Abstract
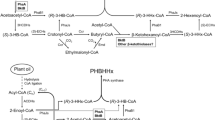
It is suggested that Δ6 fatty acid desaturase (FAD) plays a critical role in the biosynthesis of polyunsaturated fatty acids in plants and microalgae. But why does it adapt to the changed environments such as nitrogen starvation is seldom understood. One Δ6 FAD gene ( MiD6fad ) from an arachidonic acidrich microalga Myrmecia incisa Reisigl (Chlorophyta) was first heterologously expressed in Saccharomyces cerevisiae for the identification of function. The fatty acid profile of transgenic yeast detected by gas chromatography-mass spectrometry illustrated that the enzyme MiD6FAD could convert linoleic and α - linolenic acids to γ-linolenic and stearidonic acids, respectively, demonstrating that MiD6fad encoded a Δ6 FAD. A 1 965-bp fragment of the cloned 2 347-bp 5′-upstream region of MiD6fad was next subcloned and fused upstream with green fluorescent protein (GFP) gene to replace the GAL1 promoter of the vector pYES2. The generated construct was transformed into S. cerevisiae for function determination. Confocal microscopic images of the transformed line illustrated that this inserted fragment could drive GFP expression, which was further verified by fluorescence intensity quantification and Western blot analysis using anti- GFP antibody. The conversion efficiency (approximately 2% - 3%) of MiD6FAD was much lower than the reported ω 3 FAD and Δ6 elongase in this microalga, suggesting that MiD6FAD catalysed the possible ratelimiting step for ArA biosynthesis. The presence of several putative cis -acting regulatory elements in this identified promoter sheds new light on the regulation mechanism research of Δ6 FAD transcription for the ArA production in M. incisa in changing environmental factors.
Similar content being viewed by others
References
Camargo A, Llamas Á, Schnell R A, Higuera J J, González–Ballester D, Lefebvre P A, Fernández E, Galván A. 2007. Nitrate signaling by the regulatory gene NIT2 in Chlamydomonas. Plant Cell, 19 (11): 3 491–3 503.
Chen C X, Sun Z, Cao H S, Fang F L, Ouyang L L, Zhou Z G. 2015. Identification and characterization of three genes encoding acyl–CoA: diacylglycerolacyltransferase (DGAT) from the microalga Myrmecia incisa Reisigl. Algal Res earch, 12: 280–288.
Chiang T Y, Marzluf G A. 1994. DNA recognition by the NIT2 nitrogen regulatory protein: importance of the number, spacing, and orientation of GATA core elements and their flanking sequences upon NIT2 binding. Biochemistry, 33 (2): 576–582.
Dong X W, He Q F, Peng Z Y, Yu J H, Bian F, Li Y Z, Bi Y P. 2016. Production of γ–linolenic acid and stearidonic acid by Synechococcus sp. PCC7002 containing cyanobacterial fatty acid desaturase genes. Chin ese J ournal of Oceanology and Limn ology, 34(4): 772–780.
Dunn M A, White A J, Vural S, Hughes M A. 1998. Identification of promoter elements in a low–temperature–responsive gene ( blt 4.9) from barley ( Hordeum vulgare L.). Plant Molecular Biology, 38 (4): 551–564.
Guschina I A, Harwood J L. 2006. Lipids and lipid metabolism in eukaryotic algae. Prog ress in Lipid Res earch, 45 (2): 160–186.
Harwood J L, Guschina I A. 2009. The versatility of algae and their lipid metabolism. Biochimie, 91 (6): 679–684.
Hazel J R. 1995. Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annu al Rev iew of Physiology, 57: 19–42.
Higo K, Ugawa Y, Iwamoto M, Korenaga T. 1999. Plant cisacting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res earch, 27 (1): 297–300.
Houslay M D, Gordon L M. 1983. The activity of adenylate cyclase is regulated by the nature of its lipid environment. Curr ent Top ics in Membr anes and Transport, 18: 179–231.
Huang J Z, Jiang X Z, Xia X F, Yu A Q, Mao R Y, Chen X F, Tian B Y. 2011. Cloning and functional identification of delta5 fatty acid desaturase gene and its 5′–upstream region from marine fungus Thraustochytrium sp. FJN–10. Mar ine Biotechnology, 13 (1): 12–21.
Iskandarov U, Khozin–Goldberg I, Cohen Z. 2010. Identification and characterization of Δ12, Δ6, and Δ5 desaturases from the green microalga Parietochloris incisa. Lipids, 45 (6): 519–530.
Kaiser C, Michaelis S, Mitchel A. 1994. Methods in Yeast Genetics. Cold Spring Harbor. Cold Spring Harbor Laboratory Press, New York. 202p.
Kensy F, Zang E, Faulhammer C, Tan R K, Büchs J. 2009. Validation of a high–throughput fermentation system based on online monitoring of biomass and fluorescence in continuously shaken microtiter plates. Microb ial Cell Fact ories, 8: 31.
Khozin–Goldberg I, Leu S, Boussiba S. 2016. Microalgae as a source for VLC–PUFA production. In: Nakamura Y, Li–Beisson Y eds. Lipids in Plant and Algae Development. Springer, Cham. p.471–510.
Kim M J, Kim H, Shin J S, Chung C H, Ohlrogge J B, Suh M C. 2006. Seed–specific expression of sesame microsomal oleic acid desaturase is controlled by combinatorial properties between negative cis–regulatory elements in the SeFAD2 promoter and enhancers in the 5′–UTR intron. Molecular Genet ics and Genomics, 276 (4): 351–368.
Laoteng K, Ruenwai R, Tanticharoen M, Cheevadhanarak S. 2005. Genetic modification of essential fatty acids biosynthesis in Hansenula polymorpha. FEMS Microbiology Lett ers, 245 (1): 169–178.
Leonard A E, Pereira S L, Sprecher H, Huang Y S. 2004. Elongation of long–chain fatty acids. Prog ress in Lipid Res earch, 43 (1): 36–54.
Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S. 2002. PlantCARE, a database of plant cis–acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res earch, 30 (1): 325–327.
Li H, Ouyang L L, Zhou Z G. 2012. Low–temperature–induced expression of a ω3 fatty acid desaturase gene ( ω3FAD ) from Myrmecia incisa in Saccharomyces cerevisiae. J ournal of Agric ultural Biotechnology, 20 (7): 735–744. (in Chinese with English abstract)
Liu F, Li H, Li C Y, Ouyang L L, Zhou Z G. 2012. Characterization of fatty acid desaturase (FAD) genes in Myrmecia incisa and the effect of nitrogen starvation on their transcription. J ournal of Fish ery Sci ences of China, 19 (5): 729–740. (in Chinese with English abstract)
Los D A, Mironov K S, Allakhverdiev S I. 2013. Regulatory role of membrane fluidity in gene expression and physiological functions. Photosynth esis Res earch, 116 (2–3): 489–509.
Los D A, Murata N. 1998. Structure and expression of fatty acid desaturases. Biochim ica et Biophys ica Acta, 1394 (1): 3–15.
Los D A, Ray M K, Murata N. 1997. Differences in the controlof the temperature–dependent expression of four genes for desaturases in Synechocystis sp. PCC 6803. Molecular Microbiology, 25 (6): 1 167–1 175.
Lowry J A, Atchley W R. 2000. Molecular evolution of the GATA family of transcription factors: conservation within the DNA–binding domain. J ournal of Molecular Evolution, 50 (2): 103–115.
Mansilla M C, Banchio C E, De Mendoza D. 2008. Signalling pathways controlling fatty acid desaturation. In: Quinn P J, Wang X Y eds. Lipids in Health and Disease. Springer, Dordrecht. p.71–99.
Martin C E, Oh C S, Jiang Y D. 2007. Regulation of long chain unsaturated fatty acid synthesis in yeast. Biochim ica et Biophys ica Acta, 1771 (3): 271–285.
Meesapyodsuk D, Qiu X. 2012. The front–end desaturase: structure, function, evolution and biotechnological use. Lipids, 47 (2): 227–237.
Murata N, Wada H. 1995. Acyl–lipid desaturases and their importance in the tolerance and acclimatization to cold of cyanobacteria. Biochem ical J ournal, 308 (1): 1–8.
Na–Ranong S, Laoteng K, Kittakoop P, Tantichareon M, Cheevadhanarak S. 2005. Substrate specificity and preference of Δ 6–desaturase of Mucor rouxii. FEBS Lett ers, 579 (12): 2 744–2 748.
Nayeri F D, Yarizade K. 2014. Bioinformatics study of delta–12 fatty acid desaturase 2 (FAD2) gene in oilseeds. Molecular Biology Rep orts, 41 (8): 5 077–5 087.
Nishiuchi T, Nakamura T, Abe T, Kodama H, Nishimura M, Iba K. 1995. Tissue–specific and light–responsive regulation of the promoter region of the Arabidopsis thaliana chloroplast–3 fatty acid desaturase gene ( FAD7 ). Plant Molecular Biology, 29 (3): 599–609.
Nwankwo J O, Spector A A, Domann F E. 2003. A nucleotide insertion in the transcriptional regulatory region of FADS2 gives rise to human fatty acid delta–6–desaturase deficiency. J ournal of Lipid Res earch, 44 (12): 2 311–2 319.
Ouyang L L, Chen S H, Li Y, Zhou Z G. 2013a. Transcriptome analysis reveals unique C4–like photosynthesis and oil body formation in an arachidonic acid–rich microalga Myrmecia incisa Reisigl H4301. BMC Genomics, 14: 396.
Ouyang L L, Li H, Liu F, Tong M, Yu S Y, Zhou Z G. 2013b. Accumulation of arachidonic acid in a green microalga, M yrmecia incisa H4301, enhanced by nitrogen starvation and its molecular regulation mechanisms. In: Dumancas G G, Murdianti B S, Lucas E A eds. Arachidonic Acid: Dietary Sources and General Functions. Nova Science Publishers, Inc., New York. p.1–20.
Qi B X, Fraser T, Mugford S, Dobson G, Sayanova O, Butler J, Napier J A, Stobart A K, Lazarus C M. 2004. Production of very long chain polyunsaturated omega–3 and omega–6 fatty acids in plants. Nat ure Biotechnology, 22 (6): 739–745.
Rastogi R, Bate N J, Sivasankar S, Rothstein S J. 1997. Footprinting of the spinach nitrite reductase gene promoter reveals the preservation of nitrate regulatory elements between fungi and higher plants. Plant Molecular Biology, 34 (3): 465–476.
Reisigl H. 1964. Zur systematik und ökologie alpiner Bodenalgen. Ö sterreichische Botanische Zeitschrift, 111 (4): 402–499.
Reyes J C, Muro–Pastor M I, Florencio F J. 2004. The GATA family of transcription factors in Arabidopsis and rice. Plant Physiology, 134 (4): 1 718–1 732.
Russell N J. 1984. Mechanisms of thermal adaptation in bacteria: blueprints for survival. Trends in Biochem ical Sci ences, 9 (3): 108–112.
Saed Taha R, Ismail I, Zainal Z, Abdullah S N A. 2012. The stearoyl–acyl–carrier–protein desaturase promoter ( Des ) from oil palm confers fruit–specific GUS expression in transgenic tomato. J ournal of Plant Physiology, 169 (13): 1 290–1 300.
Schnell R A, Lefebvre P A. 1993. Isolation of the Chlamydomonas regulatory gene NIT2 by transposon tagging. Genetics, 134 (3): 737–747.
Sheff M A, Thorn K S. 2004. Optimized cassettes for fluorescent protein tagging in Saccharomyces cerevisiae. Yeast, 21 (8): 661–670.
Skala M C, Riching K M, Gendron–Fitzpatrick A, Eickhoff J, Eliceiri K W, White J G, Ramanujam N. 2007. In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia. Proc eedings of the Nat ional Acad emy of Sci ences of the U nited S tates of A merica, 104 (49): 19 494–19 499.
Stanier R Y, Kunisawa R, Mandel M, Cohen–Bazire G. 1971. Purification and properties of unicellular blue–green algae (order Chroococcales). Bacteriological Rev iews, 35 (2): 171–205.
Suzuki I, Los D A, Kanesaki Y, Mikami K, Murata N. 2000. The pathway for perception and transduction of lowtemperature signals in Synechocystis. EMBO J ournal, 19 (6): 1 327–1 334.
Tan L, Li S E, Zhang X Y, Ma F Y. 2015. Cloning and functional analysis of Δ6–desaturase gene and its upstream region from Mortierella sp. AGED. Journal of the Science of Food and Agriculture, 95 (15): 3 077–3 083.
Tao Y, Marzluf G A. 1999. The NIT2 nitrogen regulatory protein of Neurospora: expression and stability of nit–2 mRNA and protein. Curr ent Genet ics, 36 (3): 153–158.
Thompson G A Jr. 1989. Membrane acclimation by unicellular organisms in response to temperature change. Journal of Bioenerg etics and Biomembr anes, 21 (1): 43–60.
Tocher D R, Leaver M J, Hodgson P A. 1998. Recent advances in the biochemistry and molecular biology of fatty acyl desaturases. Prog ress in Lipid Res earch, 37 (2–3): 73–117.
Tong M, Yu S Y, Ouyang L L, Zhou Z G. 2011. Comparison of increased arachidonic acid content in Myrmecia incisa cultured during the course of nitrogen or phosphorus starvation. J ournal of Fish eries of China, 35 (5): 763–773. (in Chinese with English abstract)
Wallis J G, Watts J L, Browse J. 2002. Polyunsaturated fatty acid synthesis: what will they think of next? Trends in Biochemical Sciences, 27 (9): 467–473.
Wan X, Zhang Y B, Wang P, Jiang M L. 2011. Molecular cloning and expression analysis of a delta 6–fatty acid desaturase gene from Rhizopus stolonifer strain YF6 which can accumulate high levels of gamma–linolenic acid. J ournal of Microbiology, 49 (1): 151–154.
Warude D, Joshi K, Harsulkar A. 2006. Polyunsaturated fatty acids: biotechnology. Crit ical Rev iews in Biotechnology, 26 (2): 83–93.
Wu S J, Zhang L J, Chen X L, Miao X M, Wang J, Fu H. 2013. Identification and functional analysis of a Δ6–desaturase gene and the effects of temperature and wounding stresses on its expression in Microula sikkimensis leaves. Biosci ence, Biotechnology, and Biochem istry, 77 (9): 1 925–1 930.
Xiao G, Zhang Z Q, Yin C F, Liu R Y, Wu X M, Tan T L, Chen S Y, Lu C M, Guan C Y. 2014. Characterization of the promoter and 5′–UTR intron of oleic acid desaturase (FAD2) gene in Brassica napus. Gene, 545 (1): 45–55.
Xue W B, Liu F, Sun Z, Zhou Z G. 2016. A Δ–9 fatty acid desaturase gene in the microalga Myrmecia incisa Reisigl: cloning and functional analysis. International Journal of Molecular Sciences, 17 (7): 1143.
Ye R X, Yu Z, Shi W W, Gao H J, Bi Y H, Zhou Z G. 2014. Characterization of α–type carbonic anhydrase (CA) gene and subcellular localization of α–CA in the gametophytes of Saccharina japonica. Journal of Applied Phycology, 26 (2): 881–890.
Yu S Y, Li H, Tong M, Ouyang L L, Zhou Z G. 2012. Identification of a Δ6 fatty acid elongase gene for arachidonic acid biosynthesis localized to the endoplasmic reticulum in the green microalga Myrmecia incisa Reisigl. Gene, 493 (2): 219–227.
Zhang C J, Hou Y Q, Hao Q G, Chen H F, Chen L M, Yuan S L, Shan Z H, Zhang X J, Yang Z L, Qiu D Z, Zhou X A, Huang W J. 2015. Genome–wide survey of the soybean GATA transcription factor gene family and expression analysis under low nitrogen stress. PLoS One, 10 (4): e0125174.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (No. 31172389), the Special Project of Marine Renewable Energy from the State Oceanic Administration (No. SHME2011SW02), and the Shanghai Universities Peak Discipline Project of Aquaculture
Electronic supplementary material
343_2019_7305_MOESM1_ESM.pdf
Functional characterization of a Δ6 fatty acid desaturase gene and its 5′-upstream region cloned from the arachidonic acidrich microalga Myrmecia incisa Reisigl (Chlorophyta)
Rights and permissions
About this article
Cite this article
Zhang, L., Cao, H., Ning, P. et al. Functional characterization of a Δ6 fatty acid desaturase gene and its 5′-upstream region cloned from the arachidonic acidrich microalga Myrmecia incisa Reisigl (Chlorophyta). J. Ocean. Limnol. 36, 2308–2321 (2018). https://doi.org/10.1007/s00343-019-7305-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-019-7305-z