Abstract
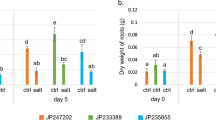
Tolerance of salt stress in potato (Solanum tuberosum L.) increased when the plants were pre-exposed to low concentrations of salt (salt acclimation). This acclimation was accompanied by increased levels of abscisic acid (ABA) in the shoot. To further study the role of roots and shoots in this acclimation process, reciprocal grafts were made between a salt-tolerant (9506) and salt-sensitive ABA(−) mutant and its ABA(+) normal sibling potato genotype. The grafted plants were acclimated with 75 or 100 mM NaCl for 3 weeks and then exposed to 150–180 mM NaCl, depending on the salt tolerance of the rootstock. After 2 weeks of exposure to the salt stress, the acclimated and unacclimated plants were compared for physiologic and morphologic parameters. The response to the salt stress was strongly influenced by the rootstock. The salt-tolerant 9506 rootstock increased the salt tolerance of scions of both the ABA-deficient mutant and its ABA(+) sibling. This salt tolerance induced by the rootstock was primarily modulated by salt acclimation and manifested in the scion via increased plant water content, stem diameter, dry matter accumulation, stomatal conductivity, and osmotic potential, and is associated with a reduction in leaf necrosis. There was also a pronounced scion effect on the rootstock. Using 9506 as a scion significantly increased root fresh and dry weights, stem diameter, and root water content of ABA(−) mutant rootstocks. Specific evidence was found of the role of exogenous ABA in the enhancement of water status in grafted plants under salt stress beyond that of grafting alone. This was verified by more positive stomatal conductivity and upward water flow in ABA-treated grafted and nongrafted plants and the absence of upward water flow in nontreated grafted plants through NMR imaging. Grafting using either salt-tolerant scions or rootstocks with inherently high ABA levels may positively modify subsequent responses of the plant under salt stress.




Similar content being viewed by others
References
Abd-Alla MH, Vuong TD, Harper JE (1998) Genotypic differences in dinitrogen fixation response to NaCl stress in intact and grafted soybean. Crop Sci 38:72–77
Amzallag GN (1996) Transmissible reproductive changes following physiological adaptation to salinity in Sorghum bicolor. New Phytol 32:317–325
Amzallag GN, Lerner HR (1995) Physiological adaptation of plants to environmental stresses. In: Pessarakli M (ed) Handbook of plant and crop physiology. Marcel Dekker, New York, pp 557–576
Amzallag GN, Lerner HR, Poljakoff-Mayber A (1990a) Exogenous ABA as a modulator of the response of Sorghum to high salinity. J Exp Bot 41:1529–1534
Amzallag GN, Lerner HR, Poljakoff-Mayber A (1990b) Induction of increased salt tolerance in Sorghum bicolor by NaCl pretreatment. J Exp Bot 41:29–34
Amzallag GN, Lerner HR, Poljakoff-Mayber A (1992) Interaction between mineral nutrients, cytokinin and gibberellic acid during growth of Sorghum at high salinity. J Exp Bot 43:81–87
Amzallag GN, Seligmann H, Lerner HR (1993) A developmental window for salt-adaptation in Sorghum bicolor. J Exp Bot 44:645–652
Asch F, Dörffling K, Dingkuhn M (1995) Response of rice varieties to soil salinity and air humidity: a possible involvement of root-borne ABA. Plant Soil 177:11–19
Azevedo Neto AD, Prisco JT, Enéas-Filho J, de Lacerda CF, Silva JV, da Costa PHA, Gomes-Filho E (2004) Effects of salt stress on plant growth, stomatal response and solute accumulation of different maize genotypes. Braz J Plant Physiol 16:31–38
Baker AJM, Grant CJ, Martin MH, Shaw SC, Whitebrook J (1986) Induction and loss of calcium tolerance in Holcus lanatus L. and other grassses. New Phytol 102:575–587
Barbera AC, Barbera A, Gallo G, Lombardo GM (1998) First results on the use of graft techniques in faba bean (Vicia faba L.) to study the process of nodulation. Part II. Workshop 12: N2 fixation in grain legumes and impact in crop rotations. p 411
Basal H, Demiral MA, Canavar O (2006) Shoot biomass production of converted race stocks of upland cotton (Gossypium hirsutum L.) exposed to salt stress. Asian J Plant Sci 5:238–242
Bayuelo-Jiménez JS, Debouck DG, Lynch JP (2003) Growth, gas exchange, water relations, and ion composition of phaseolous species grown under saline conditions. Field Crop Res 80:207–222
Behnam B, Kikuchi A, Celebi-Toprak F, Yamanaka S, Kasuga M, Yamaguchi-Shinozaki K, Watanabe KN (2006) The Arabidopsis DREB1A gene driven by the stress-inducible rd29A promoter increases salt-stress tolerance in proportion to its copy number in tetrasomic tetraploid potato (Solanum tuberosum). Plant Biotech 23:169–177
Ben Raïs L, Alpha MJ, Bahl J, Guillot-Salomon T, Dubacq JP (1993) Lipid and protein contents of jojoba leaves in relation to salt adaptation. Plant Physiol Biochem 31:547–557
Bethke PC, Drew MC (1992) Stomatal and nonstomatal components to inhibition of photosynthesis in leaves of Capsicum annuum during progressive exposure to NaCl salinity. Plant Physiol 99:219–226
Borsani O, Cuartero J, Valpuesta V, Botella MA (2002) Tomato tos1 mutation identifies a gene essential for osmotic tolerance and abscisic acid sensitivity. Plant J 32:905–914
Bray EA, Shih TY, Moses MS, Cohen A, Imai R, Plant AL (1999) Water-deficit induction of a tomato H1 histone requires abscisic acid. J Plant Growth Regul 29:35–46
Cachorro P, Remedios M, Ortiz A, Cerda A (1995) Abscisic acid and osmotic relations in Phaseolus vulgaris L. shoots under salt stress. J Plant Growth Regul 14:99–104
Chen G, Lips SH, Sagi M (2002a) Biomass production, transpiration rate and endogenous abscisic acid levels in grafts of flacca and wild-type tomato (Lycopersicon esculentum). Funct Plant Biol 29:1329–1335
Chen G, Fu X, Lips SH, Sagi M (2003) Control of plant growth resides in the shoot, and not in the root, in reciprocal grafts of flacca and wild-type tomato (Lycopersicon esculentum), in the presence and absence of salinity stress. Plant Soil 256:205–215
Chen S, Li J, Wang T, Wang S, Polle A, Hüttermann A (2002b) Osmotic stress and ion-specific effects on xylem abscisic acid and the relevance to salinity tolerance in poplar. J Plant Growth Regul 21:224–233
Cherian S, Reddy MP (2000) Salt tolerance in the halophyte Suaeda nudiflora Moq.: effect of NaCl on growth, ion accumulation and oxidative enzymes. Indian J Plant Physiol 5:32–37
CIP (2007) International Potato Center. Potato. Global production. www.Cipotato.org
Colla G, Roupahel Y, Cardarelli M, Rea E (2006) Effect of salinity on yield, fruit quality, leaf gas exchange, and mineral composition of grafted watermelon plants. HortScience 41:622–627
Conroy JP, Virgona JM, Smillie RM, Barlow EW (1988) Influence of drought acclimation and CO2 enrichment on osmotic adjustment and chlorophyll a fluorescence of sunflower during drought. Plant Physiol 86:1108–1115
Cornish K, Zeevaart JAD (1988) Phenotypic expression of wild-type tomato and three wilty mutants in relation to abscisic acid accumulation in roots and leaflets of reciprocal grafts. Plant Physiol 87:190–194
Cramer GR (2002) Response of abscisic acid mutants of Arabidopsis to salinity. Funct Plant Biol 29:561–567
Cramer GR, Quarrie SA (2002) Abscisic acid is correlated with the leaf growth inhibition of four genotypes of maize differing in their response to salinity. Funct Plant Biol 29:111–115
De Jong H, Kawchuk LM, Coleman WK, Verhaeghe CA, Russell L, Burns VJ, Tremblay-Deveau E (2001) Development and characterization of an adapted form of droopy, a diploid potato mutant deficient in abscisic acid. Am J Potato Res 78:279–290
De Lacerda CF, Cambraia J, Cano MAO, Ruiz HA (2001) Plant growth and solute accumulation and distribution in two sorghum genotypes, under NaCl stress. R Bras Fisiol Veg 13:270–284
Djanaguiraman M, Sheeba JA, Shanker AK, Devi DD, Bangarusamy U (2006) Rice can acclimate to lethal level of salinity by pretreatment with sublethal level of salinity through osmotic adjustment. Plant Soil 284:363–373
Dua RP (1997) Grafting technique in gram (Cicer arietinum) to ascertain control of root and shoot for salinity tolerance. Indian J Agric Sci 67:212–214
Dunlap JR, Binzel ML (1996) NaCl reduces indole-3-acetic acid levels in the roots of tomato plants independent of stress-induced abscisic acid. Plant Physiol 112:379–384
Durrant A (1981) Unstable genotypes. Philos Trans R Soc London Ser B 292:467–474
Eilers RG, Eilers WD, Pettapiece WW, Lelyk G (1995) Salinization of soil. In: Acton DF, Gregorich IJ (eds) The health of our soils. Toward sustainable agriculture in Canada. Centre for Land and Biological Resources Research, Agriculture and Agri-Food, Ottana, Canada, pp 77–86
Elkhatib HA, Elkhatib EA, Khalaf-Allah AM, El-Sharkawy AM (2004) Salt tolerance of four potato cultivars. J Plant Nutr 27:1575–1583
Estañ MT, Martinez-Rodriguez MM, Perez-Alfocea F, Flowers TJ, Bolarin MC (2005) Grafting raises the salt tolerance of tomato through limiting the transport of sodium and chloride to the shoot. J Exp Bot 56:703–712
Fambrini A, Castagna F, Vecchia D, Degl’Innocenti E, Ranieri A, Vernieri P, Pardossi A, Guidi L, Rascio N, Pugliesi C (2004) Characterization of a pigment-deficient mutant of sunflower (Helianthus annuusL.) with abnormal chloroplast biogenesis, reduced PS II activity and low endogenous level of abscisic acid. Plant Sci 167:79–89
Fambrini M, Vernieri P, Toncelli ML, Rossi VD, Pugliesi C (1995) Characterization of a wilty sunflower (Helianthus annuus L.) mutant. III. Phenotypic interaction in reciprocal grafts from wilty mutant and wild-type plants. J Exp Bot 46:525–530
FAO (2006) Extent and causes of salt-affected soils in participating countries. http://www.Fao
Fernández-García N, Martínez V, Cerdá A, Carvajal M (2002) Water and nutrient uptake of grafted tomato plants grown under saline conditions. Plant Physiol 159:899–905
Fernández-García N, Carvajal M, Olmos E (2004a) Graft union formation in tomato plants: peroxidase and catalase involvement. Ann Bot 93:53–60
Fernández-García N, Martínez V, Carvajal M (2004b) Effect of salinity on growth, mineral composition, and water relations of grafted tomato plants. J Plant Nutr Soil Sci 167:616–622
Fricke W, Akhiyarova G, Veselov D, Kudoyarova G (2004) Rapid and tissue-specific changes in ABA and in growth rate in response to salinity in barley leaves. J Exp Bot 55:1115–1123
Fricke W, Akhiyarova G, Wei W, Alexandersson E, Miller A, Kjellbom PO, Richardson A, Wojciechowski T, Schreiber L, Veselov D, Kudoyarova G, Volkov V (2006) The short-term growth response to salt of the developing barley leaf. J Exp Bot 57:1079–1095
Gómez-Cadenas A, Arbona V, Jacas J, Primo-Millo E, Talon M (2003) Abscisic acid reduces leaf abscission and increases salt tolerance in citrus plants. J Plant Growth Regul 21:234–240
González-Rodríguez H, Roberts JKM, Jordan WR, Drew MC (1997) Growth, water relations, and accumulation of organic and inorganic solutes in roots of maize seedlings during salt stress. Plant Physiol 113:881–893
Grillo S, Leone A, Xu Y, Tucci M, Francione R, Hasegawa PM, Monti L, Bressan RA (1995) Control of osmotin gene expression by ABA and osmotic stress in vegetative tissues of wild-type and ABA-deficient mutants of tomato. Physiol Plant 93:498–504
Guy CL (1990) Cold acclimation and freezing stress tolerance: role of protein metabolism. Annu Rev Plant Physiol Plant Mol Biol 41:187–223
Hasegawa PM, Bressan RA, Nelson DE, Samaras Y, Rhodes D (1994) Tissue culture in the improvement of salt tolerance in plants. In: Yeo AR, Flowers TJ (eds), Soil mineral stresses. Approaches to crop improvement. Monographs on theoretical and applied genetics. Springer-Verlag, Berlin, Vol 21, pp 83–125
Hassanein AA (2000) Physiological responses induced by shock and gradual salinization in rice (Oryza sativa L.) seedlings and the possible role played by glutathione treatment. Acta Bot Hung 421:139–159
He T, Cramer GR (1996) Abscisic acid concentrations are correlated with leaf area reductions in two salt-stressed rapid-cycling Brassica species. Plant Soil 179:25–33
Hmida-Sayari A, Gargouri-Bouzid R, Bidani A, Jaoua L, Savouré A, Jaoua S (2005) Overexpression of delta¹-pyrroline-5-carboxylate synthetase increases proline production and confers salt tolerance in transgenic potato plants. Plant Sci 169:746–752
Holappa LD, Walker-Simmons MK, Ho THD, Riechers DE, Beckles DM, Jones RL (2005) A Triticum tauschii protein kinase related to wheat PKABA1 is associated with ABA signaling and is distributed between the nucleus and cytosol. J Cereal Sci 41:333–346
Holbrook NM, Ahrens ET, Burns MJ, Zwieniecki MA (2001) In vivo observation of cavitation and embolism repair using magnetic resonance imaging. Plant Physiol 126:27–31
Holbrook NM, Shashidhar VR, James RA, Munns R (2002) Stomatal control in tomato with ABA-deficient roots: response of grafted plants to soil drying. J Exp Bot 53:1503–1514
Ishida N, Koizumi M, Kano H (2000) The NMR microscope: a unique and promising tool for plant science. Ann Bot 86:259–278
Jia W, Wang Y, Zhang S, Zhang J (2002) Salt-stress-induced ABA accumulation is more sensitively triggered in roots than in shoots. J Exp Bot 53:2201–2206
Jones HG, Sharp CS, Higgis KH (1987) Growth and water relations of wilty mutants of tomato (Lycopersicon esculentum Mill.). J Exp Bot 38:1848–1856
Katerji N, Van Hoorn JW, Hamdy A, Mastrorilli M (2003) Salinity effect on crop development and yield, analysis of salt tolerance according to several classification methods. Agric Water Manage 62:37–66
Khadri M, Tejera NA, Lluch C (2006) Alleviation of salt stress in common bean (Phaseolus vulgaris) by exogenous abscisic acid supply. J Plant Growth Regul 25:110–119
Khadri M, Tejera NA, Lluch C (2007) Sodium chloride-ABA interaction in two common bean (Phaseolus vulgaris) cultivars differing in salinity tolerance. Environ Exp Bot 60:211–218
Köckenberger W, Pope JM, Xia Y, Jeffrey KR, Komor E, Callaghan PT (1997) A non-invasive measurement of phloem and xylem water flow in castor bean seedlings by nuclear magnetic resonance microimaging. Planta 201:53–63
Köckenberger W, De Panfilis C, Santoro D, Dahiya P, Rawsthorne S (2004) High resolution NMR microscopy of plants and fungi. J Microsc 214:182–189
Kof EM, Vinogradova IA, Oorzhak AS, Karyagin VV, Kalibernaya ZV, Macháčková I, Kondykov IV, Chuvasheva ES (2006) ABA content in shoots and roots of pea mutants af and tl as related to their growth and morphogenesis. Russ J Plant Physiol 53:359–365
Koornneef M, Jorna ML, Brinkhorst-Van der Swan DLC, Karssen CM (1982) The isolation of abscisic acid (ABA) deficient mutants by selection of induced revertants in non-germinating gibberellin sensitive lines of Arabidopsis thaliana (L.). Heynh Theor Appl Genet 61:385–393
Kuchenbrod E, Landeck M, Thürmer F, Haase A, Zimmermann U (1996) Measurement of water flow in the xylem vessels of intact maize plants using flow-sensitive NMR imaging. Bot Acta 109:184–186
Lee JM, Oda M (2003) Grafting of herbaceous vegetable and ornamental crops. Hort Rev 28:61–124
Liu CL, Chen HP, Liu EE, Peng XX, Lu SY, Guo ZF (2003) Multiple tolerance of rice to abiotic stresses and its relationship with ABA accumulation. Acta Agro Sin 29:725–729
Maas EV, Grattan SR (1999) Crop yields as affected by salinity. American Society of Agronomy, Crop Science Society of America. Soil Science Society of America, Madison. WI Agric Drain Agronomy Monogr 38
Mandal MP, Singh RA (2001) Impact of salt stress on chlorophyll content in rice genotypes. J Res Birsa Agric Univ 13:61–63
Matsumoto K, Tamura F, Chun JP, Tanabe K (2006) Native Mediterranean Pyrus rootstock, P. amygdaliformis and P. elaeagrifolia, present higher tolerance to salinity stress compared with Asian natives. J Jpn Soc Hortic Sci 75:450–457
Matthews MA, Boyer JS (1984) Acclimation of photosynthesis to low leaf water potentials. Plant Physiol 74:161–166
Messer E (2000) Potatoes (white). In: Kiple KF, Ornelas KC (eds), The Cambridge world history of food. Cambridge University Press, Cambridge, vol I, pp 187–201
Mulholland BJ, Taylor IB, Jackson AC, Thompson AJ (2003) Can ABA mediate responses of salinity stressed tomato. Environ Exp Bot 50:17–28
Neill SJ, Horgan R (1985) Abscisic acid production and water relations in wilty tomato mutants subjected to water deficiency. J Exp Bot 36:1222–1231
Ottow EA, Brinker M, Teichmann T, Fritz E, Kaiser W, Brosche M, Kangasjaervi J, Jiang X, Polle A (2005) Populus euphratica displays apoplastic sodium accumulation, osmotic adjustment by decreases in calcium and soluble carbohydrates, and develops leaf succulence under salt stress. Plant Physiol 139:1762–1772
Pandey SK, Singh SV, Sarkar D (2005) Potato (Solanum tuberosum) for sustaining food and nutrition security in developing world. Indian J Agric Sci 75:3–18
Pardo JM, Reddy MP, Yang S, Maggio A, Huh GH, Matsumoto T, Coca MA, Paino-D’Urzo M, Koiwa H, Yun DJ (1998) Stress signalling through Ca2+/calmodulin-dependent protein phosphatase calcineurin mediates salt adaptation in plants. Proc Natl Acad Sci USA 95:9681–9686
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Safety 60:324–349
Percival GC (2005) Identification of foliar salt tolerance of woody perennials using chlorophyll fluorescence. HortScience 40:1892–1897
Peuke AD, Rokitta M, Zimmermann U, Schreiber L, Haase A (2001) Simultaneous measurement of water flow velocity and solute transport in xylem and phloem of adult plants of Ricinus communis over a daily time course by nuclear magnetic resonance spectrometry. Plant Cell Environ 24:491–503
Pruvot G, Peltier G, Rey P (1996) Effects of low temperature, high salinity and exogenous ABA on the synthesis of two chloroplastic drought-induced proteins in Solanum tuberosum. Physiol Planta 97:123–131
Quarrie SA (1982) Droopy: a wilty mutant of potato deficient in abscisic acid. Plant Cell Environ 5:23–26
Ramoliya PJ, Pandey AN (2003) Effect of salinization of soil on emergence, growth and survival of seedlings of Cordia rothii. For Ecol Manage 176:185–194
Reddy MP, Sanish S, Iyengar ERR (1992) Photosynthetic studies and compartmentation of ions in different tissues of Salicornia brachiata Roxb. under saline conditions. Photosynthetica 26:173–179
Robichaud CS, Wong J, Sussex IM (1980) Control of in vitro growth of viviparous embryo mutants of maize by abscisic acid. Dev Genet 1:325–330
Romero L, Belakbir A, Ragala L, Ruiz JM (1997) Response of plant yield and leaf pigments to saline conditions: effectiveness of different rootstocks in melon plants (Cucumis melo L.). Soil Sci Plant Nutr 43:855–862
Ross ARS, Ambrose SJ, Cutler AJ, Feurtado JA, Kermode AR, Nelson K, Zhou R, Abrams SR (2004) Determination of endogenous and supplied deuterated abscisic acid in plant tissues by high-performance liquid chromatography-electrospray ionization tandem mass spectrometry with multiple reaction monitoring. Anal Biochem 329:324–333
Ruiz JM, Blasco B, Rivero RM, Romero L (2005) Nicotine-free and salt-tolerant tobacco plants obtained by grafting to salinity-resistant rootstocks of tomato. Physiol Plant 124:465–475
Sagi M, Fluhr R, Lips SH (1999) Aldehyde oxidase and xanthine dehydrogenase in a flacca tomato mutant with deficient abscisic acid and wilty phenotype. Plant Physiol 120:571–577
Santa-Cruz A, Martínez-Rodríguez MM, Bolarín MC, Cuartero J (2001) Response of plant yield and leaf ion contents to salinity in grafted tomato plants. Acta Hort 559:413–417
Santa-Cruz A, Martinez-Rodriguez MM, Perez-Alfocea F, Romero-Aranda R, Bolarin MC (2002) The rootstock effect on the tomato salinity response depends on the shoot genotype. Plant Sci 162:825–831
Scheenen T, Heemskerk A, de Jager A, Vergeldt F, Van As H (2002) Functional imaging of plants: a nuclear magnetic resonance study of a cucumber plant. Biophys J 82:481–492
Schmutz U, Lüdders P (1999) Effect of NaCl salinity on growth, leaf gas exchange, and mineral composition of grafted mango rootstocks (var. ‘13-1’ and ‘Turpentine’). Gartenbauwissenschaft 64:60–64
Schneider H, Manz B, Westhoff M, Mimietz S, Szimtenings M, Neuberger T, Faber C, Krohne G, Haase A, Volke F, Zimmermann U (2003) The impact of lipid distribution, composition and mobility on xylem water refilling of the resurrection plant Myrothamnus flabellifolia. New Phytol 159:487–505
Sharp RE, LeNoble ME (2002) ABA, ethylene and the control of shoot and root growth under water stress. J Exp Bot 53:33–37
Shaterian J, Georges F, Hussain A, Waterer D, De Jong H, Tanino KK (2005a) Root to shoot communication and abscisic acid in calreticulin (CR) gene expression and salt-stress tolerance in grafted diploid potato clones. Environ Exp Bot 53:323–332
Shaterian J, Waterer D, De Jong H, Tanino KK (2005b) Differential stress responses to NaCl salt application in early- and late-maturing diploid potato (Solanum sp.) clones. Environ Exp Bot 54:202–212
Sibole JV, Montero E, Cabot C, Poschenrieder C, Barceló J (1998) Role of sodium in the ABA-mediated long-term growth response of bean to salt stress. Physiol Plant 104:299–305
Silveira JAG, Cardoso BB, Melo ARB, Viégas RA (1999) Salt-induced decrease in nitrate uptake and assimilation in cowpea plants. Braz J Plant Physiol 11:77–82
Silveira JAG, Melo ARB, Viégas RA, Oliveira JTA (2001) Salinity-induced effects on nitrogen assimilation related to growth in cowpea plants. Environ Exp Bot 46:171–179
Simmonds NW (1965) Mutant expression in diploid potatoes. Heredity 20:65–72
Smith JD, McDaniel S, Lively S (1978) Regulation of embryo growth by abscisic acid in vitro. Maize Genet Coop Newsl 52:107–108
Steduto P, Albrizio R, Giorio P, Sorrentino G (2000) Gas-exchange response and stomatal and non-stomatal limitations to carbon assimilation of sunflower under salinity. Environ Exp Bot 44:243–255
Strognov BP (1964) Practical means for increasing salt tolerance of plants as related to type of salinity in the soil. In: Poljakoff-Mayber A, Meyer AM (eds), Physiological basis of salt tolerance of plants. Israel Program for Scientific Translations Ltd., Jerusalem, pp 218–244
Suzuki M, Settles MA, Tseung CW, Li QB, Latshaw S, Wu S, Porch TG, Schmelz EA, James MG, McCarty DR (2006) The maize viviparous15 locus encodes the molybdopterin synthase small subunit. Plant J 45:264–274
Tal M (1967) The grafting relations of three wilting tomato mutants: sitiens, flacca and notabilis. Rep Tomato Genet Coop 17:55–56
Tal M, Nevo Y (1973) Abnormal stomatal behaviour and root resistance, and hormonal imbalance in three wilty mutants of tomato. Biochem Genet 8:291–300
Teixeira J, Pereira S, Queirós F, Fidalgo F (2006) Specific roles of potato glutamine synthetase isoenzymes in callus tissue grown under salinity: molecular and biochemical responses. Plant Cell Tissue Organ Cult 87:1–7
Thomas DS, Eamus D (1999) The influence of predawn leaf water potential on stomatal responses to atmospheric water content at constant Ci and on stem hydraulic conductance and foliar ABA concentrations. J Exp Bot 50:243–251
Thompson AJ, Andrews J, Mulholland BJ, McKee JMT, Hilton HW, Horridge JS, Farquhar GD, Smeeton RC, Smillie IRA, Black CR, Taylor IB (2007) Overproduction of abscisic acid in tomato increases transpiration efficiency and root hydraulic conductivity and influences leaf expansion. Plant Physiol 143:1905–1917
Umezawa T, Shimizu K, Kato M, Ueda T (2000) Enhancement of salt tolerance in soybean with NaCl pretreatment. Physiol Plant 110:59–63
Umezawa T, Shimizu K, Kato M, Ueda T (2001) Effects of non-stomatal components on photosynthesis in soybean under salt stress. Jpn J Trop Agric 45:57–63
Umezawa T, Okamoto M, Kushiro T, Nambara E, Oono Y, Seki M, Kobayashi M, Koshiba T, Kamiya Y, Shinozaki K (2006) CYP707A3, a major ABA 8’-hydroxylase involved in dehydration and rehydration response in Arabidopsis thaliana. Plant J 46:171–182
Velagaleti RR, Marsh S, Kramer D, Fleischman D, Corbin J (1990) Genotypic differences in growth and nitrogen fixation among soybean (Glycine max (L.) Merr.) cultivars grown under salt stress. Trop Agric 67:169–177
Velikanov GA, Belova LP (2005) Regulation of water permeability of vacuolar symplast. Russian J Plant Physiol 52:758–764
Verslues PE, Guo Y, Dong CH, Ma W, Zhang JK (2006) Mutation of SAD2, an importin β-domain protein in Arabidopsis, alters abscisic acid sensitivity. Plant J 47:776–787
Wahome PK, Jesch HH, Grittner I (2000) Effect of NaCl on the vegetative growth and flower quality of roses. J Appl Bot Angew Bot 74:38–41
Wahome PK, Jesch HH, Grittner I (2001) Mechanisms of salt stress tolerance in two rose rootstocks: Rosa chinensis ‘Major’ and R. rubiginosa. Sci Hortic 87:207–216
Waisel Y (2001) Salinity: a major enemy of sustainable agriculture. In: Breckle SW, Maik V, Walter W (eds), Sustainable land use in deserts. Springer-Verlag, New York, pp 166–173
Wang TL, Donkin ME, Martin ES (1984) The physiology of a wilty pea: abscisic acid production under water stress. J Exp Bot 35:1222–1232
Wilkinson S, Davies WJ (1997) Xylem sap pH increase: a drought signal received at the apoplastic face of the guard cell that involves the suppression of saturable abscisic acid uptake by the epidermal symplast. Plant Physiol 113:559–573
Zhu C, Schraut D, Hartung W, Schäffner AR (2005) Differential responses of maize MIP genes to salt stress and ABA. J Exp Bot 56:2971–2981
Acknowledgments
This study was funded by the Iranian Agricultural Research Organization and Natural Resources and University of Saskatchewan. ABA content was assessed in cooperation with Dr. Sue Abrams and NRC-PBI. The greenhouse assistance by Tom Ward and John Peters and secretarial help by Mary Lee, Sharon Steven, Carolyn Quellet were appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Etehadnia, M., Waterer, D., De Jong, H. et al. Scion and Rootstock Effects on ABA-mediated Plant Growth Regulation and Salt Tolerance of Acclimated and Unacclimated Potato Genotypes. J Plant Growth Regul 27, 125–140 (2008). https://doi.org/10.1007/s00344-008-9039-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-008-9039-6