Abstract
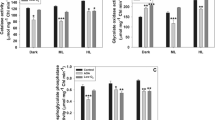
Elevated carbon dioxide (CO2) has been shown to enhance the photosynthesis of plants, while inducing stomatal closure in leaves. Stomatal closure is always a key issue regarding plants’ responses against stress under elevated CO2. This paper examined the mechanism underlying CO2 elevation-induced stomatal closure in Arabidopsis. Endogenous nitric oxide (NO) in guard cells was detected by the specific probe 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate. The Arabidopsis wild type, nitric oxide synthase (NOS) mutant atnos1, and nitrate reductase (NR) mutant nia1nia2 were used as experimental materials. The present study shows that elevated CO2-induced NO accumulation in guard cells by 120 % and decreased the aperture of stomata by 32 % compared with ambient CO2. CO2 elevation-induced stomatal closure was reversed by scavenging NO. Here, we suggested that NO plays an important role in stomatal closure of Arabidopsis induced by elevated CO2. The pharmacological and genetic evidence shows that both the NOS inhibitor L-NAME and NR inhibitor tungstate significantly decreased NO accumulation in guard cells and inhibited stomatal closure, whereas the stomatal aperture of both the atnos1 and nia1nia2 mutants decreased slightly under elevated CO2, indicated that both NOS-like and NR were involved in CO2 elevation-induced NO accumulation. Therefore, we conclude from these findings that elevated CO2 increases the level of NO through both NOS-like and NR, which then induces stomatal closure in Arabidopsis under elevated CO2.





Similar content being viewed by others
References
Asai S, Ohta K, Yoshioka H (2008) MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana. Plant Cell 20:1390–1406
Bethke PC, Gubler F, Jacobsen JV, Jones RL (2004) Dormancy of Arabidopsis seeds and barley grains can be broken by nitric oxide. Planta 219:847–855
Betts RA, Boucher O, Collins M, Cox PM, Falloon PD, Gedney N, Hemming DL, Huntingford C, Jones CD, Sexton DM (2007) Projected increase in continental runoff due to plant responses to increasing carbon dioxide. Nature 448:1037–1041
Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45:113–122
Ceulemans R, Praet LV, Jiang X (1995) Effects of CO2 enrichment, leaf position and clone on stomatal index and epidermal cell density in poplar (Populus). New Phytol 131:99–107
Corpas FJ, Barroso JB, Carreras A, Valderrama R, Palma JM, León AM, Sandalio LM, del Río LA (2006) Constitutive arginine-dependent nitric oxide synthase activity in different organs of pea seedlings during plant development. Planta 224:246–254
Crawford NM (2006) Mechanisms for nitric oxide synthesis in plants. J Exp Bot 57:471–478
De Souza AP, Gaspar M, Da Silva EA, Ulian EC, Waclawovsky AJ, Dos Santos RV, Teixeira MM, Souza GM, Buckeridge MS (2008) Elevated CO2 increases photosynthesis, biomass and productivity, and modifies gene expression in sugarcane. Plant Cell Environ 31:1116–1127
Dean JV, Harper JE (1986) Nitric oxide and nitrous oxide production by soybean and winged bean during the in vivo nitrate reductase assay. Plant Physiol 82:718–723
Del Río LA, Corpas FJ, Barroso JB (2004) Nitric oxide and nitric oxide synthase activity in plants. Phytochemistry 65:783–792
Desikan R, Griffiths R, Hancock JT, Neill SJ (2002) A new role for an old enzyme: nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc Natl Acad Sci 99:16314–16318
Desikan R, Cheung MK, Bright J, Henson D, Hancock JT, Neill SJ (2004) ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. J Exp Bot 55:205–212
Ferrario-Méry S, Thibaud MC, Betsche T, Valadier MH, Foyer CH (1997) Modulation of carbon and nitrogen metabolism, and of nitrate reductase, in untransformed and transformed Nicotiana plumbaginifolia during CO2 enrichment of plants grown in pots and in hydroponic culture. Planta 202:510–521
Ferris R, Taylor G (1994) Stomatal characteristics of four native herbs following exposure to elevated CO2. Ann Bot 73:447–453
Fonseca F, Bowsher CG, Stulen I (1997) Impact of elevated atmospheric carbon dioxide on nitrate reductase transcription and activity in leaves and roots of Plantago major. Physiol Plantarum 100:940–948
Foresi NP, Laxalt AM, Tonón CV, Casalongué CA, Lamattina L (2007) Extracellular ATP induces nitric oxide production in tomato cell suspensions. Plant Physiol 145:589–592
Garcia-Mata C, Lamattina L (2007) Abscisic acid (ABA) inhibits light-induced stomatal opening through calcium- and nitric oxide-mediated signaling pathways. Nitric Oxide 17:143–151
Gedney N, Cox P, Betts R, Boucher O, Huntingford C, Stott P (2006) Detection of a direct carbon dioxide effect in continental river runoff records. Nature 439:835–838
Geiger M, Liu PW, Engels C, Harnecker J, Schulze ED, Ludewig F, Sonnewald U, Scheible WR, Stitt M (1998) Enhanced carbon dioxide leads to a modified diurnal rhythm of nitrate reductase activity in older plants, and a large stimulation of nitrate reductase activity and higher levels of amino acids in young tobacco plants. Plant Cell Environ 21:253–268
Gonugunta VK, Srivastava N, Puli MR, Raghavendra AS (2008) Nitric oxide production occurs after alkalization during stomatal closure induced by abscisic acid. Plant Cell Environ 31:1717–1724
Guo FQ, Crawford NM (2005) Arabidopsis nitric oxide synthase1 is targeted to mitochondria and protects against oxidative damage and dark-induced senescence. Plant Cell 17:3436–3450
Guo FQ, Okamoto M, Crawford NM (2003) Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science 302:100–103
Jarvis A, Mansfield T, Davies WJ (1999) Stomatal behaviour, photosynthesis and transpiration under rising CO2. Plant Cell Environ 22:639–648
Jin CW, Du ST, Chen WW, Li GX, Zhang YS, Zheng SJ (2009) Elevated carbon dioxide improves plant iron nutrition through enhancing the iron-deficiency-induced responses under iron-limited conditions in tomato. Plant Physiol 150:272–280
Kolla VA, Raghavendra AS (2007) Nitric oxide is a signaling intermediate during bicarbonate-induced stomatal closure in Pisum sativum. Physiol Plant 130:91–98
Lamattina L, García-Mata C, Graziano M, Pagnussat G (2003) Nitric oxide: the versatility of an extensive signal molecule. Ann Rev Plant Biol 54:109–136
Mayer B, Hemmens B (1997) Biosynthesis and action of nitric oxide in mammalian cells. Trends Biochem Sci 22:477–481
McAinsh MR, Clayton H, Mansfield TA, Hetherington AM (1996) Changes in stomatal behavior and guard cell cytosolic free calcium in response to oxidative stress. Plant Physiol 111:1031–1042
Meehl GA, Stocker TF, Collins WD, Friedlingstein P, Gaye AT, Gregory JM, Kitoh A, Knutti R, Murphy JM, Noda A (2007) Global climate projections. In: Solomon S, Qin D, Manning M et al (eds) Climate change: the physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, pp 747–845
Merilo E, Laanemets K, Hu HH, Xue SW, Jakobson L, Tulva I, Gonzalez-Guzman M, Rodriguez PL, Schroeder JI, Broschè M, Kollist H (2013) PYR/RCAR receptors contribute to ozone-, reduced air humidity-, darkness-, and CO2-induced stomatal regulation. Plant Physiol 162:1652–1668
Morgan JA, LeCain DR, Pendall E, Blumenthal DM, Kimball BA, Carrillo Y, Williams DG, Heisler-White J, Dijkstra FA, West M (2011) C4 grasses prosper as carbon dioxide eliminates desiccation in warmed semi-arid grassland. Nature 476:202–205
Neill SJ, Desikan R, Clarke A, Hancock JT (2002) Nitric oxide is a novel component of abscisic acid signaling in stomatal guard cells. Plant Physiol 128:13–16
Neill SJ, Desikan R, Hancock JT (2003) Nitric oxide signalling in plants. New Phytol 159:11–35
Neill SJ, Hancock JT, Desikan R (2006) Preface to nitric oxide signalling: plant growth and development. J Exp Bot 57:462
Niu YF, Jin CW, Jin GL, Zhou QY, Lin XY, Tang CX, Zhang YS (2011) Auxin modulates the enhanced development of root hairs in Arabidopsis thaliana (L.) Heynh. under elevated CO2. Plant Cell Environ 34:1304–1317
Pillitteri LJ, Sloan DB, Bogenschutz NL, Torii KU (2006) Termination of asymmetric cell division and differentiation of stomata. Nature 445:501–505
Planchet E, Sonoda M, Zeier J, Kaiser WM (2006) Nitric oxide (NO) as an intermediate in the cryptogein-induced hypersensitive response—a critical re-evaluation. Plant Cell Environ 29:59–69
Prior S, Torbert H, Runion G, Mullins G, Rogers H, Mauney J (1998) Effects of carbon dioxide enrichment on cotton nutrient dynamics. J Plant Nutr 21:1407–1426
She XP, Song XG (2006) Cytokinin- and auxin-induced stomatal opening is related to the change of nitric oxide levels in guard cells in broad bean. Physiol Plant 128:569–579
Tian QY, Sun DH, Zhao MG, Zhang WH (2007) Inhibition of nitric oxide synthase (NOS) underlies aluminum-induced inhibition of root elongation in Hibiscus moscheutos. New Phytol 174:322–331
Underwood W, Melotto M, He SY (2007) Role of plant stomata in bacterial invasion. Cell Microbiol 9:1621–1629
Wang RC, Tischner R, Gutiérrez RA, Hoffman M, Xing XJ, Chen MS, Coruzzi G, Crawford NM (2004) Genomic analysis of the nitrate response using a nitrate reductase-null mutant of Arabidopsis. Plant Physiol 136:2512–2522
Wang WH, Yi XQ, Han AD, Liu TW, Chen J, Wu FH, Dong XJ, He JX, Pei ZM, Zheng HL (2012) Calcium-sensing receptor regulates stomatal closure through hydrogen peroxide and nitric oxide in response to extracellular calcium in Arabidopsis. Plant Cell Environ 63:177–190
Wang H, Xiao WD, Niu YF, Jin CW, Chai RS, Tang CX, Zhang YS (2013) Nitric oxide enhances development of lateral roots in tomato (Solanum lycopersicum L.) under elevated carbon dioxide. Planta 237:137–144
Webb AA, McAinsh MR, Mansfield TA, Hetherington AM (1996) Carbon dioxide induces increases in guard cell cytosolic free calcium. Plant J 9:297–304
Wendehenne D, Durner J, Klessig DF (2004) Nitric oxide: a new player in plant signalling and defence responses. Curr Opin Plant Biol 7:449–455
Woodward F (1987) Stomatal numbers are sensitive to increases in CO2 from pre-industrial levels. Nature 327:617–618
Xiong J, Lu H, Lu K, Duan YX, An LY, Zhu C (2009) Cadmium decreases crown root number by decreasing endogenous nitric oxide, which is indispensable for crown root primordia initiation in rice seedlings. Planta 230:599–610
Young JJ, Mehta S, Israelsson M, Godoski J, Grill E, Schroeder JI (2013) CO2 signaling in guard cells: calcium sensitivity response modulation, a Ca2+-independent phase, and CO2 insensitivity of the gca2 mutant. Proc Natl Acad Sci 103:7506–7511
Acknowledgments
This work was financially supported by the National Key Project on Science and Technology of China [2012BAC17B02], the Chinese Ministry of Agriculture [201103004] and the Project of Scientific Emissary of Zhejiang Province [2012T2T209]. We are thankful to the 985-Institute of Agrobiology and Environmental Sciences of Zhejiang University for providing the experimental equipment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H., Xiao, W., Niu, Y. et al. Elevated Carbon Dioxide Induces Stomatal Closure of Arabidopsis thaliana (L.) Heynh. Through an Increased Production of Nitric Oxide. J Plant Growth Regul 34, 372–380 (2015). https://doi.org/10.1007/s00344-014-9473-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-014-9473-6