Abstract
Biological control and induced resistance are two of the promising approaches to the control of postharvest diseases. This study was conducted to evaluate the efficacy of salicylic acid (SA) alone or in combination with an antagonistic yeast, Cryptococcus laurentii, in controlling the blue mold disease caused by Penicillium expansum on apple fruit wounds. SA alone significantly inhibited the spore germination of P. expansum in vitro when its concentration was increased to 1000 μg ml−1, but it was not effective in controlling the disease in vivo. Simultaneous application of SA and C. laurentii to the wounds on the apple fruit surface showed that SA could improve the efficacy of C. laurentii against P. expansum in a concentration-dependent manner, being most effective at 10 μg ml−1 but less effective at a higher or lower concentrations. Besides reducing the blue mold incidence in the local wound sites, the combination of C. laurentii with SA at 10 μg ml−1 also had a synergistic effect on the induction of fruit resistance to the disease, which might be associated with a rapid increase in peroxidase, phenylalanineamonialyase and lipoxygenase activities. In addition, SA at 100 μg ml−1 or above showed an adverse effect on the growth of C. laurentii in vitro and in vivo, whereas it had no effect when its concentration was decreased to 10 μg ml−1 or lower. This suggested that SA could enhance the biological activity of C. laurentii in apple fruit by inducing resistance to pathogens based on the antagonistic activity of C. laurentii.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Blue mold, caused by Penicillium expansum Link, is a major postharvest disease of apple fruits. The most effective control strategy is the postharvest treatment of apples with fungicides. However, fungicide toxicity, along with the development of fungicide resistance by pathogens, has caused the public considerable concern requiring that alternatives to synthesized fungicides be developed.
Recently, biological control with antagonistic yeasts, especially for controlling wound-invading pathogens, has emerged as a promising method of controlling the postharvest decay of fruits and vegetables. But to be an economically feasible alternative, the biocontrol yeasts must be further developed to enhance their performance in controlling postharvest diseases (Janisiewicz and Korsten 2002; Droby and others 2003).
At the same time, considerable attention has also been placed on the induction of resistance as an important manageable form of plant protection and control of postharvest diseases of fruits and vegetables (Wilson and others 1994; Kuć 2001; Kogel and Langen 2005). Several selected postharvest biocontrol yeasts have been shown to be capable of inducing resistance in harvested fruits (Droby and others 2002; El Ghaouth and others 2003). Furthermore, it has been reported that several physical- and chemical-based elicitors such as ultraviolet light, chitosan, harpin, methyl jasmonate, and salicylic acid (SA) can augment the biocontrol activities of selected biocontrol yeasts (de Capdeville and others 2002; Qin and others 2003; Yao and Tian 2005).
The involvement of SA as a signal molecule in local defenses and in systemic acquired resistance (SAR) has been extensively studied. Exogenous application of SA can also activate resistance in plants without pathogen-inoculation (Durrant and Dong 2004). In postharvest systems, previous data showed that SA had multifunctional effectiveness, including direct antimicrobial activity in various fruits (Terry and Joyce 2004), suppression of banana fruit ripening (Srivastava and Dwivedi 2000), and induction of disease resistance in sweet cherry fruit (Qin and others 2003).
In this study, we used SA at various concentrations alone or in combination with a biocontrol yeast Cryptococcus laurentii to control the postharvest blue mold of apple fruit and investigated its possible modes of action through the measurements of SA on C. laurentii, P. expansum, and fruit in vivo and in vitro.
MATERIALS AND METHODS
Microorganisms and Fruit Material
Fuji apple fruits (Malus domestica Borkh.) were hand-picked at harvest maturity. The fruit samples were sorted to remove any with apparent injuries or infections and then stored at 4°C under high humidity before being used in biocontrol experiments. After they were immersed in a solution of 0.1% sodium hypochlorite (actual concentration of available chlorine ≥ 52 μg ml−1) for 1 min, the samples were washed with inflowing fresh water for 10 min and then were allowed to air dry at room temperature (22°C). The yeast antagonist C. laurentii (Kufferath) Skinner was isolated from the surfaces of the apple fruits and identified by the VITEK 32 Automicrobic System (bioMerieux Company) and maintained at 4°C on nutrient yeast dextrose agar (NYDA) medium (containing 8 g nutrient broth, 5 g yeast extract, 10 g glucose, and 20 g agar in 1 l of distilled water). The yeast was grown with the liquid cultures in 250-ml Erlenmeyer flasks containing 50 ml of nutrient yeast dextrose broth (NYDB). After inoculation with a loop of the culture, the flasks were incubated on a rotary shaker at 28°C for 24 hours. After incubation, the cells were centrifuged at 3000 × g for 10 min and were washed twice with a solution of 50 ml of sterile distilled water to remove the growth medium. The cell pellets were re-suspended in sterile distilled water and were counted to 1 × 108 cells per ml by means of a hemocytometer. The pathogen P. expansum was isolated from an apple fruit infected by the typical blue mold and cultured on potato-dextrose agar (PDA) medium. After the spores were removed from a 1-week-old culture with a bacteriological loop, they were suspended in sterile distilled water. Spore concentration was determined with a hemocytometer.
Efficacy of C. laurentii and SA on Control of Blue Mold in Fruit Wounds
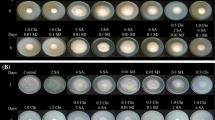
The apples were wounded (5-mm-diameter by 3-mm-deep wound) on the equator using the tip of a sterile dissecting needle and then treated with 30 μl of one of the following: (1) cell suspensions of C. laurentii at 108 cells ml−1; (2) 1, 10, 100, or 1000 μg ml−1 of SA; (3) cell suspensions of C. laurentii at 108 cells ml−1 containing 1, 10, 100, or 1000 μg ml−1of SA, (4) sterile distilled water as a control. Two hours later, 20 μl of 1 × 105 spores/ml suspension of P. expansum were challenge-inoculated onto each wound. After air drying, the apples were stored in enclosed plastic trays at 22°C with a relative humidity of about 95%. The number of infected fruits and their lesion diameters were recorded after 5 days of inoculation. There were four replicates of 24 apples per replicate (four wounds per fruit, total of 96 wounds per replicate), and the experiment described above was conducted twice.
Effect of SA on P. expansum Spore Germination In Vitro
The effect of SA on the spore germination of P. expansum was assessed in potato dextrose broth (PDB). Aliquots (100 μl) of the spore suspension (5 × 106 spores per ml) of the pathogen were transferred to a 10 ml glass tube containing 2 ml PDB. Treatments were evaluated at the concentrations of 1, 10, 100, and 1000 μg ml−1 plus sterile distilled water as a control. After filter sterilizing, the SA solutions were added to give the final concentrations. In each replicate 150–200 spores were observed microscopically and their germination was evaluated following 12 h of incubation at 28°C on a rotary shaker (200 rpm). The treatments were replicated three times and the experiment described above was conducted twice.
Effects of SA on Populations of C. laurentii In Vitro
The 25 ml-Erlenmeyer flasks containing 5 ml of NYDB were prepared and the filter-sterilized SA solutions were added at the selected concentrations (0, 1, 10, 100, and 1000 μg ml−1). The shake-flask cultures were started with 1 × 104 cells per ml of C. laurentii and incubated on a rotary shaker (200 rpm) at 28°C. The samples were collected after 24 h, and the cell suspension was counted using a hemacytometer. The treatments were replicated three times and the experiment described above was conducted twice.
Effects of SA on Population Growth of Antagonists in Wounds
The fruit samples were treated and wounded as described above. Then, the wounds were treated with 30 μl of a cell suspension of C. laurentii at 108 cells ml−1 containing 0, 1, 10, 100, or 1000 μg ml−1 of SA. The samples were taken at different times (0, 12, 24, 48, 72, and 96 h at 22°C) after the treatment. The tissue was removed with a cork borer (1 cm diameter by 1 cm deep) and ground with a mortar and pestle in 10 ml of sterile distilled water. The cells was counted using a haemacytometer. There were three replicates per treatment, and the experiment described above was conducted twice.
Effects of C. laurentii and SA on Induced Disease Resistance
Evaluation of induced disease resistance by C. laurentii and SA was performed according to the method of Droby and others (2002). The samples were gently wounded (5 mm diameter by 3 mm deep) on the equator using the tip of a sterile dissecting needle, after which a 30-μl of C. laurentii at 108 cells per ml alone, or SA at 10 μg ml alone, or the combination was piped into each wound. Wounds treated with the same amount of sterile distilled sterilized water served as a control. After 0, 24, 48, 72, and 96 h at 22°C, four fresh wounds were made at a point 5 mm from the edge of the site pre-treated with SA and C. laurentii (+5 mm); these wounds were inoculated with 20 μl of a spore suspension of P. expansum (1 × 105 spores per ml) to make sure that the pre-treatment agents and the pathogen were injected into spatially separated wounds. The fruit samples were then incubated in enclosed plastic trays at 22°C under humid conditions (approximately 95%). For each treatment at each inoculation time, the number of the infected wounds was recorded every 4 days after inoculation with the pathogen. Each treatment consisted of three replications of 12 fruit samples per treatment (four wounds per fruit, total of 48 wounds per replicate and the experiment described above was conducted twice.
Effects of C. laurentii and SA on Defense-related Enzyme Activities
The fruit samples were treated and wounded as described above. The wounds were then treated with 30 μl of a solution of cell suspension of C. laurentii at 108 cells ml−1 alone, SA at 10 μg ml−1 alone, C. laurentii at 108 cells ml−1 in combination with SA at 10 μg ml−1, and sterile distilled water as a control. The enzymes were extracted at 4°C from one gram of fresh tissue from the wound site and the +5-mm site with 10 ml of cold (4°C) 50 mmol l−1 sodium phosphate buffer (PH 7.8) containing 1.33 mmol l−1 EDTA and 1% polyvinyl-polypyrrolidone (PVPP) in a mortar and pestle at different times (0, 24, 48, 72, and 96 h at 22°C) after the treatments. The homogenates were centrifuged at 4°C for 15 min at 27,000 × g, and the supernatant was used for assay of the enzyme activities and protein content. There were three replicates per treatment and six fruit samples per replicate; the experiment described above was conducted twice.
Following the method described by Lurie and others (1997) with some modifications, peroxidase (POD) activity was measured using guaiacol as substrate. The reaction mixtures contained 3 ml of 50 m mol/l sodium phosphate buffer (pH 6.4), 220 μl of 0.3% guaiacol, 60 μl of 0.3% H2O2, and 20 μl of crude enzyme extract. The reaction was initiated immediately by adding H2O2 at 30°C incubated in a water bath. The reaction was allowed to proceed for 5 min, and measurements of A470 were taken once every 30 seconds beginning 1 min after addition of H2O2 to the substrate. A cuvette containing all components except H2O2 was used as control. One unit of POD activity is defined as the amount of enzyme extract producing an increase of A470 by 0.01 in 1 min, and the activity is expressed as U mg−1 protein.
Phenylalanine amonialyase (PAL) activity was measured according to the method of Camm and Towers (1973) with some modifications. The reaction mixtures contained 1 ml supernatant with 4 ml 0.1 mol.l−1 borate buffer (pH 8.8) and 10 mmol.l−1 L-phenylalanine. After incubation of the mixtures at 30°C for 30 min, the reaction was terminated by the addition of 0.1 ml of 5N HCl. The increase in absorbance at 290 nm, due to the formation of trans-cinnamate, was measured spectrophotometrically. One unit of PAL activity is defined as the amount of enzyme extract producing an increase of A290 by 0.01 in 1 h; the activity is expressed as U mg−1 protein.
Lipoxygenase (LOX) activity was measured according to the method described by Todd and others (1990) with some modifications. The reaction mixture contained 200 μl Tween 20 and 40 μl of linoleic acid in 4.0 ml of 0.1 M phosphate, pH 7.0. Lipoxygenase activity was measured spectrophotometrically by monitoring the change in absorbance at 234 nm over a 5-min period taken once every 30 seconds. One unit of LOX activity is defined as the amount of the enzyme that causes an increase in absorption by 0.01 min−1 at 25°C, and the activity is expressed as U mg−1 protein.
The protein content was determined according to the method of Bradford (1976) using bovine serum albumin (Sigma-Aldrich) as a standard.
Statistical Analyses
The data were analyzed by analysis of variance (ANOVA) in the Statistical Program SPSS/PC ver. II.x. Statistical significance was applied at the level p < 0.05. When the analysis was statistically significant, Duncan’s multiple range test was applied to the separate means.
RESULTS
Effect of SA and C. laurentii on the Control of Blue Mold
As shown in Table 1, treatment with SA alone at all the selected concentrations wasn’t effective in reducing blue mold incidence and severity in vivo. However, the addition of SA at the concentration of 10 μg ml−1 or lower to a cell suspension of C. laurentii produced a significant increase in the activity of C. laurentii against P. expansum in apple fruit (p < 0.05). At the optimal concentration (10 μg ml−1), the percentage of infected fruits in the combined treatment was reduced significantly from 41.6 ± 7.6% to 12.9 ± 4.9%, as compared with that of C. laurentii alone. In addition, the efficacy of the combined treatment was weakened as the concentration of SA was decreased to 1 μg ml−1 (p < 0.05). What is more, when combined with SA at 1000 μg ml−1, the efficacy of C. laurentii was entirely lost, which was similar to that of SA applied alone (p > 0.05). As for the combination of C. laurentii with SA at 100 μg ml−1, the decay incidence was also much higher than that of C. laurentii alone (p < 0.05). But it should be noted that there was no significant difference of lesion diameters in the treatments of C. laurentii with SA at the concentration range from 0 to 100 μg ml−1 (p > 0.05).
Effect of SA on Spore Germination of P. expansum in vitro
The results (Figure 1) showed that at the concentrations of 1000 μg ml−1, a marked inhibition in the percentage of spore germinations was observed in PDB with SA (p < 0.05). However, when the concentration was decreased to 100 μg ml−1 or lower, there was just a slight inhibition. And there was little difference in spore germination of P. expansum in the treatment with SA from 0 to 10 μg ml−1 (p > 0.05).
Spore germinations of Penicillium expansum in potato-dextrose broth (PDB) containing salicylic acid (SA) at the selected concentrations (1000, 100, 10, 1, 0 μg ml−1). Standard deviations of three replications are given as the short bars. Different letters indicate significant differences (p < 0.05) according to Duncan’s multiple range test.
Effect of SA on C. laurentii Growth In Vitro and In Vivo
In NYDB (Figure 2), the growth of C. laurentii was not influenced by SA at the lower concentrations used (p > 0.05). After 24 h of incubation, the populations of C. laurentii were all increased nearly 104-fold in the presence of SA from 0 to 10 μg ml−1. When increased up to 100 μg ml−1 or higher, SA had an obvious negative effect on the growth of C. laurentii (p < 0.05). At the highest concentration of 1000 μg ml−1, SA nearly completely inhibited the growth of C. laurentii.
Populations of Cryptococcus laurentii in nutrient yeast dextrose broth (NYDB) containing salicylic acid (SA) at the selected concentrations (1000, 100, 10, 1, 0 μg ml−1). Standard deviations of three replications are given as the short bars. Different letters indicate significant differences (p < 0.05) according to Duncan’s multiple range test.
As shown in Figure 3, the ability of C. laurentii to grow in apple wounds in the presence of SA was similar to the results observed in vitro.
Population growths of Cryptococcus laurentii on apple wounds in presence of salicylic acid (SA) at the selected concentrations (1000, 100, 10, 1, 0 μg ml−1). Standard deviations of three replications are given as the short bars. An asterisk indicates that the difference of the population in the treatments is significant (p < 0.05) according to Duncan’s multiple range test.
Effect of SA and C. laurentii on the Induction of Resistance
The results (Figure 4) showed that all treatments, when applied either immediately or 24 h before challenge by P. expansum, had no significant effect on reduction of the decay incidence (p > 0.05). However, when applied 72 or 96 h prior to inoculation with P. expansum, the combined treatment with SA (10 μg ml−1) and C. laurentii effectively reduced the decay incidence in comparison with the treatment with SA and C. laurentii alone or the control (p < 0.05). By 96 h, the blue mold incidence in the combined treatment was 38%, 57 %, or 50 % of that in the control, C. laurentii, or SA alone, respectively.
Effects of salicylic acid (SA) and Cryptococcus laurentii on inducing resistance to blue mold on apple wounds. Standard deviations of three replications are given as the short bars. An asterisk indicates that the difference of the decay incidence between the co-treatment with SA and C. laurentii and any other treatment is significant (p < 0.05) according to Duncan’s multiple range test.
Effects of SA and C. laurentii on POD Activity
At the local wound site (Figure 5A), the combination of C. laurentii with SA induced an approximately eightfold increase in POD activity within 24 h of inoculation (p < 0.05) and then dropped by 48 h followed by a notable increase again (p > 0.05). However, in the tissue treated with SA alone, an induction of POD activity was also observed after 72 h of inoculation (p < 0.05). In the control or C. laurentii-treatment alone, a relatively small increase in POD activity with time was detected. As shown in Figure 5B, the treatment with SA alone also resulted in an induction of POD activity, which was increased approximately 4.6-fold within 24 h of inoculation at the +5 mm site (p < 0.05), although this was still lower than that of the combined treatment with C. laurentii (p < 0.05). However, in the tissue treated with C. laurentii alone, an induction of POD activity was observed by 72 h of inoculation (p < 0.05).
Effects of salicylic acid (SA) and Cryptococcus laurentii on the peroxidase activities in apple wounds at the local wound site (A) and at + 5 mm from the edge of the wound site (B). Standard deviations of three replications are given as the short bars. An asterisk indicates that the difference between the co-treatment with SA and C. laurentii and any other treatment is significant (p < 0.05) according to Duncan’s multiple range test.
Effects of SA and C. laurentii on PAL activity
At the local wound site (Figure 6A), the combined treatment induced a peak of PAL activity within 24 h of inoculation and was fivefold higher than that measured in the control (p < 0.05). However, no significant increase was induced by the treatment with SA or C. laurentii alone (p > 0.05). At the +5 mm site (Figure 6B), the treatment with SA or C. laurentii alone, or the combination stimulated an increase in PAL activity of approximately 3.0-, 2.2-, and 5.2-fold higher than that of the control within 24 h of inoculation, respectively (p < 0.05).
Effects of salicylic acid (SA) and Cryptococcus laurentii on the phenylalanine amonialyase activities in apple wounds at the local wound site (A) and at +5 mm from the edge of the wound site (B). Standard deviations of three replications are given as the short bars. An asterisk indicates that the difference between the co-treatment with SA and C. laurentii and any other treatment is significant (p < 0.05) according to Duncan’s multiple range test.
Effects of SA and C. laurentii on LOX Activity
At the local wound site (Figure 7A), all treatments increased LOX activities within 24 h of inoculation, but no significant differences in them were observed within 72 h of inoculation (p > 0.05). In the treatment with SA alone or in combination with C. laurentii, LOX activity increased approximately 3-fold and 3.2-fold, respectively, compared to that observed in the control after 72 h (p < 0.05). At the +5-mm site (Figure 7B), LOX activity in the combined treated tissue showed a rapid and strong increase of approximately 9.6-fold within 24 h of inoculation (p < 0.05). However, in the tissues treated with yeast or SA as well as water alone, a moderate induction was observed only after 48 h of inoculation (p < 0.05).
Effect of salicylic acid (SA) and Cryptococcus laurentii on the lipoxygenase activities in apple wounds at the local wound site (A) and at +5 mm from the edge of the wound site (B). Standard deviations of three replications are given as the short bars. An asterisk indicates that the difference between the co-treatment with SA and C. laurentii and any other treatment is significant (p < 0.05) according to Duncan’s multiple range test.
DISCUSSION
In this study, spore germination of P. expansum in PDB was significantly influenced by SA when its concentration was increased up to 100 μg ml−1 or higher (Figure 1), but blue mold incidence and lesion diameter caused by P. expansum were not influenced by SA at concentrations from 0 to 1000 μg ml−1 in vivo (Table 1). This rather different effect in vivo and in vitro was similar to that of 2-deoxy-D-glucose reported by El-Ghaouth and others (2000a). This is not surprising because results from in vitro studies do not always accurately represent the efficacy of that in the in vivo situation (Rotem and other 1978).
The antagonistic yeasts C. laurentii and P. expansum have been selected mainly for their ability to rapidly colonize and grow in the surface wounds, and subsequently to compete with the pathogen for nutrients and space, which is believed to be a major component of the mode of action of C. laurentii (Janisiewicz and Korsten 2002; Zhang and others 2005). In the present study, the efficacy of C. laurentii was reduced markedly (Table 1) when its growth was inhibited significantly in the presence of SA at 100 μg ml−1 or above (Figure 2, Figure 3), which was similar to the result from chitosan chloride reported by El-Ghaouth and others (2000b).
SA alone at the relatively low concentration of 10 μg ml−1 did not affect the populations of C. laurentii in vitro (Figure 2) or in vivo (Figure 3), or the spore germination of P. expansum in vitro (Figure 1). Interestingly, the combination of SA at this concentration (10 μg ml−1) with C. laurentii significantly enhanced control of blue mold disease on apple fruit wounds, as compared with C. laurentii alone (Table 1). Moreover, this combined treatment produced a useful result in reducing the blue mold incidence when applied 72 h or more prior to the inoculation with P. expansum by inducing resistance in the fruit rather than by directly affecting the pathogen (Figure 4), because no direct interactions could occur when the pretreatment agents and the pathogen were inoculated into the spatially separated wounds (de Capdeville and others 2002; Droby and others 2002; El Ghaouth and others 2003).
The effectiveness of the combined treatment lay in reducing the disease incidence rather than the lesion diameters (Table 1), which was in agreement with the effect of other biologically based elicitors reported by Fajardo and others (1998). It indicated that the induced resistance could be effective, particularly during the early phase of disease progression, and that once the fruit samples were visibly infected by the pathogen, the elicitors did not reduce the fungal colonization or the severity of symptoms (Fajardo and others1998).
Additionally, the results in Table 1 also showed that the SA improved the biocontrol activity of C. laurentii most effectively at a concentration of 10 μg ml−1 but less at lower concentrations (1 μg ml−1), findings in agreement with the general phenomenon that plant growth regulators are most effective when applied at optimal concentration.
The induction of disease resistance is a sensitization process that primes the plant for more rapid deployment of defenses, which has been shown to coincide with the activation of a biochemical defense system for disease resistance in plants, such as PAL, POD, and LOX (Kuć 2001; Conrath and others 2002). Our results showed that the combined treatment provided a rapid and strong induction of these defense-related reactions (Figure 5, 6, and 7). It may facilitate formation of appropriate defense-related reactions in the fruit to the potential attacking pathogen, because the success in the defense response depends on the speed at which the plant recognizes the attacking pathogen and the intensity with which the appropriate defense mechanism is activated (Conrath and others 2002; Ton and others 2005). However, the mechanism for the different biochemistry reactions at different sites remains to be elucidated.
In conclusion, the results presented in this study showed that the biological efficacy of C. laurentii against P. expansum on apple fruit could be significantly enhanced by adding SA, which was most effective at 10 μg ml−1. The mechanism of action might be related to induced resistance to pathogens based on the antagonistic activity of C. laurentii. These results offer a potential for minimizing apple fruit decay in an integrated pest management strategy.
References
Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Ana1 Biochem 72:248–254
Camm EC, Towers GHN. 1973. Phenylalanineammonialyase. Phytochemistry 12:961–973
Conrath U, Pieterse CMJ, Mauch-Mani B. 2002. Priming in plant–pathogen interactions. Trends Plant Sci 7:210–216
de Capdeville G, Beer SV, Wilson CL, Aist JR. 2002. Alternative disease control agents induce resistance to blue mold in harvested ‘Red Delicious’ apple fruit. Phytopathology 92:900–908
Droby S, Vinokur V, Weiss B, Cohen L, Daus A, and others. 2002. Induction of resistance to Penicillium digitatum in grapefruit by the yeast biocontrol agent Candida oleophila. Phytopathology 92:393–399
Droby S, Wsiniewski M, Ei-Ghaouth A, Wilson C. 2003. Biological control of postharvest diseases of fruits and vegetables: current achievements and future challenges. Acta Hort 628:703–713
Durrant WE, Dong X. 2004. Systemic acquired resistance. Annu Rev Phytopathol 42:185–209
El-Ghaouth A, Smilanick JL, Wisniewski M, Wilson CL. 2000a. Improved control of apple and citrus fruit decay with a combination of Candida saitoana and 2-deoxy-D-glucose. Plant Dis 84:249–253
El-Ghaouth A, Smilanick JL, Wilson CL. 2000b. Enhancement of the performance of Candida saitoana by the addition of glycolchitosan for the control of postharvest decay of apple and citrus fruit. Postharvest Biol Technol 19:103–110
El Ghaouth A, Wilson CL, Wisniewski M. 2003. Control of postharvest decay of apple fruit with Candida saitoana and induction of defense responses. Phytopathology 93:344–348
Fajardo JE, McCollum TG, McDonald RE, Mayer RT. 1998. Differential induction of proteins in orange Flavedo by biologically based elicitors and challenged by Penicillium digitatum Sacc. Biol Control 13:143–151
Janisiewicz WJ, Korsten L. 2002. Biological control of postharvest diseases of fruits. Annu Rev Phytopathol 40:411–441
Kogel K, Gregor L. 2005. Induced disease resistance and gene expression in cereals. Cell Microbiol 7:1555–1564
Kuć J. 2001. Concepts and direction of induced systemic resistance in plants and its application. Eur J Plant Pathol 107:7–12
Lurie S, Fallik E, Handros A, Shapira R. 1997. The possible involvement of peroxidase in resistance to Botrytis cinerea in heat treated tomato fruit. Physiol Mol Plant Pathol 50:141–149
Qin GZ, Tian SP, Xu Y, Wan YK. 2003. Enhancement of biocontrol efficacy of antagonistic yeasts by salicylic acid in sweet cherry fruit. Physiol Mol Plant Pathol 62:147–154
Rotem J, Cohen Y, Bashi E. 1978. Host and environmental influences on sporulation in vivo. Annu Rev Phytopathol 16:83–101
Srivastava MK, Dwivedi UN. 2000. Delayed ripening of banana fruit by salicylic acid. Plant Sci 158:87–96
Terry L A, Joyce DC. 2004. Elicitors of induced disease resistance in postharvest horticultural crops: a brief review. Postharvest Biol Technol 32:1–13
Todd TF, Paliyath G, Thompson JE. 1990. Characteristics of a membrane associated lipoxygenase in tomato fruit. Plant Physiol 94:1225–1232
Ton J, Jakab G, Toquin V, Flors V, Iavicoli A, and others. 2005. Dissecting the β-aminobutyric acid–induced priming phenomenon in Arabidopsis. Plant Cell 17:987–999
Wilson CL, Ei-Ghaouth A, Chalutz E, Droby S, Stevens C, and others. 1994. Potential of induced resistance to control postharvest diseases of fruits and vegetables. Plant Dis 78:837–844
Yao HJ, Tian SP. 2005. Effects of a biocontrol agent and methyl jasmonate on postharvest diseases of peach fruit and the possible mechanisms involved. J Appl Microbiol 98:941–950
Zhang HY, Zheng XD, Fu CX, Xi YF. 2005. Postharvest biological control of gray mold rot of pear with Cryptococcus laurentii. Postharvest Biol Technol 35:79–86
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China (NNSFC- 30571301) and the Ph.D. Programs Foundation of Ministry of Education of China PPFMEC-20040335025).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Yu, T., Zheng, X.D. Salicylic Acid Enhances Biocontrol Efficacy of the Antagonist Cryptococcus laurentii in Apple Fruit. J Plant Growth Regul 25, 166–174 (2006). https://doi.org/10.1007/s00344-005-0077-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-005-0077-z