Abstract
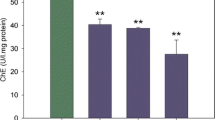
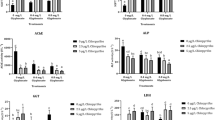
Endosulfan, an organochlorine pesticide, is highly toxic and effective at controlling pests in agriculture, horticulture, and public health programs. In this study, static bioassays were used to evaluate the toxicity of endosulfan to freshwater prawns (Macrobrachium rosenbergii) of various lengths (1.5±0.03, 4±0.08, and 7±0.06 cm). Additionally, the activities of peroxidase (POD), acid phosphatase (ACP), alkaline phosphatase, acetylcholinesterase (AChE), and Na+/K+-ATPase were analyzed to reflect the effects of endosulfan exposure. The 96 h LC50 of endosulfan for prawns 1.5, 4, and 7 cm long were 1.86, 4.53, and 6.09 μg/L, respectively, improved tolerance to endosulfan with growth. The POD activities of test organisms exposed to low concentrations of endosulfan were inhibited, indicating the presence of oxygen damaged tissue. Moreover, a notable decrease in AChE activity was observed due to overstimulation of neurotransmission, which might result in abnormal behavior. The effect caused by endosulfan on phosphatase production in the hepatopancreas of prawns 1.5, 4, and 7 cm long was different because the ability of nonspecific immune regulation increased with growth. The 96 h LC50 values obtained in this study could be used in the formulation of water-quality criteria in China. Moreover, the changes in enzymes activities of M. rosenbergii under stress of endosulfan could be applied in the establishment of early warning indicators for bio-safety.
Similar content being viewed by others
References
Achuthankutty C T, Nair S R S, Krishnakumari L. 1993. Growth of juvenile shrimp Metapenaeus monoceros fed with squid and mussel. Indian J. Mar. Sci., 22(4): 283–286.
Agrahari S, Gopal K. 2008. Inhibition of Na+-K+-ATPase in different tissues of freshwater fish Channa punctatus (Bloch) exposed to monocrotophos. Pestic Biochem. Physiol., 92(2): 57–60.
Ahmad I, Hamid T, Fatima M, Chand H S, Jain S K, Athar M, Raisuddin S. 2000. Induction of hepatic antioxidants in freshwater catfish (Channa punctatus Bloch) is a biomarker of paper mill effluent exposure. Biochim. Biophys. Acta, 1523(1): 37–48.
Almeida M G, Fanini F, Davino S C, Aznar A E, Koch O R, Barros S B. 1997. Pro- and anti-oxidant parameters in rat liver after short term exposure to hexachlorobenzene. Hum. Exp. Toxicol., 16(5): 257–261.
Arufea M, Arellanoa J M, Garcíaa L, Albendína G, Sarasqueteb C. 2007. Cholinesterase activity in gilthead seabream (Sparus aurata) larvae: characterization and sensitivity to the organophosphate azinphosmethyl. Aquat. Toxicol., 84(3): 328–336.
Atli G, Canli M. 2007a. Enzymatic responses to metal exposures in a freshwater fish Oreochromis niloticus. Comp. Biochem. Physiol. C Toxicol. Pharmacol., 145(2): 282–287.
Atli G, Canli M. 2011b. Metals (Ag+, Cd2+, Cr6+) affect ATPase activity in the gill, kidney, and muscle of freshwater fish Oreochromis niloticus following acute and chronic exposures. Environ. Toxicol., http://dx.doi.org/10.1002/tox.20766.
Bacchetta C, Cazenave J, Parma M, Biancucci G. 2011. Biochemical stress responses in tissues of the cichlid fish Cichlasoma dimerus exposed to a commercial formulation of endosulfan. Arch. Environ. Contam. Toxicol., 61(3): 453–460.
Bagchi M, Hassoun E A, Bagchi D, Stohs S J. 1992. Endrininduced increases in hepatic lipid peroxidation, membrane microviscosity, and DNA damage in rats. Arch. Environ. Contam. Toxicol., 23(1): 1–5.
Ballesteros M L, Durando P E, Nores M L, Diaz M P, Bistoni M A, Wunderlin D A. 2009a. Oxidative stress responses in different organs of Jenynsia multidentata exposed to endosulfan. Ecotoxicol. Environ. Saf., 72(1): 199–205.
Ballesterosa M L, Gonzalezb M, Wunderlinc D A, Bistonia M A, Miglioranzab K S B. 2011b. Uptake, tissue distribution and metabolism of the insecticide endosulfan in Jenynsia multidentata (Anablepidae, Cyprinodontiformes). Environ. Pullut., 159(6): 1 709–1 714.
Bernet D, Schmidt H, Wahli T, Burkhardt-Holm P. 2001. Effluent from a sewage treatment works causes changes in serum chemistry of brown trout (Salmo trutta L.). Ecotoxicol. Environ. Saf e., 48(2): 140–147.
Bhavan P S, Geraldine P. 2001. Biocheical stress response in tissues of the Prawn Macrobrachium malcolmsonii on exposure of endosulfan. P estic. Biochem. Phys., 16(1): 5–7.
Bianchini A, Castilho P C. 1999. Effects of zinc exposure on oxygen consumption and gill Na+ /K+ ATPase of the estuarine crab Chasmagnatus granulata Dana, 1851(Decapoda-Grapsidae). Bull. Environ. Contam. Toxicol., 62(1): 63–69.
Borges A, Scotti L V, Siqueira D R, Zanini R, Amaral F, Jurinitz D F, Wassermann G F. 2007. Changes in hematological and serum biochemical values in jundia Rhamdia quelen due to sub-lethal toxicity of cypermethrin. Chemosphere, 69(6): 920–926.
Brunelli E, Bernab C N I, Berg C, Lundstedt-Enkel K, Bonacci A, Tripepi S. 2009. Environmentally relevant concentrations of endosulfan impair development, metamorphosis and behavior in Bufo bufo tadpoles. Aquat. Toxicol., 91(2): 135–142.
Capkin E, Altinok I, Karahan S. 2006. Water quality and fish size affect toxicity of endosulfan, an organochlorine pesticide, to rainbow trout. Chemosphere, 64(10): 1 793–1 800.
Das B K, Mukherjee S C. 2003. Toxicity of cypermethrin in Labeo rohita fingerlings: biochemical, enzymatic and haematological consequences. Comp. Biochem. Physiol. C., 134(1): 109–121.
DeLorenzo M E, De Leon R G, 2010. Toxicity of the insecticide etofenprox to three life stages of the grass shrimp, Palaemonetes pugio. Arch. Environ. Con. Tox., 58(4): 985–990.
Dutta H M, Arends D A. 2003. Effects of endosulfan on brain acetylcholinesterase activity in juvenile bluegill sunfish. Environ. Res., 91(3): 157–162.
Fagotti A, Morosi L, Di Rosa I, Clarioni R, Simoncelli F, Pascolini R, Pellegrino R, Guex G D, Hotz H. 2005. Bioaccumulation of organochlorine pesticides in frogs of the Rana esculenta complex in central Italy. Amphibia Reptilia, 26(1): 93–104.
Food and Agriculture Organization of the United Nations. 2010. Fisheries and Aquaculture Department. Aquaculture production (Quantities and Values) 1950-2010. FAO Release March on 2012, http://www.fao.org/fishery/statistics/globalaquaculture-production/query/zh. Accessed on 2013-01-23.
Giusi G, Facciolo R M, Alò R, Carelli A, Madeo M, Brandmayr P, Canonaco M. 2005. Some environmental contaminants influence motor and feeding behaviors in the ornate wrasse (Thalassoma pavo) via distinct cerebral histamine receptor subtypes. Environ. Health Perspect., 113(11): 1 522–1 529.
Hii Y S, Lee M Y, Chuah T S. 2007. Acute toxicity of organochlorine insecticide endosulfan and its effect on behavior and some hematological parameters of Asian swamp eel (Monopterus albus, Zuiew). Pestic Biochem. Physiol., 89(1): 46–53.
Jia H L, Li Y F, Wang D G, Cai D J, Yang M, Ma J M, Hu J X. 2009. Endosulfan in China 1-gridded usage inventories. Environ. Sci. Pollut. Res. Int., 16(3): 295–301.
Key P B, Chung K W, Opatkiewicz A D, Wirth E F, Fulton M H. 2003. Toxicity of the insecticides fipronil and endosulfan to selected life stages in the grass shrimp (Palaemonetes pugio). Bull. Environ. Contam. Toxicol., 70(3): 533–540.
Kumar A, Doan H, Barnes M, Chapman J C, Kookana R S. 2010. Response and recovery of acetylcholinesterase activity in freshwater shrimp, Paratya australiensis (Decapoda: Atyidae) exposed to selected anticholinesterase insecticides. Ecotoxicol. Environ. Safe., 73(7): 1 503–1 510.
Lee S E, Kim J S, Kennedy I R, Park J W, Kwon G S, Koh S C, Kim J E. 2003. Biotransformation of an organochlorine insecticide, endosulfan, by Anabaena species. J. Agr ic. Food. Chem., 51(5): 1 336–1 340.
Lombardi J V, Machado-Neto J G, Bross-Garcia A L, Marques H L, Kubo E. 2001. Acute toxicity of the pesticides endosulfan and ametryne to the freshwater prawn Macrobrachium rosenbergii De Man. Bull. Environ. Contam. Toxicol., 67(5): 665–671.
Matos P, Fontainhas-Fernandes A, Peixoto F, Carrola J, Rocha E. 2007. Biochemical and histological hepatic changes of Nile tilapia Oreochromis niloticus exposed to carbaryl. Pestic. Biochem. Phys., 89(1): 73–80.
McKinlay R, Plant J A, Bell J N B, Voulvoulis N. 2008. Endocrine disrupting pesticides: implications for risk assessment. Environ. Int., 34(2): 168–183.
McLeese D, Metcalfe C. 1980. Toxicities of eight organochlorine compounds in sediment and seawater to Crangon septemspinosa. Bull. Environ. Contam. Toxicol., 25(1): 921–928.
Mersie W, Seybold C A, McNamee C, Lawson M A. 2003. Abating endosulfan from runoff using vegetative filter strips: the importance of plant species and flow rate. Agric. Ecosys. Environ., 97(1): 215–223.
Natarajan E, Biradar R S, George J P. 1992. Acute toxicity of pesticides to giant freshwater prawn Macrobrachium rosenbergii (De Man). J. Aqua. Trop., 7(2): 183–188.
Pandey S, Nagpure N S, Kumar R, Sharma S, Srivastava S K, Verma M S. 2006. Genotoxicity evaluation of acute doses of endosulfan to freshwater teleost Channa punctatus (Bloch) by alkaline single-cell gel electrophoresis. Ecotoxicol. Environ. Saf e., 65(1): 56–61.
Perez F J, Maureira V. 2003. Inactivation in vivo of basic peroxidase and increased content of H2O2 in grapevine leaves post treatment with DTT and paraquat. J. Plant Physiol., 160(6): 645–650.
Pillai B R, Das Mahapatra K, Ponzoni R W, Sahoo L, Lalrinsanga P L, Nguyen N H, Mohanty S, Sahu S, Vijaykumar, Sahu S, Khaw H L, Patra G, Patnaik S, Rath S C. 2011. Genetic evaluation of a complete diallel cross involving three populations of freshwater prawn (Macrobrachium rosenbergii) from different geographical regions of India. Aquaculture, 319(3–4): 347–354.
Rao J V. 2006. Sublethal effects of an organophosphorus insecticide (RPR-II) on biochemical parameters of tilapia, Oreochromis mossambicus. Comb. Biochem. Physiol C., 143(4): 492–498.
Rosenshield M L, Jofr E M B, Karasov W H. 1999. Effects of polychlorinated biphenyl 126 on green frog (Rana clamitans) and leopard frog (Rana pipiens) hatching success, development, and metamorphosis. Environ. Toxicol. Chem., 18(11): 2 478–2 486.
Saiyed H, Dewan A, Bhatnagar V, Shenoy U, Shenoy R, Rajmohan H, Patel K, Kashyap R, Kulkarni P, Rajan B, Lakkad B. 2003. Effect of endosulfan on male reproductive development. Environ. Health Perspect., 111(16): 1 958–1 962.
Sucahyo D, van Straalen N M, Krave A, van Gestel C A. 2008. Acute toxicity of pesticides to the tropical freshwater shrimp Caridina laevis. Ecotoxicol. Environ. Sa f e., 69(3): 421–427.
Suryavanshi U, Sreepada R A, Ansari Z A, Nigam S, Badesab S. 2009. A study on biochemical changes in the penaeid shrimp, Metapenaeus monoceros (Fabricius) following exposure to sublethal doses of organochlorine pesticide (endosulfan). Chemosphere., 77(11): 1 540–1 550.
Sutherland T D, Horne I, Lacey M J, Harcourt R L, Russell R J, Oakeshott J G. 2000. Enrichment of an endosulfandegrading mixed bacterial culture. Appl. Environ. Microbiol., 66(7): 22–28.
Suvetha L, Ramesh M, Saravanan. 2010. Influence of cypermethrin toxicity on ionic regulation and gill Na+/K+-ATPase activity of a freshwater teleost fish Cyprinus carpio. Environ. Toxicol. Phar., 29(1): 44–49.
Tumburu L, Shepard E F, Strand A E, Browdy C L. 2012. Effects of endosulfan exposure and Taura Syndrome virus infection on the survival and molting of the marine penaeid shrimp, Litopenaeus vannamei. Chemosphere, 86(9): 912–918.
Wan M T, Kuo J N, Buday C, Schroeder G, Van Aggelen G, Pasternak J. 2005. Toxicity of alpha-, beta-, (alpha+beta)-endosulfan and their formulated and degradation products to Daphnia magna, Hyalella azteca, Oncorhynchus mykiss, Oncorhynchus kisutch, and biological implications in streams. Environ. Toxicol. Chem., 24(5): 1 146–1 154.
Wang B, Huang J, Lu Y, Arai S, Iino F, Morita M, Yu G. 2012a. The pollution and ecological risk of endosulfan in soil of Huai’an city, China. Environ. Monit. Assess., 184(12): 7 093–7 101.
Wang D L, Zuo D, Wang L M, Sun T, Wang Q, Zhao Y L. 2012b. Effects of white spot syndrome virus infection on immuno-enzyme activities and ultrastructure in gills of Cherax quadricarinatus. Fish. Shellfish Immun., 32(5): 645–650.
Werner E. 1994. Ontogenetic scaling of competitive relations: size-dependent effects and responses in two anuran larvae. Ecology, 75(1): 197–213.
Wirth E F, Lund S A, Fulton M H, Scott G I. 2001. Determination of acute mortality in adults and sublethal embryo responses of Palaemonetes pugio to endosulfan and methoprene exposure. Aquatic Toxicol., 53(1): 9–18.
Zhong L, Chao L, Yang W, Liang P, Shi X, Gao X. 2011. Effects of imidacloprid and omethoate on the activity of peroxidase, glutathione reductase and catalase in wheat seedlings. Chinese J. Pestic Sci., 13(3): 276–280. (in Chinese with English abstract)
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by Key Project of Shanghai Municipal Science and Technology Commission, China (No. 11391901400)
Rights and permissions
About this article
Cite this article
Dai, X., Xiong, Z., Xie, J. et al. Acute toxicity of organochlorine insecticide endosulfan to the giant freshwater prawn Macrobrochium rosenbergii . Chin. J. Ocean. Limnol. 32, 111–119 (2014). https://doi.org/10.1007/s00343-014-3081-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-014-3081-y