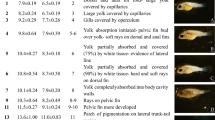
Abstract
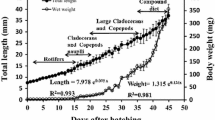
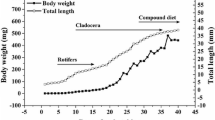
Ontogenetic changes in digestive enzyme activities and the amino acid (AA) profile of starry flounder, Platichthys stellatus, were investigated and limiting amino acids were estimated compared with the essential AA profile between larvae and live food to clarify starry flounder larval nutritional requirements. Larvae were collected at the egg stage and 0, 2, 4, 7, 12, 17, 24 days after hatching (DAH) for analysis. Larvae grew from 1.91 mm at hatching to 12.13 mm at 24 DAH. Trypsin and chymotrypsin activities changed slightly by 4 DAH and then increased significantly 4 DAH. Pepsin activity increased sharply beginning 17 DAH. Lipase activity increased significantly 4 DAH and increased progressively with larval growth. Amylase activity was also detected in newly hatched larvae and increased 7 DAH followed by a gradual decrease. High free amino acid (FAA) content was detected in starry flounder eggs (110.72 mg/g dry weight). Total FAA content dropped to 43.29 mg/g in 4-DAH larvae and then decreased gradually to 13.74 mg/g in 24-DAH larvae. Most FAAs (except lysine and methionine) decreased >50% in 4-DAH larvae compared with those in eggs and then decreased to the lowest values in 24-DAH larvae. Changes in the protein amino acid (PAA) profile were much milder than those observed for FAAs. Most PAAs increased gradually during larval development, except lysine and phenylalanine. The percentages of free threonine, valine, isoleucine, and leucine decreased until the end of the trial, whereas the protein forms of these four AAs followed the opposite trend. A comparison of the essential AA composition of live food (rotifers, Artemia nauplii, and Artemia metanauplii) and larvae suggested that methionine was potentially the first limiting AA. These results may help develop starry flounder larviculture methods by solving the AA imbalance in live food. Moreover, the increased digestive enzyme activities indicate the possibility of introducing artificial compound feed.
Similar content being viewed by others
References
Alvarez-González C A, Moyano-López F J, Civera-Cerecedo R, Carrasco-Chávez V, Ortiz-Galindo J L, Dumas S. 2008. Development of digestive enzyme activity in larvae of spotted sand bass Paralabrax maculatofasciatus. 1. Biochemical analysis. Fish Physiol. Biochem., 34(4): 373–384.
Applebaum S L, Perez R, Lazo J P, Holt G J. 2001. Characterization of chymotrypsin activity during early ontogeny of larval red drum (Sciaenops ocellatus). Fish Physiol. Biochem., 25(4): 291–300.
Aragão C, Conceição L E C, Martins D et al. 2004. A balanced dietary amino acid profile improves amino acid retention in post-larval Senegalese sole (Solea senegalensis). Aquaculture, 233(1-4): 293–304.
Aragão C, Conceição L, Fyhn H J, Dinis M T. 2000. Whole body amino acid profile of Solea senegalensis larvae changes during ontogeny. Comparative Biochemistry and Physiology-Part A: Molecular & Integrative Physiology, 126 (S1): 6.
Bolasina S, Pérez A, Yamashita Y. 2006. Digestive enzymes activity during ontogenetic development and effect of starvation in Japanese flounder, Paralichthys olivaceus. Aquaculture, 252(2-4): 503–515.
Bradford M M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal yt. Biochem., 72(1-2): 248–254.
Brown M R, Battaglene S C, Morehead D T et al. 2005. Ontogenetic changes in amino acid and vitamins during early larval stages of striped trumpeter (Latris lineata). Aquaculture, 248: 263–274.
Cara J B, Moyano F J, Cárdenas S, Fernández-Díaz C, Yúfera M. 2003. Assessment of digestive enzyme activities during larval development of white bream. J. Fish Biol., 63(1): 48–58.
Conceição L E C, Grasdalen H, Rønnestad I. 2003. Amino acid requirements of fish larvae and post-larvae: new tools and recent findings. Aquaculture, 227(1-4): 221–232.
Conceição L E C, Ozório R O A, Suurd E A et al. 1998. Amino acid profiles and amino acid utilization in larval African catfish (Clarias gariepinus): effects of ontogeny and temperature. Fish Physiol. Biochem., 19(1): 43–58.
Conceição L E C, Rønnestad I, Tonheim S K. 2001. Metabolic budgets for lysine and glutamate in unfed herring (Clupea harengus) larvae. Aquaculture, 206(3-4): 305–312.
Conceição L E C, van der Meeren T, Verreth J A J et al. 1997. Amino acid metabolism and protein turnover in larval turbot (Scophthalmus maximus) fed natural zooplankton or Artemia. Mar. Biol., 129(2): 255–265.
Dayal J S, Ali S A, Thirunavukkarasu A R et al. 2003. Nutrient and amino acid profiles of egg and larvae of Asian seabass, Lates calcarifer (Bloch). Fish Physiol. Biochem., 29(2): 141–147.
Douglas S E, Mandla S, Gallant J W. 2000. Molecular analysis of the amylase gene and its expression during the development in the winter flounder, Pleuronectes americanus. Aquaculture, 190(3-4): 247–260.
Fang H H, Wang B. 2011. Histological studies on the development of digestive system in larval and juvenile starry flounder. Chinese Agricultural Science Bulletin, 27(14): 50–54. (in Chinese with English abstract)
Finn R N, Fyhn H J, Henderson R J et al. 1996. The sequence of catabolic substrate oxidation and enthalpy balance of developing embryos and yolk-sac larvae of turbot (Scophthalmus maximus L.). Comp. Biochem. Physiol., 115(2): 133–151.
Finn R N. 1994). Physiological energetics of developing marine fish embryos and larvae. University of Bergen, Bergen, Norway.
Helland S, Terjesen B F, Berg L. 2003. Free amino acid and protein content in the planktonic copepod Temora longicornis compared to Artemia franciscana. Aquaculture, 215(1-4): 213–228.
Infante J L Z, Cahu C. 1994. Development and response to a diet change of some digestive enzymes in sea bass (Dicentrarchus labrax) larvae. Fish Physiol. Biochem., 12(5): 399–408.
James C, Dias P, Salman A E. 1987. The use of marine yeast (Candida sp.) and bakers’ yeast (Sac c haromyces cerevisiae) in combination with Chlorella sp. for mass culture of the rotifer Brachionus pliatilis. Hydrobiologia, 147: 263–268.
Jeeja P K, Joseph I, Raj R P. 2011). Nutritional composition of rotifer (Brachionus plicatilis Müller) cultured using selected natural diets.
Indian J. Fish., 58(2): 59–65.
Kamaci H O, Suzer C, Coban D, Firat K, Saka S. 2009. Organogenesis and enzymatic functionality of exocrine pancreas in cultured Gilthead Sea Bream (Sparus aurata) Larvae. J. Anim. Vet. Adv., 8(12): 2477–2484.
Krautz M C, Vásquez S, Castro L R et al. 2010. Changes in metabolic substrates during early development in anchoveta Engraulis ringens (Jenyn. 1842. in the Humboldt Current. Mar. Biol., 157(5): 1137–1149.
Krogdahl Å, Sundby A. 1999). Characteristics of pancreatic function in fish. In: Pierzynowski S G, Zabielski R eds. Biology of the pancreas in growing animals. Elsevier Science, Amsterdam. p.437–458.
Lazo J P, Holt G J, Arnold C R. 2000. Ontogeny of pancreatic enzymes in larval red drum Sciaenops ocellatus. Aquac. Nutr., 6(3): 183–192.
Liu Z H, Wang B, Yao Z G, Sun P X, Liu P, Wang Z L. 2008. Morphological development and growth of larval and juvenile fish of starry flounder, Platichthys stellatus. Advances in Marine Science, 26(1): 90–97. (in Chinese with English abstract)
Ma P, Sivaloganathan B, Reddy P K, Chan W K, Lam T J. 2001. Ontogeny of a-amylase gene expression in sea bass larvae (Lates calcarifer). Mar. Biotechnol., 3(5): 463–469.
Mambrini M, Kaushik S J. 1995. Indispensable amino acid requirements of fish: correspondence between quantitative data and amino acid profiles of tissue proteins. J. Appl. Ichthyol., 11(3-4): 240–247.
Murray H M, Gallant J W, Perez-Casanova J C, Johnson S C, Douglas S E. 2003. Ontogeny of lipase expression in winter flounder. J. Fish Bio l., 62(4): 816–833.
Naz M. 2008. The changes in the biochemical compositions and enzymatic activities of rotifer (Brachionus plicatilis, Müller) and Artemia during the enrichment and starvation periods. Fish Physiol. Biochem., 34(4): 391–404.
Oozeki Y, Bailey K M. 1995. Ontogenetic development of digestive enzyme activities in larval walleye pollock, Theragra chalcogram m a. Mar. Biol., 122(2): 177–186.
Portella M C, Takata R, Leitão N J et al. 2013. Free amino acids in Pacu, Piaractus mesopotamicus, eggs and larvae. J. World Aquacult. Soc., 44(3): 425–434
Rønnestad I, Fyhn H J. 1993. Metabolic aspects of free amino acids in developing marine fish eggs and larvae. Rev. Fish. Sci., 1(3): 239–259.
Rønnestad I, Rojas-Garcia C R, Tonheim S K et al. 2001. In vivo studies of digestion and nutrient assimilation in marine fish larvae. Aquaculture, 201(1-2): 161–175.
Saavedra M, Beltran M, Pousão-Ferreira P, Dinis M T, Blasco J, Conceição L E C. 2007. Evaluation of bioavailability of individual amino acids in Diplodus puntazzo larvae: towards the ideal dietary amino acid profile. Aquaculture, 263(1-4): 192–198.
Saavedra M, Conceição L E C, Pousão-Ferreira P, Dinis M T. 2006. Amino acid profiles of Diplodus sargus (L. 1758. larvae: implications for feed formulation. Aquaculture, 261(2): 587–593.
Sæle Ø, Nordgreen A, Olsvik P A, Hamre K. 2010. Characterization and expression of digestive neutral lipases during ontogeny of Atlantic cod (Gadus morhua). Comp. Biochem. Physiol. A. Mol. Integr. Physiol., 157(3): 252–259.
Skjærven K H, Finn R N, Kryvi H et al. 2003. Yolk resorption in developing plaice (Pleuronectes platessa). In: Browman H I, Skiftesvik A B eds. The Big Fish Bang. Proceedings of the 26th Annual Larval Fish Conference. Institute of Marine Research, Bergen, Norway. ISBN 82-7461-059-8.
Thorsen A, Kjesbu O S, Fyhndr H J et al. 1996. Physiological mechanisms of buoyancy in eggs from brackish water cod. J. Fish Biol., 48(3): 457–477.
Tonheim S K, Koven W, Rønnestad I. 2000. Enrichment of Artemia with free methionine. Aquaculture, 190(3-4): 223–235.
Tulli F, Tibaldi E. 1997. Changes in amino acids and essential fatty acids during early larval rearing of dentex. Aquacult. Int., 5(3): 229–236.
Welch A, Hoenig R, Stieglitz J et al. 2013. Growth rates of larval and juvenile bigeye scad Selar crumenophthalmus in captivity. Springer Plus, 2:634.
Weltzien F A, Planas M, Cunha I et al. 1999. Free amino acid and protein contents of start-feeding larvae of turbot (Scophthalmus maximus) at three temperatures. Mar. Biol., 133(2): 327–336.
Wilson R P, Poe W E. 1985. Relationship of whole body and egg essential amino acid patterns to amino acid requirement patterns in channel catfish, Ictalurus punctatus. Comp. Biochem. Physiol. B. Comp. Biochem., 80(2): 385–388.
Yamashita Y T, Aritaki M, Kurita Y, Tanaka M. 2014. Early growth and development of reciprocal hybrids of the starry flounder Platichthys stellatus and stone flounder Kareius bicoloratus. J. Fish Biol., 84(5): 1503–1518.
Zamani A, Hajimoradloo A, Madani R, Farhangi M. 2009. Assessment of digestive enzymes activity during the fry development of the endangered Caspian brown trout Salmo caspius. J. Fish Biol., 75(4): 932–937.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supported by the Scientific and Technological Development Plan Project of Yantai City (No. 2013ZH348), the Aquatic Animal Nutrition and Feed Research and Innovation Demonstration Platform (No. 201301001), and the National Special Research Fund for Non-Profit Marine Sector (No. 1205025)
Rights and permissions
About this article
Cite this article
Song, Z., Wang, J., Qiao, H. et al. Ontogenetic changes in digestive enzyme activities and the amino acid profile of starry flounder Platichthys stellatus . Chin. J. Ocean. Limnol. 34, 1013–1024 (2016). https://doi.org/10.1007/s00343-016-5031-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-016-5031-3