Abstract
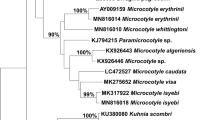
Shell-boring species Polydora brevipalpa Zachs, 1933 is redescribed based on morphological observations and molecular approach for future unambiguous identification. Genetic distance analyses showed that the interspecific polydorid variation (16.7%–25.6%) was at least 15 times higher than the intraspecific one (0.2%–0.9%) based on the cytochrome c oxidase subunit I (CO1) gene sequences of polydorids. However, 18S rDNA variation pattern demonstrated a rather narrow barcoding gap, with the interspecific polydorid variation (0.5%–5.6%) being very close to the intraspecific one (0.0%–0.4%). As such, the CO1 gene exhibited better DNA barcode for identification of polydorids than the 18S rDNA gene because of the sufficiently large barcoding gaps. Analysis of molecular variance results based on CO1 gene sequences showed that most variations in sequences (97.79%) lay within groups of adult worms and egg capsules rather than between them. This indicated that egg capsules from Crassostrea gigas (Thunberg, 1793) in Ningbo and Nantong were related to the adult worms from Patinopecten yessoensis (Jay, 1857) in Dalian, and both of them belonged to P. brevipalpa. This result was further supported by parsimony network analysis, which showed that egg capsules collected from different localities and adult worms shared a single haplotype. This study was the first to report both P. brevipalpa infestation on C. gigas and to utilise the known CO1 sequences of the adult polydorids to validate morphologically unidentified egg capsules or early larvae. P. brevipalpa was most possibly brought to Chinese waters through transportation of Pa. yessoensis brood stock from Japan.
Similar content being viewed by others
References
Bandelt H J, Forster P, Rohl A. 1999. Median–joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution, 16 (1): 37–48.
Bely A E, Wray G A. 2004. Molecular phylogeny of naidid worms (Annelida: Clitellata) based on cytochrome oxidase I. Molecular Phylogenetics and Evolution, 30 (1): 50–63.
Blake J A, Kudenov J D. 1978. The Spionidae (Polychaeta) from southeastern Australia and adjacent areas with a revision of the genera. Memoirs of the National Museum of Victoria, 39: 171–280.
Blake J A. 1996. Family Spionidae Grube, 1850. Including a review of the genera and species from California and a revision of the genus Polydora Bosc, 1802. In: Blake J A, Hilbig B, Scott P H eds. Taxonomic Atlas of the Benthic Fauna of the Santa Maria Basin and Western Santa Barbara Channel. Volume 6. The Annelida Part 3–Polychaeta: Orbiniidae to Cossuridae. Santa Barbara Museum of Natural History, Santa Barbara, California. p.81–223.
Blank M, Bastrop R. 2009. Phylogeny of the mud worm genus Marenzelleria (Polychaeta, Spionidae) inferred from mitochondrial DNA sequences. Zoologica Scripta, 38 (3): 313–321.
Bucklin A, Steinke D, Blanco–Bercial L. 2011. DNA barcoding of marine metazoa. Annual Review of Marine Science, 3 (1): 471–508.
David A A, Williams J D. 2012. Morphology and natural history of the cryptogenic sponge associate Polydora colonia Moore, 1907 (Polychaeta: Spionidae). Journal of Natural History, 46 (23–24): 1 509–1 528.
Erséus C, Kvist S. 2007. COI variation in Scandinavian marine species of Tub i fic oides (Annelida: Clitellata: Tubificidae). Journal of the Marine Biological Association of the United Kingdom, 87 (5): 1 121–1 126.
Excoffier L, Lischer H E L. 2010. Arlequin Suite ver 3.5, a new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources, 10: 564–567.
Gao Y, Zhang T, Yang H S, Zhang X F. 2011. The study on morphological and anatomic observation of Polydora ciliat e. Marine Sciences, 35 (10): 103–109. (in Chinese with English abstract)
Hall T A. 1999. BioEdit: a user–friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41: 95–98.
Hebert P D N, Cywinska A, Ball S L, de Waard J R. 2003. Biological identifications through DNA barcodes. Proceedings of the Royal Society B: Biological Sciences, 270 (1512): 313–321.
Hebert P D N, Stoeckle M Y, Zemlak S T, Francis C M. 2004. Identification of birds through DNA barcodes. PLoS Biology, 2 (10): e312.
Li Q, Xu K F, Yu R H. 2007. Genetic variation in Chinese hatchery populations of the Japanese scallop ( Patinopecten yessoensis ) inferred from microsatellite data. Aquaculture, 269 (1–4): 211–219.
Librado P, Rozas J. 2009. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics, 25 (11): 1 451–1 452.
Luttikhuizen P C, Dekker R. 2010. Pseudo–cryptic species Arenicola defodiens and Arenicola marina (Polychaeta: Arenicolidae) in Wadden Sea, North Sea and Skagerrak: morphological and molecular variation. Journal of Sea Research, 63 (1): 17–23.
Medlin L, Elwood H J, Stickel S, Sogin M L. 1988. The characterization of enzymatically amplified eukaryotic 16S–like rRNA–coding regions. Gene, 71 (2): 491–499.
Mori K, Sato W, Nomura T, Imajima M. 1985. Infestation of the Japanese scallop Patinopecten yessoensis by the boring polychaetes, Polydora, on the Okhotsk Sea coast of Hokkaido, especially in Abashiri waters. Bulletin of Japanese Society of Science of Fishery, 51 (3): 371–380.
Norlinder E, Nygren A, Wiklund H, Pleijel F. 2012. Phylogeny of scale–worms (Aphroditiformia, Annelida), assessed from 18SrRNA, 28SrRNA, 16SrRNA, mitochondrial cytochrome c oxidase subunit I (COI), and morphology. Molecular Phylogenetics and Evolution, 65 (2): 490–500.
Nygren A, Eklöf J, Pleijel F. 2010. Cryptic species of Notophyllum (Polychaeta: Phyllodocidae) in Scandinavian waters. Organisms Diversity and Evolution, 10 (3): 193–204.
Nygren A, Pleijel F. 2011. From one to ten in a single stroke–resolving the European Eumida sanguinea (Phyllodocidae, Annelida) species complex. Molecular Phylogenetics a nd Evolution, 58 (1): 132–141.
Pérez–Losada M, Breinholt J W, Aira M, Domínguez J. 2015. An updated multilocus phylogeny of the Lumbricidae (Annelida: Clitellata: Oligochaeta) earthworms. Journal of Phylogenetics and Evolutionary Biology, 3: 140.
Polzin T, Daneschmand S V. 2003. On Steiner trees and minimum spanning trees in hypergraphs. Operations Research Letters, 31 (1): 12–20.
Radashevsky V I, Lana P C, Nalesso R C. 2006. Morphology and biology of Polydora species (Polychaeta: Spionidae) boring into oyster shells in South America, with the description of a new species. Zootaxa, 1353: 1–37.
Radashevsky V I, Nogueira J M M. 2003. Life history, morphology and distribution of Dipolydora armata (Polychaeta: Spionidae). Journal of the Marine Biological Association of the United Kingdom, 83 (2): 375–384.
Radashevsky V I, Olivares C. 2005. Polydora uncinata (Polychaeta: Spionidae) in Chile: an accidental transportation across the Pacific. Biological Invasions, 7 (3): 489–496.
Radashevsky V I, Pankova V V. 2013. Shell–boring versus tube–dwelling: is the mode of life fixed or flexible? Two cases in spionid polychaetes (Annelida, Spionidae). Marine Biology, 160 (7): 1 619–1 624.
Radashevsky V I. 1993. Revision of the genus Polydora and related genera from the North West Pacific (Polychaeta: Spionidae). Publications of the Seto Marine Biological Laboratory, 36 (1–2): 1–60.
Radashevsky V I. 2012. Spionidae (Annelida) from shallow waters around the British Islands: an identification guide for the NMBAQC Scheme with an overview of spionid morphology and biology. Zootaxa, 3152: 1–35.
Read G B. 2010. Comparison and history of Polydora websteri and P. haswelli (Polychaeta: Spionidae) as mud–blister worms in New Zealand shellfish. New Zealand Journal of Marine and Freshwater Research, 44 (2): 83–100.
Rice S A, Karl S, Rice K A. 2008. The Polydora cornuta complex (Annelida: Polychaeta) contains populations that are reproductively isolated and genetically distinct. Invertebrate Biology, 127 (1): 45–64.
Sato–Okoshi W, Abe H. 2012. Morphological and molecular sequence analysis of the harmful shell boring species of Polydora (Polychaeta: Spionidae) from Japan and Australia. Aquaculture, 368–369: 40–47.
Sato–Okoshi W, Abe H. 2013. Morphology and molecular analysis of the 18S rRNA gene of oyster shell borers, Polydora species (Polychaeta: Spionidae), from Japan and Australia. Journal of the Marine Biological Association of the United Kingdom, 93 (5): 1 279–1 286.
Sato–Okoshi W, Okoshi K, Abe H, Li J Y. 2013. Polydorid species (Polychaeta, Spionidae) associated with commercially important mollusk shells from eastern China. Aquaculture, 406–407: 153–159.
Sato–Okoshi W, Okoshi K, Koh B S, Kim Y H, Hong J S. 2012. Polydorid species (Polychaeta: Spionidae) associated with commercially important mollusk shells in Korean waters. Aquaculture, 350–353: 82–90.
Sato–Okoshi W, Okoshi K, Shaw J. 2008. Polydorid species (Polychaeta: Spionidae) in south–western Australian waters with special reference to Polydora uncinata and Boccardia knoxi. Journal of the Marine Biological Association of the United Kingdom, 88 (3): 491–501.
Sato–Okoshi W, Sugawara Y, Nomura T. 1990. Reproduction of the boring polychaete Polydora variegata inhabiting scallops in Abashiri Bay, North Japan. Marine Biology, 104 (1): 61–66.
Sato–Okoshi W. 1999. Polydorid species (Polychaeta: Spionidae) in Japan, with descriptions of morphology, ecology and burrow structure. 1. Boring species. Journal of the Marine Biological Association of the U nited K ingdom, 79 (5): 831–848.
Silina A V. 2006. Tumor–like formations on the shells of Japanese scallops Patinopecten yessoensis (Jay). Marine Biology, 148 (4): 833–840.
Simon C A, Thornhill D J, Oyarzun F, Halanych K M. 2009. Genetic similarity between Boccardia proboscidea from Western North America and cultured abalone, Haliotis midae, in South Africa. Aquaculture, 294 (1–2): 18–24.
Simon C A. 2011. Polydora and Dipolydora (Polychaeta: Spionidae) associated with molluscs on the south coast of South Africa, with descriptions of two new species. African Invertebrates, 52 (1): 39–50.
Surugiu V. 2012. Systematics and ecology of species of the Polydora–complex (Polychaeta: Spionidae) of the Black Sea. Zootaxa, 3518: 45–65.
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30 (12): 2 725–2 729.
Tang B, Ye L T, Cao C, Yang B L, Wang J Y. 2015. Morphological and anatomic observation of Polydora brevipalpa in Patinopecten yessoensis. South China Fisheries Science, 11 (4): 95–101. (in Chinese with English abstract)
Teramoto W, Sato–Okoshi W, Abe H, Nishitani G, Endo Y. 2013. Morphology, 18S rRNA gene sequence and life history of a new Polydora species (Polychaeta: Spionidae) from northeastern Japan. Aquatic Biology, 18 (1): 31–45.
Thompson J D, Gibson T J, Plewniak F, Jeanmougin F, Higgins D G. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research, 25 (24): 4 876–4 882.
Walker L M. 2011. A review of the current status of the Polydora–complex (Polychaeta: Spionidae) in Australia and a checklist of recorded species. Zootaxa, 275 1: 40–62.
Ye L T, Cao C, Tang B, Yao T, Wang R X, Wang J Y. 2017. Morphological and molecular characterization of Polydora websteri (Annelida: Spionidae), with remarks on relationship of adult worms and larvae using mitochondrial COI gene as a molecular marker. Pakistan Journal of Zoology, 4 9 (2): 699–710.
Ye L T, Tang B, Wu K C, Su Y L, Wang R X, Yu Z N, Wang J Y. 2015. Mudworm Polydora lingshuiensis sp. n is a new species that inhabits both shell burrows and mudtubes. Zootaxa, 3986: 88–100.
Acknowledgement
We are very grateful to Prof. JI Hongjiu (Institution of Oceanology and Marine Fisery, Jiangsu) and Prof. YAN Xiwu (Dalian Ocean University) for their assistance in the collection of samples. Special thanks are extended to two anonymous reviewers, who give us many constructive suggestions and critical comments, and spend a lot of energy to polish our manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (No. 31301863), the Pearl River S&T Nova Program of Guangzhou (No. 201710010166), the Natural Science Foundation of Guangdong Province (No. 2018A030313349), the Special Scientific Research Funds for Central Non-profit Institutes, South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences (No. 2017YB08), and the earmarked fund for Modern Agro-industry Technology Research System (No. CARS-49)
Rights and permissions
About this article
Cite this article
Ye, L., Yao, T., Wu, L. et al. Morphological and molecular diagnoses of Polydora brevipalpa Zachs, 1933 (Annelida: Spionidae) from the shellfish along the coast of China. J. Ocean. Limnol. 37, 713–723 (2019). https://doi.org/10.1007/s00343-019-7381-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-019-7381-0