO4 175; KCl 5; H3BO3 2.5; MnSO4 0.2; ZnSO4 0.2; Na2MoO4 0.05; buffered solution to pH 6.0 with 2.5 mM 2-(N-morpholino)ethanesulfonic acid (MES)-KOH; Pinton et al. 1999) under hydroponic conditions. Copper and Fe were added to nutrient solution depending on nutritional treatment (Table 1). To limit as far as possible Cu contamination, analytical grade reagents were used to prepare nutrient solution (Merck KGaA, Darmstadt, Germany).
In the first experiment (Experiment I), Cu was added at different concentrations: 0 µM CuSO4 (−CuS + FeE, Cu-deficiency treatment), 0.05 µM CuSO4 (+ CuS + FeE, Cu-sufficiency treatment) or 100 µM CuSO4 (++ CuS + FeE, Cu-excess treatment); Fe was always supplied as 100 µM of Fe chelated by ethylenediaminetetraacetic acid (EDTA). Variations in morphophysiological parameter, plant growth, antioxidant enzyme activities, and ionomic composition of Cu-deficient and Cu-excess conditions were compared to Cu-sufficient plants (+ CuS + FeE used as control).
In the second experiment (Experiment II), Cu was added at different concentrations and forms as described in Table 1: 0.05 µM CuSO4 or Cu-EDTA (+ CuS or + CuE, Cu-sufficiency treatments); 100 µM CuSO4 or Cu-EDTA (++ CuS or ++ CuE, Cu-excess treatments). Fe was supplied chelated by EDTA or by citrate, as 100 µM Fe-EDTA or 100 µM Fe-citrate (+ FeE or + FeC, Fe-sufficiency treatments). Copper-EDTA and Fe-EDTA were purchased already in the chelated form from Merck KGaA (Cu-EDTA: 03,668-500G; Fe-EDTA: 03,650-1 KG; Darmstadt, Germany). Iron-citrate was prepared according to von Wirén et al. (1994) by mixing an aliquot of FeCl3 with citrate (in 10% chelate excess). Variations in morphophysiological parameter, plant growth, antioxidant enzyme activities and ionomic composition of plants exposed to Cu-excess were compared to the relative Cu-sufficiency condition, depending on the Cu and Fe form applied to nutrient solution (++ CuS + FeE Cu-excess condition was compared with the + CuS + FeE Cu-sufficiency condition; ++CuS + FeC vs. + CuS + FeC; ++CuE + FeE vs. + CuE + FeE; ++ CuE + FeC vs. + CuE + FeC).
Before harvesting (21-day-old plants), light transmittance of fully expanded leaves was measured and values are shown as “Soil–Plant Analyzer-Development” index values (SPAD‐502, Minolta, Osaka, Japan; Zanin et al. 2015). For the multielemental analyses, plant roots were washed accordingly to Bienfait et al. (1985) to remove root apoplastic metals (especially Fe and Cu) using 1.2 g L−1 Na2S2O4; 1.5 mM 2,2’-bipyridyl; 0.5 mM Ca(NO3)2; 1 mM MES. Roots and shoots were harvested, and fresh (FW) and dry weights (DW) were assessed.
In Silico Evaluation of Cu- and Fe-Forms in Nutrient Solution
The evaluation of Cu- and Fe-forms most abundant in nutrient solution were predicted by in silico analyses performed by Visual MINTEQ software (v 3.1, https://vminteq.lwr.kth.se/).
Multielement Analysis
Roots and shoots were oven dried at 105 °C and digested with HNO3 in a microwave oven (MARS Xpress, CEM, Matthews, NC, USA) as reported in Buoso et al. (2021). Macro- and micro-nutrients were measured by either Inductively Coupled Plasma (ICP)-Optical Emission Spectroscopy or ICP-Mass Emission Spectroscopy (ICP-OES: Varian Vista Pro axial, USA; and ICP-MS: NexION™ 300, PerkinElmer Inc., USA, respectively).
Antioxidant Enzyme Activities
Antioxidant enzyme activities were analyzed in maize leaves after 21 days of growth. Total superoxide dismutase (SOD) activity was assayed as described by Elavarthi and Martin (2010), whereas the activity of catalase (CAT) and peroxidase (POX) was analyzed as reported by Hippler et al. (2016). The total protein content was determined by Bradford assay, using as standard the bovine serum albumin (BSA; Bradford 1976). Moreover, to discriminate different isoforms, SOD activity was also visualized on non-denaturing PAGE gel as described by Dourado et al. (2014) and Hippler et al. (2016). Electrophoresis was performed on a 12% polyacrylamide gel loading equal amounts of proteins (20 μg of total proteins for each gel lane).
Statistical Analyses and Data Elaboration
Analyses were performed on three independent experiments, a pool of three plants was used for each sample. One-way analysis of variance (ANOVA) was performed to statistically analyzed data using Holm–Sidak test using SigmaPlot Version 12.0 software (P < 0.05, N = 3). Data clustering and PCA analyses were determined using ClustVis web tool (https://biit.cs.ut.ee/clustvis/; Metsalu and Vilo 2015).
Results
Experiment I—Maize Response to Different Cu Availability
Alteration of plant morphology, ionomic and biochemical parameters was analyzed in conditions conducting to Cu-deficiency (− CuS + FeE), Cu-sufficiency (+ CuS + FeE), and Cu-excess (++ CuS + FeE). Copper2+ was supplied into the nutrient solution ranging from 0 to 100 μM (Experiment I, Table 1). Iron was supplied at a concentration of 100 µM that is adequate to meet plant nutritional needs (Vangronsveld and Clijsters 1994). In comparison to Cu-sufficient plants (+ CuS + FeE), the absence of Cu in the nutrient solution (− CuS + FeE) determined a slight reduction of SPAD index values in shoots, whereas biomass accumulation of − CuS + FeE treated plants were comparable to that recorded for + CuS + FeE ones (Fig. 1, Supplementary Fig. S1). On the other hand, the addition of Cu in excess (++ CuS + FeE) caused alterations in plant development with changes in shoot and root morphology and a strong limitation of biomass production (fresh and dry weights, Supplementary Figure S1). The Cu toxicity in maize plants led to a drastic limitation of root elongation and to a significant increase in occurrence of interveinal chlorosis at the leaf level (Fig. 1, Supplementary Fig. S1).
Multielement analyses of roots and shoots allowed the identification and quantification of most abundant elements in maize tissues (Fig. 2). Copper-free treatment (− CuS + FeE) did not induce substantial changes on plant morphology, but the Cu concentration in roots was more than halved in comparison to Cu-sufficient plants (+ CuS + FeE). On the other hand, the use of Cu-excess in nutrient solution determined a sturdy increase of Cu content in plants (especially in roots ++ CuS + FeE). Concerning the other elements, Cu-limiting condition (− CuS + FeE) determined a significant reduction of Zn, Mn, and Ca content in leaves in comparison to Cu-sufficient ones (+ CuS + FeE). The Cu-excess treatment (++ CuS + FeE) determined a strong limitation of Fe, Zn, Ca (in roots and shoots), Mn and P (in shoots) concentrations vs control condition (+ CuS + FeE), and a slight increase of S concentration was observed mainly in shoots (Fig. 2).
Multielemental analyses of maize plants grown under different Cu availability (Experiment I). Total concentration (mg kg−1 of dry weight) of micro- and macro-nutrients in roots (A, C) and shoots (B, D) of Cu-deficient (− CuS + FeE), Cu-sufficient (+ CuS + FeE), and Cu-excess (++ CuS + FeE) maize plants. Data are means + SD, for each element letters indicate a significant difference (ANOVA Holm-Sidak; N = 3, P < 0.05)
While Cu-deficiency did not affect the activity of antioxidant enzymes (SOD, CAT, and POX), as − CuS + FeE showed similar activities to those recorded in Cu-sufficient leaves (+ CuS + FeE), a significant increase of all three enzymatic activities was measured in leaves of ++ CuS + FeE. In particular, under Cu-excess, SOD and POX were at least two times more active than in Cu-sufficient leaves (Experiment I, Fig. 3).
Experiment II—Influence of Cu- and Fe-Forms on Maize Response Under Cu-Excess Condition
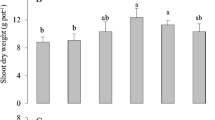
Regarding the Experiment II, the use of different Cu- and Fe-forms affected the morphology and morphometric parameters of maize plants (Fig. 4, Supplementary Fig. S2). Under Cu-excess, the use of CuSO4 compromised plant growth and development, stunted growth of roots and shoots, yellowing and chlorosis on leaves were observed in comparison to their Cu-sufficient controls (+ CuS + FeE and + CuS + FeC). Moreover, these symptoms were more evident in presence of Fe-EDTA (++ CuS + FeE) than with Fe-citrate (++ CuS + FeC, as shown by a significant reduction of SPAD index values and root growth; Fig. 4 and Supplementary Fig. S2). When Cu-EDTA was used in excess (++ CuE + FeE and ++ CuE + FeC), maize plants grew better than when the same amount of Cu was applied in form of CuSO4 (++ CuS + FeE and ++ CuS + FeC), although a slight reduction of shoot and root weights were observed in comparison to their controls (+ CuE + FeE and + CuE + FeC, respectively). Moreover, the use of Fe-citrate along with Cu-EDTA led to a significant reduction of SPAD index values in comparison to Cu-sufficient control (++ CuE + FeC vs. + CuE + FeC); this pattern was not observed when Fe-EDTA was applied along with Cu-EDTA excess (++ CuE + FeE vs. + CuE + FeE; Supplementary Fig. S2).
Shoots (A) and roots (B) of maize plants grown under Cu toxicity, different Cu and Fe sources were applied to nutrient solution (Experiment II). From the left to the right: + CuS + FeE (0.05 µM CuSO4 and 100 µM Fe-EDTA); + CuS + FeC (0.05 µM CuSO4 and 100 µM Fe-citrate); + CuE + FeE (0.05 µM Cu-EDTA and 100 µM Fe-EDTA); + CuE + FeC (0.05 µM Cu-EDTA and 100 µM Fe-citrate); ++ CuS + FeE (100 µM CuSO4 and 100 µM Fe-EDTA); ++ CuS + FeC (100 µM CuSO4 and 100 µM Fe-citrate); ++ CuE + FeE (100 µM Cu-EDTA and 100 µM Fe-EDTA); ++ CuE + FeC (100 µM Cu-EDTA and 100 µM Fe-citrate)
Simulations of the elemental chemical speciation and complexes obtained from the Visual MINTEQ chemical speciation software indicate that, under Cu-excess (100 µM Cu and 100 µM Fe), Cu and Fe compete for chelating agent (Supplementary Table S1). When Fe is applied in form of Fe-citrate, the occurrence of a metal chelate exchange process between the two elements may occur at equilibrium leading to the formation of Cu-citrate as most abundant citrate form (++ CuS + FeC; ++ CuE + FeC; Supplementary Table S1). When nutrients are use chelated to EDTA (+ CuS + FeE; + CuE + FeE; + CuE + FeC; ++ CuS + FeE; ++ CuE + FeE; ++ CuE + FeC), the equilibrium constant promotes the formation of Fe-EDTA complex over than Cu-EDTA, this latter occur in solution mainly when EDTA exceed Fe moiety (++ CuE + FeE). Therefore, the computational prediction indicates that in EDTA containing nutrient solutions: ++ CuS + FeE, Cu is mainly present as Cu2+ (64%) and Cu-EDTA2− (24%) and Fe is in form of Fe-EDTA− (74%) and Fe(OH)2+ (23%); ++ CuS + FeC, Cu is in form of Cu-citrate− (66%) and Cu2+ (28%) and Fe is in form of FeOH2+ (62%) and Fe-citrate (35%); ++ CuE + FeE, Cu is in form of Cu-EDTA2− (100%) and Fe in form of Fe-EDTA− (98%); ++ CuE + FeC, Cu is in form of Cu-citrate− (72%) and Cu-EDTA2− (16%) and Fe is in form of Fe-EDTA− (82%) and Fe-citrate (10%, Supplementary Table S1).
Multielement analyses indicated that all four Cu-excess treatments determined an overall increase of Cu concentration in maize, although under ++ CuE + FeE maize plants did not over accumulate Cu as much as under the other treatments (Fig. 5). When Cu-excess was applied in form of CuSO4 (++ CuS + FeE; ++ CuS + FeC) an overall reduction of Fe, Zn, Mn, Ca (in roots and shoots), and P (in shoots) was observed in comparison to their controls (+ CuS + FeE; + CuS + FeC), regardless to Fe form applied. On the other hand, the use of Cu-EDTA in excess applied in combination to Fe-EDTA (++ CuE + FeE) determine a reduction of Fe (in roots) and Ca in maize while the concentration of all the other elements was not affected by Cu-excess in comparison to the control plants (+ CuE + FeE). In the treatment ++ CuE + FeC, where the excess of Cu was applied in form chelated by EDTA and in conjunction to Fe-citrate, Zn and Mn concentrations were negatively affected but at the same time P and S concentrations increased in roots, whereas the elemental composition in shoots was not influenced by this treatment in comparison to + CuE + FeC (++ CuE + FeC vs + CuE + FeC). Under normal Cu condition, Cu and Mn concentration in maize plants increased when Fe was applied as Fe-citrate (+ CuS + FeC, + CuE + FeC) instead of Fe-EDTA (+ CuS + FeE; + CuE + FeE).
Multielemental analyses of maize plants grown under Cu toxicity: different Cu and Fe sources were applied to nutrient solution (Experiment II). Total concentration (mg kg−1 of dry weight) of micro- and macro-nutrients in roots (A, C) and shoots (B, D) of maize plants. Letters indicate a significant difference (ANOVA Holm-Sidak; N = 3, P < 0.05)
In order to highlight possible differences and similarities among samples, a multivariate analysis (PCA) was carried out on the elemental composition dataset of shoots and roots (Fig. 6). The PCA generated an eight-component model, the first four components (PC1-PC4) explained 95% and 91% of total variance in both shoots and roots, respectively. The first two components (PC1 and PC2) explaining over 70% of the total variance have been chosen to show sample distribution. In both shoots and roots, the PCA analyses discriminate different groups along PC1, all samples grown under Cu-sufficiency (+ Cu) clustered together regardless to Fe-source applied to nutrient solution. Along PC1 separate clusters were observed for plants grown under Cu-excess (++ Cu) and the separation was marked when CuSO4 was used as Cu form more than Cu-EDTA which clustered closed to samples grown under normal Cu condition.
PCA analyses and heatmap clustering based on elemental composition of maize shoots (A) and roots (B). Unit variance scaling is applied to rows; SVD with imputation is used to calculate principal components. X and Y axis of PCA plots show principal component 1 and principal component 2 that explain 48.9% and 22.6% of the total variance, respectively, in shoots (A); and explain 47% and 25% of the total variance, respectively, in roots (B). Prediction ellipses are such that with probability 0.95, a new observation from the same group will fall inside the ellipse. In heatmaps elements are centered; unit variance scaling is applied to elements; both elements and samples are clustered using correlation distance and average linkage
Regarding antioxidant enzymes, the highest activities were observed under CuSO4 excess (++ CuS + FeE; ++ CuS + FeC) for all three tested enzymes (SOD; CAT, POX); while the use of Cu-EDTA excess (++ CuE + FeE; ++ CuE + FeC) did not significantly change the enzymatic activities in comparison Cu-sufficient leaves (+ CuE + FeE; + CuE + FeC) (Fig. 7).
Evaluation of Cu toxicity on the activity of antioxidant enzymes (A superoxide dismutase, SOD; B catalase, CAT; and C peroxidase, POX) in leaves of maize when different Cu and Fe sources were applied to nutrient solution. Data are means + SD, for each element letters indicate a significant difference (ANOVA Holm-Sidak; N = 3, P < 0.05)
Different isoenzymes of SOD were detected using native-PAGE and SOD staining (Supplementary Figure S3). When Cu was in excess and in form of CuSO4, the most abundant and active isoform in maize leaves was referred to Cu/Zn SOD. In agreement with total enzymatic activity (Fig. 7), an overall low activity ascribable to all SOD isoforms was observed under + CuE + FeE treatment (Supplementary Fig. S3).
Discussion
Copper-Free Nutrient Solution Does Not Affect Maize Growth
In crops, Cu-deficiency symptoms are related to bluish-green leaves which become chlorotic near the tips and along both sides of the midrib (Alloway and Tills 1984). Despite − CuS + FeE plants showed a reduction of SPAD index values and root Cu concentration (in comparison to Cu-sufficient plants, + CuS + FeE), no changes on maize biomass accumulation were observed when plants were grown in a Cu-free nutrient solution (Supplementary Fig. S1). This behavior could be explained by a high abundance of Cu as contaminant (Gries et al. 1998). Furthermore, maize could have an endogenous amount of Cu deriving from the seed storage that sustains plant needs during first vegetative stages. Thus, it is plausible suppose that Cu-deficient symptoms may become more evident in the latter phonological stages (e.g., flowering and ripening of the seeds; Graham 1975; Dell 1981).
In tobacco and in lupin, the deprivation of some micronutrients (Cu, Zn, or Mn) impaired the activities of antioxidant enzymes, as SOD isoforms, depending on the type and severity of the deficiency stress (Yu et al. 1998; Yu and Rengel 1999). Under our condition, Cu-free nutrient solution (− CuS + FeE) did not affect significantly the activity of tested-antioxidant enzymes (Fig. 3) and did not determine any substantial change on elemental composition of maize plants, except for a slight reduction of Zn, Mn, and Ca content in shoots (Fig. 2). Overall, these results suggest that, under our experimental conditions (15 days of treatment), Cu deprivation did not impact maize growth and development.
Maize Plants are Susceptible to Cu-Excess in Nutrient Solution
In the present study, Cu-excess condition strongly impaired maize growth and induced leaf chlorosis in ++ CuS + FeE plants in comparison to control plants (Cu-sufficient condition + CuS + FeE, Fig. 1, Supplementary Fig. S1). The effects of Cu toxicity on growth and development have been reported in several crops, including maize (Mocquot et al. 1996; Ouzounidou et al. 1998; Adrees et al. 2015; Marastoni et al. 2019b). As expected, maize plants over accumulated Cu when exposed to Cu-excess in nutrient solution, and the element was mainly accumulated in roots (Fig. 2). The high Cu concentration in roots (rather than in shoots) might be due to the Cu accumulation in the apoplast (Krzesłowska 2011; Marastoni et al. 2019a). Allan and Jarrell (1989) explained that the adsorption of Cu on the root surface could occur in cationic form interacting with negative charges of the cell-wall.
The nutritional status of maize plants was strongly affected by Cu toxicity as the concentrations of P, Ca, Fe, Zn, and Mn markedly decreased in comparison to Cu-sufficient plants (+ CuS + FeE, Fig. 2). The antagonistic effect of Cu on the content of other nutrients was reported in several species and was mainly referred to a competitive effect of Cu on the uptake of ions (Michaud et al. 2008; Keller et al. 2015; Azeez et al. 2015; Ali et al. 2002; Feil et al. 2020). When highly abundant in the apoplast, Cu ions tend to displace Ca2+ ions from exchange sites in the root free space (Jensén and Aòalsteinsson 1989). Moreover, multielement analyses indicated that Cu-excess condition also determine an increase of S concentration in maize shoots (Fig. 2). In cabbage, the high S levels under Cu toxicity correlated with the upregulation of sulfate transporters (Shahbaz et al. 2010) that may be due to a higher requirement of S-containing molecules to counteract oxidative stress.
In agreement with previous evidence (Devi and Prasad 1998; Martins and Mourato 2006; Liu et al. 2018), an increase of SOD activity in maize leaves was observed under Cu toxicity in comparison to Cu-sufficient plants (+ CuS + FeE, Fig. 3) and this activity was mainly linked to Cu/Zn SOD isoform (as confirmed by in-gel activity Supplementary Fig. S3). The SOD activity operates the production of free radicals in plant cells, that in turn lead to lipid peroxidation and destabilization of thylakoid membranes (Van Assche and Clijsters 1990). Other antioxidant enzymes (such as CAT and POX) increased their activity under Cu-excess condition, confirming their important role in ROS detoxification (Yruela 2009; Pantola and Shekhawat 2012; Adrees et al. 2015).
Maize Response to Cu Toxicity is Dependent on Fe- and Cu-Forms
The use of different Cu- and Fe-forms allowed us to evaluate the sensitivity of maize plants to Cu-excess condition (Experiment II, Table 1). Considering the occurrence of chelate-exchange processes at the rhizosphere and their role to influence the availability of nutrients, the effect of two different Cu-sources (CuSO4 and Cu-EDTA) and two different Fe-forms (Fe-EDTA and Fe-citrate) were compared. In agreement with previous evidence (Fig. 4), maize plants showed phytotoxicity by high Cu-levels in nutrient solution, and the toxicity symptoms were mainly observed in plants exposed to CuSO4 rather than to Cu-EDTA. Under CuSO4 excess, main symptoms involved a sharp reduction of fresh and dry weights, stunted growth of roots, leaf deformation, and necrosis of apical meristems (Fig. 4, Supplementary Fig. S2). Confirming data of the Experiment I (see Sect. “Maize Plants are Susceptible to Cu-Excess in Nutrient Solution”), the root exposure to high Cu-levels reduced in maize the concentration of other nutrients in comparison to Cu-sufficient plants, showing an antagonistic effect of Cu-excess on nutritional levels of other elements. Multivariate analysis (PCA) on ionomic data pointed out a clear separation of samples treated with high CuSO4 from the others (Fig. 6), confirming that the CuSO4-containing treatments clearly distinguished the ionomic profile of maize plants. The acquisition of Fe, Zn, Ca, and P were significantly affected in plants when Cu was applied in excess as CuSO4 rather than Cu-EDTA (regardless to chelating agent of Fe, Fig. 5). The ionomic profiles may explain the severe growth reduction observed in CuSO4 treated plants. Under ++ CuS + FeE, the evaluation of equilibrium constants suggests that Cu was mainly available for root uptake as free ion (Cu2+, Supplementary Table S1). In this form, Cu may compete with other cations (such as Fe) for their binding site on PS and for their root acquisition (Zhang et al., 1991; Ma and Nomoto 1996). Especially under soil alkaline conditions (such as in calcareous soils), high Cu-levels may compromise Fe bioavailability and, in turn, limit the root uptake of Fe in grasses (that is mainly operated via PS-complexation based mechanisms; Reichman and Parker 2005).
The evaluation of stability constants of Fe and Cu metal complexes in solution indicates that the Cu availability for root acquisition is also influenced by Fe form (Supplementary Table S1). The use of Fe-citrate can determine an exchange of chelating ligand, where at the equilibrium the predominant form in nutrient solution is likely to be Cu-citrate (regardless to the Cu form applied). Furthermore, it has been reported that under high Cu-levels, grasses promote root exudation of citrate, a metal ligand that becomes available for the complexation of nutrients at the rhizosphere (De Conti et al. 2020). Therefore, under ++ CuS + FeC and ++ CuE + FeC, maize roots were likely exposed to an excess of Cu in form of Cu-citrate while Fe occurred mainly as Fe(OH)2+ or Fe-EDTA, respectively. In form of Fe-hydroxide, this nutrient is poorly available for root uptake as its solubility is strongly compromised at neutral/alkaline pH (Lindsay and Schwab 1982). In this way, it is plausible suppose that the strong reduction of plant growth observed in ++ CuS + FeC in comparison to ++ CuE + FeC is linked to Cu toxicity exacerbated by limiting Fe availability.
Taken together, these results indicate that both CuSO4-treatments (++ CuS + FeE and ++ CuS + FeC) resulted in a low Fe acquisition by roots (likely due to competition of Cu ions with PS-binding sites or due to chelate-exchange processes between Cu ions and Fe-citrate) leading to Fe shortage in plants. As reported by Waters and Armbrust (2013), ++ CuS + FeE or ++ CuS + FeC plants may have been more susceptible to high levels of Cu (in comparison to the other treatments) due to the Fe deficiency condition which leads plants to be more sensitive to the toxicity of the metal.
The simultaneous use of Cu-EDTA and Fe-EDTA led to a beneficial effect on Cu-levels in shoots and roots as Cu concentration in plants was halved by ++ CuE + FeE treatment in comparison to all other Cu-excess treatments. In silico analyses indicate that at the equilibrium the synthetic chelating agent EDTA was mainly bounded by Fe (with the formation of Fe-EDTA complex), whereas Cu-EDTA complex may occur when EDTA exceed Fe moiety (a condition referring to ++ CuE + FeE treatment).
Up to date, knowledge on Cu-acquisition systems may indicate that the Cu metal can be taken up by roots through a reductive mechanism (as Cu+) as well by non-reductive way (as Cu2+) or by metal-complex transporters. Therefore, Cu-complex may be directly taken up by roots or become substrate of Cu-chelated reductases located on plasma membrane of root cells. Our data suggests that, in plants, the Cu acquisition from Cu-EDTA is limited in comparison to Cu-citrate and this response may be due to a different affinity of the chelating agent to the molecular components involved in the Cu-acquisition system.
The production of ROS and antioxidant molecules in plants can be considered as a marker of metal phytotoxicity and their entity depends upon plant species, severity, and the duration of Cu stress (Sharma and Dietz 2009). Adrees et al. (2015) reported that the increase of Cu-levels in the growth medium caused a dose-dependent increase in ROS generation. At high CuSO4 concentration, the activities of SOD, CAT, and POX increased (as also reported in Chen et al. 2000), while all the Cu-EDTA excess plants (++ CuE + FeE and ++ CuE + FeC) did not show significant changes in the activity of ROS-detoxification enzymes in comparison to Cu-EDTA sufficient plants (+ CuE + FeE and + CuE + FeC, Fig. 7). The activities of antioxidant enzymes support morphological and multielement observations, as the use of Cu-EDTA allowed to prevent phytotoxic effects on plants even when high Cu-levels occur in solution (in maize, present work, and in Brassica napus, Habiba et al. 2015). This data suggests that the application of exogenous synthetic chelates, as EDTA, to crops might be a valid strategy to overcome toxicity in grasses on contaminated soils. As in the next years agriculture has claimed to have a sustainable view, particular attention should be paid to the nature of metal chelates released in the environment. Therefore, the evaluation of strong metal chelates with natural origin, such as (phyto)siderophores, lignosulphonates, polyphenols, and humic substances, might have great relevance to limit environmental impact and alleviate Cu toxicity on contaminated soils, especially for organic farming.
Conclusion
In the present research, maize response to Cu-limiting or excess conditions have been characterized. Maize plants showed severe symptoms of Cu toxicity when plants were exposed to an excess of Cu depending on Cu- and Fe-forms applied. According to different Cu- and Fe-forms, the effect of high Cu-levels on plant nutrition are shown in a schematic representation (Cu and Fe-forms availability, root nutrient concentrations, and leaf enzymatic activities are summarized in Fig. 8). The use of excess of CuSO4 determined marked symptoms of stunted growth, leaf chlorosis, and root length inhibition, while same Cu concentration in form of Cu-EDTA neither limit plant growth nor seems to be affected by ROS. Depending on metals and metal-chelating agents available at the rhizosphere, the occurrence of chelate-exchange processes and cross connection between Cu and Fe acquisition systems might be relevant to alleviate Cu toxicity in contaminated soils.
Schematic indication of Cu- and Fe-forms present at equilibrium in nutrient solution under different treatments (only Cu-excess conditions have been considered): ++ CuS + FeE, ++ CuS + FeC; ++ CuE + FeE, ++ CuE + FeC. Heatmap of elemental concentration in roots is shown with color intensity normalized on row z-score. High concentration values are indicated in red, low concentrations in yellow. The activity of detoxifying enzymes (SOD, CAT, POX) increased (red arrow) by ++ CuS + FeE and ++ CuS + FeC in comparison to + CuS + FeE and + CuS + FeC (see Fig. 7)
The study of the plant responses to Cu toxicity under low Fe availability would be a future perspective of the work as most vineyards are cultivated on calcareous soils and most of them had been contaminated over the years by repeated applications of Cu-based fungicides. Understanding the interplay between Cu and other nutrients will allow to evaluate the best management practices of contaminated soils toward a sustainable crop production.
Data Availability
All data generated or analyzed during this study are included in this published article.
Change history
19 July 2022
Open access funding note has been updated.
Abbreviations
- CAT:
-
Catalase
- COPT:
-
Copper transporter
- Cu:
-
Copper
- CuE:
-
Cu-EDTA
- CuS:
-
CuSO4
- DW:
-
Dry weight
- EDTA:
-
Ethylenediaminetetraacetic acid
- Fe:
-
Iron
- FeC:
-
Fe-citrate
- FeE:
-
Fe-EDTA
- FRO:
-
Fe(III)-chelate reductase
- FW:
-
Fresh weight
- HMA:
-
Heavy metal ATPases
- IRT:
-
Fe transporter
- POX:
-
Peroxidase
- PS:
-
Phytosiderophores
- SOD:
-
Superoxide dismutase
- SPAD:
-
Soil–plant analyzer-development
- YS1:
-
Yellow stripe 1 transporter
References
Adrees M, Ali S, Rizwan M, Ibrahim M, Abbas F, Farid M, Bharwana SA (2015) The effect of excess copper on growth and physiology of important food crops: a review. Environ Sci Pollut Res 22:8148–8162
Adriano DC (2001) Trace elements in terrestrial environments: biochemistry, bioavailability and risks of metals, 2nd edn. Springer, New York, pp 219–261
Ali NA, Bernal MP, Ater M (2002) Tolerance and bioaccumulation of copper in Phragmites australis and Zea mays. Plant Soil 239:103–111
Allan DL, Jarrell WM (1989) Proton and copper adsorption to maize and soybean root cell walls. Plant Physiol 89:823–832
Alloway BJ, Tills AR (1984) Copper deficiency in world crops. Outlook Agric 13:32–42
Azeez M, Adesanwo O, Adepetu J (2015) Effect of copper (Cu) application on soil available nutrients and uptake. Afr J Agric Res 10:359–364
Bashir K, Inoue H, Nagasaka S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2006) Cloning and characterization of deoxymugineic acid synthase genes from graminaceous plants. J Biol Chem 281:32395–32402
Bernal M, Casero D, Singh V, Wilson GT, Grande A, Yang H (2012) Transcriptome sequencing identifies SPL7-regulated Cu acquisition genes FRO4/FRO5 and the Cu dependence of Fe homeostasis in Arabidopsis. Plant Cell 24:738–761
Bienfait HF, Van den Briel W, Mesland-Mul NT (1985) Free space iron pools in roots. Plant Physiol 78:596–600
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bravin MN, Garnier C, Lenoble V, Gérard F, Dudal Y, Hinsinger P (2012) Root-induced changes in pH and dissolved organic matter binding capacity affect copper dynamic speciation in the rhizosphere. Geochim Cosmochim Acta 84:256–268
Brun LA, Maillet J, Hinsinger P, Pepin M (2001) Evaluation of copper availability to plants in copper contaminated vineyard soils. Environ Pollut 111:293–302
Brunetto G, de Melo GWB, Terzano R, Del Buono D, Astolfi S, Tomasi N, Pii Y, Mimmo T, Cesco S (2016) Copper accumulation in vineyard soils: rhizosphere processes and agronomic practices to limit its toxicity. Chemosphere 162:293–307
Buoso S, Tomasi N, Said-Pullicino D, Arkoun M, Yvin JC, Pinton R, Zanin L (2021) Characterization of physiological and molecular responses of Zea mays seedlings to different urea-ammonium ratios. Plant Physiol Biochem 162:613–623
Burkhead JL, Gogolin Reynolds KA, Abdel-Ghany SE, Cohu CM, Pilon M (2009) Copper homeostasis. New Phytol 182:799–816
Chaignon V, Sanchez-Neira I, Herrmann P, Jaillard B, Hinsinger P (2003) Copper bioavailability and extractability as related to chemical properties of contaminated soils from a vine-growing area. Environ Pollut 123:229–238
Chen LM, Lin CC, Kao CH (2000) Cu toxicity in rice seedlings: changes in antioxidative enzyme activities, H2O2 level and cell wall peroxidase activity in roots. Bot Bull Acad Sin 41:99–103
Chen Y, Shi J, Tian G, Zheng S, Lin Q (2004) Fe deficiency induces Cu uptake and accumulation in Commelina communis. Plant Sci 166:1371–1377
Chopin EIB, Alloway BJ (2007) Distribution and mobility of trace elements in soils and vegetation around the mining and smelting areas of Tharsis, Riotinto and Huelva, Iberian Pyrite Belt, SW Spain. Water Air Soil Pollut 182:245–261
Chopin EIB, Marin B, Mkoungafoko R, Rigaux A, Hopgood MJ, Delannoy E, Laurain M (2008) Factors affecting distribution and mobility of trace elements (Cu, Pb, Zn) in a perennial grapevine (Vitis vinifera L.) in the Champagne region of France. Environ Pollut 156:1092–1098
Curie C, Cassin G, Couch D, Divol F, Higuchi K, Le Jean M, Mission J, Schikora A, Czernic P, Mari S (2009) Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Ann Bot 103:1–11
De Conti L, Cesco S, Mimmo T, Pii Y, Valentinuzzi F, Melo GWB, Ceretta CA, Trentin E, Marques ACR, Brunetto G (2020) Iron fertilization to enhance tolerance mechanisms to copper toxicity of ryegrass plants used as cover crop in vineyards. Chemosphere 243:125298
Dell B (1981) Male sterility and anther wall structure in copper-deficient plants. Ann Bot 48:599–608
Devi SR, Prasad MNV (1998) Copper toxicity in Ceratophyllum demersum L. (Coontail), a free floating macrophyte: response of antioxidant enzymes and antioxidants. Plant Sci 138:157–165
Dourado MN, Souza LA, Martins PF, Peters LP, Piotto FA, Azevedo RA (2014) Burkholderia sp. SCMS54 triggers a global stress defense in tomato enhancing cadmium tolerance. Water Air Soil Pollut 225:2159
Elavarthi S, Martin B (2010) Spectrophotometric assays for antioxidant enzymes in plants. Plant stress tolerance. Humana Press, Totowa, pp 273–280
Feigl G, Kumar D, Lehotai N, Tugyi N, Molnár Á, Ördög A, Szepesi A, Gémes K, Laskay G, Erdei L, Kolbert Z (2013) Physiological and morphological responses of the root system of Indian mustard (Brassica juncea L. Czern.) and rapeseed (Brassica napus L.) to copper stress. Ecotox Environ Saf 94:179–189
Feil SB, Pii Y, Valentinuzzi F, Tiziani R, Mimmo T, Cesco S (2020) Copper toxicity affects phosphorus uptake mechanisms at molecular and physiological levels in Cucumis sativus plants. Plant Physiol Biochem 157:138–147
Graham RD (1975) Male sterility in wheat plants deficient in copper. Nature 254:514
Gries D, Klatt S, Runge M (1998) Copper-deficiency-induced phytosiderophore release in in the calcicole grass Hordelymus europaeus. New Phytol 140:95–101
Grotz N, Guerinot ML (2006) Molecular aspects of Cu, Fe and Zn homeostasis in plants. Biochim Biophys Acta 1763:595–608
Habiba U, Ali S, Farid M, Shakoor MB, Rizwan M, Ibrahim M, Abbasi GH, Hayat T, Ali B (2015) EDTA enhanced plant growth, antioxidant defense system, and phytoextraction of copper by Brassica napus L. Environ Sci Pollut Res Int 22:1534–1544
Hippler FW, Cipriano DO, Boaretto RM, Quaggio JA, Gaziola SA, Azevedo RA, Mattos-Jr D (2016) Citrus rootstocks regulate the nutritional status and antioxidant system of trees under copper stress. Environ Exp Bot 130:42–52
Hofmann NR (2012) Nicotianamine in zinc and iron homeostasis. Plant Cell 24:373
Irtelli B, Petrucci WA, Navari-Izzo F (2009) Nicotianamine and histidine/proline are, respectively, the most important copper chelators in xylem sap of Brassica carinata under conditions of copper deficiency and excess. J Exp Bot 60:269–277
Jensén P, Aòalsteinsson S (1989) Effects of copper on active and passive Rb+ influx in roots of winter wheat. Physiol Plantarum 75:195–200
Jung MC, Thornton I (1996) Heavy metal contamination of soils and plants in the vicinity of a lead-zinc mine. Korea Appl Geochem 11:53–59
Keller C, Rizwan M, Davidian JC, Pokrovsky OS, Bovet N, Chaurand P, Meunier JD (2015) Effect of silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 μM Cu. Planta 241:847–860
Kochian L (1991) Mechanisms of micronutrient uptake and translocation in plants. In: Mortvedt J, Cox F, Shuman L, Welch R (eds) Micronutrients in agriculture. Soil Science Society of America, Madison, pp 119–296
Kopittke PM, Menzies NW (2006) Effect of Cu toxicity on growth of cowpea (Vigna unguiculata). Plant Soil 279:287–296
Korshunova YO, Eide D, Clark WG, Guerinot ML, Pakrasi HB (1999) The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol Biol 40:37–44
Krzesłowska M (2011) The cell wall in plant cell response to trace metals: polysaccharide remodeling and its role in defense strategy. Acta Physiol Plant 33:35–51
Lequeux H, Hermans C, Lutts S, Verbruggen N (2010) Response to copper excess in Arabidopsis thaliana: impact on the root system architecture, hormone distribution, lignin accumulation and mineral profile. Plant Physiol Biochem 48:673–682
Liao MT, Hedley MJ, Woolley DJ, Brooks RR, Nichols MA (2000) Copper uptake and translocation in chicory (Cichorium intybus L. cv Grasslands Puna) and tomato (Lycopersicon esculentum Mill. cv Rondy) plants grown in NFT system. II. The role of nicotianamine and histidine in xylem sap copper transport. Plant Soil 223:243–252
Lindsay WL, Schwab AP (1982) The chemistry of iron in soils and its availability to plants. J Plant Nutr 5:821–840
Liu J, Wang J, Lee S, Wen R (2018) Copper-caused oxidative stress triggers the activation of antioxidant enzymes via ZmMPK3 in maize leaves. PLoS ONE 13:e0203612
Ma JF, Nomoto K (1996) Effective regulation of iron acquisition in graminaceous plants. The role of mugineic acids as phytosiderophores. Physiol Plantarum 97:609–617
Mackie KA, Müller T, Kandeler E (2012) Remediation of copper in vineyards–a mini review. Environ Pollut 167:16–26
Marastoni L, Sandri M, Pii Y, Valentinuzzi F, Brunetto G, Cesco S, Mimmo T (2019a) Synergism and antagonisms between nutrients induced by copper toxicity in grapevine rootstocks: Monocropping vs. intercropping. Chemosphere 214:563–578
Marastoni L, Tauber P, Pii Y, Valentinuzzi F, Astolfi S, Simoni A, Brunetto G, Cesco S, Mimmo T (2019b) The potential of two different Avena sativa L. cultivars to alleviate Cu toxicity. Ecotoxicol Environ Saf 182:109430
Marschner H (1995) Mineral nutrition of higher plants. Academic Press, London, pp 344–346
Marschner P (2011) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic Press, London
Marschner H, Römheld V (1994) Strategies of plants for acquisition of iron. Plant Soil 165:261–274
Marschner H, Römheld V, Kissel M (1986) Different strategies in higher plants in mobilization and uptake of iron. J Plant Nutr 9:695–713
Martins LL, Mourato MP (2006) Effect of excess copper on tomato plants: growth parameters, enzyme activities, chlorophyll, and mineral content. J Plant Nutr 29:2179–2198
Metsalu T, Vilo J (2015) Clustvis: a web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res 43:W566–W570
Michaud AM, Chappellaz C, Hinsinger P (2008) Copper phytotoxicity affects root elongation and iron nutrition in durum wheat (Triticum turgidum durum L.). Plant Soil 310:151–165
Mocquot B, Vangronsveld J, Clijsters H, Mench M (1996) Copper toxicity in young maize (Zea mays L.) plants: effects on growth, mineral and chlorophyll contents, and enzyme activities. Plant Soil 182:287–300
Ouzounidou G, Ilias I, Tranopoulou H, Karataglis S (1998) Amelioration of copper toxicity by iron on spinach physiology. J Plant Nutr 21:2089–2101
Pantola RC, Shekhawat GS (2012) Copper induced antioxidative enzyme indices in leaves of Brassica juncea seedlings. J Pharm Biomed Sci 15:1–6
Pätsikkä E, Kairavuo M, Šeršen F, Aro EM, Tyystjärvi E (2002) Excess copper predisposes photosystem II to photoinhibition in vivo by outcompeting iron and causing decrease in leaf chlorophyll. Plant Physiol 129:1359–1367
Pilon M, Tapken W (2013) Copper homeostasis: regulation in plants. In: Scott RDA (ed) Encyclopedia of inorganic and bioinorganic chemistry
Pinton R, Cesco S, Iacolettig G, Astolfi S, Varanini Z (1999) Modulation of NO3- uptake by water-extractable humic substances: involvement of root plasma membrane H+ ATPase. Plant Soil 215:155–161
Printz B, Lutts S, Hausman J-F, Sergeant K (2016) Copper trafficking in plants and its implication on cell wall dynamics. Front Plant Sci 7:601
Rahimi A, Bussler W (1973) The effect of copper deficiency on the tissue structure of higher plants. Z Pflanzenerachr 135:183–195
Rehman M, Liu L, Wang Q, Saleem MH, Bashir S, Ullah S, Peng D (2019) Copper environmental toxicology, recent advances, and future outlook: a review. Environ Sci Pollut Res 26:18003–18016
Reichman SM, Parker DR (2005) Metal complexation by phytosiderophores in the rhizosphere. Biogeochemistry of trace elements in the rhizosphere. Elsevier, Amsterdam, pp 129–156
Ryan BM, Kirby JK, Degryse F, Harris H, McLaughlin MJ, Scheiderich K (2013) Copper speciation and isotopic fractionation in plants: uptake and translocation mechanisms. New Phytol 199:367–378
Sancenón V, Puig S, Mira H, Thiele DJ, Peñarrubia L (2003) Identification of a copper transporter family in Arabidopsis thaliana. Plant Mol Biol 51:577–587
Sauvé S, McBride MB, Norvell WA, Hendershot WH (1997) Copper solubility and speciation of in situ contaminated soils: effects of copper level, pH and organic matter. Water Air Soil Pollut 100:133–149
Schaaf G, Ludewig U, Erenoglu BE, Mori S, Kitahara T, von Wirén N (2003) ZmYS1 functions as a proton-coupled symporter for phytosiderophore- and nicotianamine-chelated metals. J Biol Chem 29:9091–9096
Schenkeveld WDC, Oburger E, Gruber B, Schindlegger Y, Hann S, Puschenreiter M, Kraemer SM (2014) Metal mobilization from soils by phytosiderophores–experiment and equilibrium modeling. Plant Soil 383:59–71
Schramel O, Michalke B, Kettrup A (2000) Study of the copper distribution in contaminated soils of hop fields by single and sequential extraction procedures. Sci Total Environ 263:11–22
Shahbaz M, Tseng MH, Stuiver CE, Koralewska A, Posthumus FS, Venema JH, Parmar S, Schat H, Hawkesford MJ, De Kok LJ (2010) Copper exposure interferes with the regulation of the uptake, distribution and metabolism of sulfate in Chinese cabbage. J Plant Physiol 167:438–446
Sharma SS, Dietz KJ (2009) The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci 14:43–50
Thiel H, Finck A (1973) Ermittlung von Grenzwerten optimaler Kupfer-Versorgung für Hafer und Sommergerste. Zeitschrift Fu Ér Pflanzenerna Éhrung Und Bodenkunde 134:107–125
Uren NC (1982) Chemical reduction at the root surface. J Plant Nutr 5:515–520
Van Assche F, Clijsters H (1990) Effects of metals on enzyme activity in plants. Plant Cell Environ 13:195–206
Vangronsveld J, Clijsters H (1994) Toxic effects of metals. In: Farago ME (ed) Plants and the chemical elements. Biochemistry, uptake, tolerance and toxicity. pp 150–177
Vinit-Dunand F, Epron D, Alaoui-Sossé B, Badot PM (2002) Effects of copper on growth and on photosynthesis of mature and expanding leaves in cucumber plants. Plant Sci 163:53–58
von Wirén N, Mori S, Marschner H, Romheld V (1994) Iron inefficiency in maize mutant ys1 (Zea mays L. cv Yellow-Stripe) is caused by a defect in uptake of iron phytosiderophores. Plant Physiol 106:71–77
Waters BM, Armbrust LC (2013) Optimal copper supply is required for normal plant iron deficiency responses. Plant Signal Behav 8:e26611
Welch RM, Shuman L (1995) Micronutrient nutrition of plants. Crit Rev Plant Sci 14:49–82
Welch RM, Norvell WA, Schaefer SC, Shaff JE, Kochian LV (1993) Induction of iron (III) and copper (II) reduction in pea (Pisum sativum L.) roots by Fe and Cu status: does the root-cell plasmalemma Fe (III)-chelate reductase perform a general role in regulating cation uptake? Planta 190:555–561
Wintz H, Fox T, Wu YY, Feng V, Chen W, Chang HS, Vulpe C (2003) Expression profiles of Arabidopsis thaliana in mineral deficiencies reveal novel transporters involved in metal homeostasis. J Biol Chem 278:47644–47653
Yruela I (2009) Copper in plants: acquisition, transport and interactions. Funct Plant Biol 36:409–430
Yu Q, Rengel Z (1999) Micronutrient deficiency influences plant growth and activities of superoxide dismutases in narrow-leafed lupins. Ann Bot 83:175–182
Yu Q, Osborne L, Rengel Z (1998) Micronutrient deficiency changes activities of superoxide dismutase and ascorbate peroxidase in tobacco plants. J Plant Nutr 21:1427–1437
Zanin L, Tomasi N, Rizzardo C, Gottardi S, Terzano R, Alfeld M, Janssens K, De Nobili M, Mimmo T, Cesco S (2015) Iron allocation in leaves of Fe-deficient cucumber plants fed with natural Fe complexes. Physiol Plant 154:82–94
Zanin L, Venuti S, Zamboni A, Varanini Z, Tomasi N, Pinton R (2017) Transcriptional and physiological analyses of Fe deficiency response in maize reveal the presence of Strategy I components and Fe/P interactions. BMC Genom 18:154
Zhang FS, Römheld V, Marschner H (1991) Diurnal rhythm of release of phytosiderophores and uptake rate of zinc in iron-deficient wheat. Soil Sci Plant Nutr 37:671–678
Acknowledgements
This research did not receive any specific Grant from funding agencies in the public, commercial, or not-for-profit sectors.
Funding
Open access funding provided by Università degli Studi di Udine within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
AF, SB, LZ, RP, NT contributed to the study conception, design, data collection, analyses, and manuscript preparation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Handling Editor: Andrzej Bajguz.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Franco, A., Buoso, S., Zanin, L. et al. Copper Toxicity in Maize: The Severity of the Stress is Reduced Depending on the Applied Fe-Chelating Agent. J Plant Growth Regul 42, 1567–1581 (2023). https://doi.org/10.1007/s00344-022-10641-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-022-10641-1