Abstract
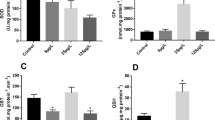
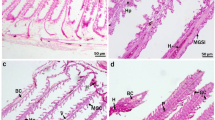
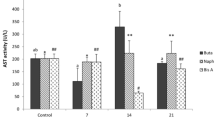
The effect of the organophosphate fenitrothion (FS) on the non-target freshwater prawn Palaemonetes argentinus was studied. Initially, the 96-h lethal concentration (LC50) of FS was determined in adult prawns. Inhibition of cholinesterase (ChE) in the muscle and hemolymph was assessed. Then, in the hepatopancreas, the activities of superoxide dismutase (SOD), catalase (CAT), and glutathione-S-transferase (GST) were analyzed. Lipid peroxidation (LPO) was also determined in the hepatopancreas. The 96-h LC50 value was 1.12 μg/L. Hemolymph ChE activity showed a significant decrease in exposed prawns to FS compared to the control group, while no significant differences in the muscle were observed between groups (p < 0.05). FS caused a significant increase in the activity of antioxidant enzymes SOD, CAT, and GST compared to the control group (p < 0.02). By contrast, LPO levels were not affected by the pesticide (p < 0.05). These results indicate that P. argentinus is very sensitive to organophosphorus which alter biochemical parameters that are related to antioxidant status. Thus, these parameters could be used as biomarkers for assessing water pollution.



Similar content being viewed by others
References
Aebi, H. (1984). Catalase in vitro. Methods in Enzymology, 105, 121–126.
Bianchini, A., & Monserrat, J. M. (2007). Effects of methyl parathion on Chasmagnathus granulatus hepatopancreas: protective role of sesamol. Ecotoxicology and Environmental Safety, 67, 100–108.
Blat, A., Almar, M. M., & Romero, F. J. (1988). The effect of two sulphur-containing pesticides, fenitrothion and endosulphan, on glutathione (GSH) content and on GSH S-transferase and gamma-glutamyl transpeptidase activities in midgut gland of the American red crayfish Procambarus clarkii. Drug Metabolism Drug Interaction, 6, 383–394.
Boschi, E. E. (1981). Decapoda: Natantia. In E. E. Boschi (Ed.), Fauna Argentina de agua dulce (pp. 19–61). Buenos Aires: FECIC.
Brown, M., Davies, I. M., Moffat, C. F., Redshaw, J., & Craft, J. A. (2004). Characterisation of choline esterases and their tissue and subcellular distribution in mussel (Mytilus edulis). Marine Environmental Research, 57, 155–169.
Buege, J. A., & Aust, S. D. (1978). Microsomal lipid peroxidation. Methods in Enzymology, 52, 302–309.
Chang, C., Lee, P., Liu, C., & Cheng, W. (2006). Trichlorfon, an organophosphorus insecticide, depresses the immune responses and resistance to Lactococcus garvieae of the giant freshwater prawn Macrobrachium rosenbergii. Fish Shellfish Immunology, 20, 574–585.
Chiodi Boudet, L., Polizzi, P., Romero, M., Robles, A., & Gerpe, M. (2013). Lethal and sublethal effects of cadmium in the white shrimp Palaemonetes argentinus: a comparison between populations from contaminated and reference sites. Ecotoxicology and Environmental Safety, 89, 52–58.
Collins, P. (1999). Feeding of Palaemonetes argentinus (Nobili) (Decapoda: Palaemonidae) in flood valley of river Parana Argentina. Journal of Crustacean Biology, 19, 485–492.
Collins, P., & Cappello, S. (2006). Cypermethrin toxicity to aquatic life: bioassays for the freshwater prawn Palaemonetes argentinus. Archives of Environmental Contamination and Toxicology, 51, 79–85.
Collins, P., & Paggi, J. C. (1998). Feeding ecology of Macrobrachium borellii (Nobili) (Decapoda: Palaemonidae) in flood valley of river Parana Argentina. Hydrobiologia, 362, 21–30.
Crane, M., Sildanchandra, W., Kheir, R., & Callaghan, A. (2002). Relationship between biomarker activity and developmental endpoints in Chironomus riparius Meigen exposed to an organophosphate insecticide. Ecotoxicology and Environmental Safety, 53, 361–369.
Damasio, J., Guilhermino, L., Soares, A. M., Riva, M. C., & Barata, C. (2007). Biochemical mechanisms of resistance in Daphnia magna exposed to the insecticide fenitrothion. Chemosphere, 70, 74–82.
Day, K., & Scott, I. (1990). Use of AChE to detect sublethal toxicity in stream invertebrates exposed to low concentrations of organophosphorus pesticides. Aquatic Toxicology, 18, 101–104.
Delatte, H., Paupy, C., Dehecq, J. S., Thiria, J., Failloux, A. B., & Fontenille, D. (2008). Aedes albopictus, vector of chikungunya and dengue viruses in Reunion Island: biology and control. Parasite Journal Society Francaise of Parasitology, 15, 3–13.
Devi, K. P., Pandian, S. K., & Kumar, N. S. (2005). Cholinesterase activity in clam Meretrix casta: possible biomarker for organophosphate pesticide pollution. Bulletin of Environmental Contamination and Toxicology, 74, 250–255.
Di Giulio, R. T., Benson, W. H., Sanders, B. M., & Van Veld, P. A. (1995). Biochemical mechanisms: Metabolism, adaptation, and toxicity. In G. M. Rand (Ed.), Fundamentals of aquatic toxicology: Effects, environmental fate, and risk assessment (pp. 523–561). Bristol: Taylor & Francis.
Dias Bainy, A. C. (2000). Biochemical responses in penaeids caused by contaminants. Aquaculture, 191, 163–168.
Domingues, I., Satapornvanit, K., Yakupitiyage, A., Soares, A. M. V. M., & Nogueira, A. J. A. (2008). In situ assay with the midge Kiefferulus calligaster for contamination evaluation in aquatic agro-systems in central Thailand. Chemosphere, 71, 1877–1887.
Drach, P., & Tchernigovtzeff, C. (1967). Sur le méthode de la détermination des stades d’intermue et son application générale aux Crustacés. Vie et Milieu., 18, 595–610.
Ellman, G. L., Courtney, K. O., Anders, V., & Featherstone, R. M. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemistry and Pharmacology, 7, 88–95.
Escartin, E., & Porte, C. (1996). Acetylcholinesterase inhibition in the crayfish Procambarus clarkii exposed to fenitrothion. Ecotoxicology and Environmental Safety, 34, 160–164.
Finney, D. J. (1971). Probit analysis. New York: Cambridge University Press.
Frasco, M. F., Fournier, D., Carvalho, F., & Guilhermino, L. (2006). Cholinesterase from the common prawn (Palaemon serratus) eyes: catalytic properties and sensitivity to organophosphate and carbamate compounds. Aquatic Toxicology, 77, 412–421.
Fulton, M. H., & Key, P. B. (2001). Acetylcholinesterase inhibition in estuarine fish and invertebrates as an indicator of organophosphorus insecticide exposure and effects. Environmental Toxicology and Chemistry, 20, 37–45.
Gagnaire, B., Geffard, O., Xuereb, B., Margoum, C., & Garric, J. (2008). Cholinesterase activities as potential biomarkers: characterization in two freshwater snails, Potamopyrgus antipodarum (Mollusca, Hydrobiidae, Smith 1889) and Valvata piscinalis (Mollusca, Valvatidae, Muller 1774). Chemosphere, 71, 553–560.
García-de la Parra, L. M., Bautista-Covarrubias, J. C., Rivera-de la Rosa, N., Betancourt-Lozano, M., & Guilhermino, L. (2006). Effects of methamidophos on acetylcholinesterase activity, behavior, and feeding rate of the white shrimp (Litopenaeus vannamei). Ecotoxicology and Environmental Safety, 65, 372–380.
González- Baró, M. R., Garda, H., & Pollero, R. (1997). Effect of fenitrothion on hepatopancreas microsomal membrane fluidity in Macrobrachium borellii. Pestlclde Blochemtstry and Physiology, 58, 133–143.
Graça, M., Rodrigues, C. A., Ocón, C. S., & Gómez, N. (2002). In situ tests for water quality assessment: a case study in Pampean rivers. Water Research, 36, 4033–4040.
Habig, C., & Di Giulio, R. D. (1991). Biochemical characteristics of cholinesterases in aquatic organisms. In P. Mineau (Ed.), Cholinesterase inhibiting insecticides: Their impact on wildlife and the environment (Chemicals in agriculture, Vol. 2, pp. 19–34). New York: Elsevier.
Habig, W., Pabst, M. J., & Jakoby, W. B. (1974). Glutathione-S-transferases. Journal of Biology and Chemistry, 22, 7130–7139.
Karami-Mohajeri, S., & Abdollahi, M. (2010). Toxic influence of organophosphate, carbamate and organochlorine pesticides on cellular metabolism of lipids, proteins and carbohydrates: a systematic review. Human Experimental Toxicology, 30, 1119–1140.
Key, P. B., & Fulton, M. H. (2002). Characterization of cholinesterase activity in tissues of the grass shrimp (Palaemonetes pugio). Pesticide Biochemistry and Physiology, 72, 186–192.
Key, P., Wirth, E., & Fulton, M. (2006). A review of grass shrimps as, Palaemonetes spp., as a bioindicator of anthropogenic impacts. Environmental Bioindicators, 1, 115–128.
Kristoff, G., Barrionuevo, D. C., Cacciatore, L. C., Verrengia Guerrero, N. R., & Cochon, A. C. (2012). In vivo studies on inhibition and recovery of B-esterase activities in Biomphalaria glabrata exposed to azinphos-methyl: analysis of enzyme, substrate and tissue dependence. Aquatic Toxicology, 112-113, 19–26.
Lacorte, S., Lartiges, S. B., Garrigues, P., & Barcelo, D. (1995). Degradation of organophosphorus pesticides and their transformation products in estuarine waters. Environmental Science of Technology, 29, 431–438.
Lavarías, S., García, F., Crespo, R., Pedrini, N., & Heras, H. (2013). Study of biochemical biomarkers in freshwater prawn Macrobrachium borellii (Crustacea: Palaemonidae) exposed to organophosphate fenitrothion. Ecotoxicology and Environmental Safety, 96, 10–16.
Leboulanger, C., Schwartz, C., Somville, P., Diallo, A. O., & Pagano, M. (2011). Sensitivity of two Mesocyclops (Crustacea, Copepoda, Cyclopidae), from tropical and temperate origins, to the herbicides, diuron and paraquat, and the insecticides, temephos and fenitrothion. Bulletin of Environmental Contamination and Toxicology, 87, 487–493.
Lee, Y. M., Park, T. J., Jung, S. O., Seo, J. S., Park, H. G., Hagiwara, A., Yoon, Y. D., & Lee, J. S. (2006). Cloning and characterization of glutathione S-transferase gene in the intertidal copepod Tigriopus japonicus and its expression after exposure to endocrine-disrupting chemicals. Marine Environmental Research, 62, 219–223.
Leelaja, B. C., & Rajini, P. S. (2013). Biochemical and physiological responses in aenorhabditis elegans exposed to sublethal concentrations of the organophosphorus insecticide, monocrotophos. Ecotoxicology and Enviromental Safety, 94, 8–13.
Lignot, J. H., Charmantier, G., & Cochard, J. C. (1998). Effect of an organophosphorous insecticide, fenitrothion, on survival, osmoregulation, and acetylcholinesterase activity in different life stages of two penaeid shrimps: Penaeus stylirostris and Penaeus vannamei (Crustacea Decapoda). Journal of Shellfish Research, 17, 1251–1258.
Liu, H., Yuan, B., & Li, S. (2012). Altered quantities and in vivo activities of cholinesterase from Daphnia magna in sub-lethal exposure to organophosphorus insecticides. Ecotoxicology and Environmental Safety, 80, 118–125.
Lowry, O. H., Rosenbrough, N. J., Farr, A. L., & Randall, R. (1951). Protein measurement with the Folin phenol reagent. Journal of Biology and Chemistry, 193, 265–275.
Misra, H. P., & Fridovich, I. (1972). The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. Journal of Biology and Chemistry, 247, 3170–3175.
Monserrat, J. M., Bianchini, A., & Rebelo, M. (1997). Toxicity and anticholinesterase effect of formulated methyl parathion to the estuarine crab Chasmagnathus granulata (Decapoda, Grapsidae) pre-exposed to sesamol. Comparative Biochemistry and Physiology. C, 118(3), 329–334.
Monserrat, J. M., Martínez, P. E., Geracitano, L. A., Amado, L. L., Martins, C. M. G., Lopes Leães Pinho, G., Soares Chaves, I. S., Ferreira-Cravo, M., Ventura-Lima, J., & Bianchini, A. (2007). Pollution biomarkers in estuarine animals: critical review and new perspectives. Comparative Biochemistry and Physiology. C, 146, 221–234.
Montagna, M., & Collins, P. (2007). Survival and growth of Palaemonetes argentinus (Decapoda; Caridea) exposed to insecticides with chlorpyrifos and endosulfan as active element. Archives of Environmental Contamination and Toxicology, 53, 371–378.
Mugni, H., Ronco, A., & Bonetto, C. (2011). Insecticide toxicity to Hyalella curvispina in run off and stream water within a soybean farm (Buenos Aires, Argentina). Ecotoxicology and Environmental Safety, 74, 350–354.
Ohkawa, H., Ohishi, N., & Yagi, K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analiytical Biochemistry, 95, 351–358.
Oliveira, C., Almeida, J. R., Guilhermino, L., Soares, A. M. V. M., & Gravato, C. (2013). Swimming velocity, avoidance behavior and biomarkers in Palaemon serratus exposed to fenitrothion. Chemosphere, 90, 936–944.
Petersen, D. R., & Doorn, J. A. (2004). Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radical Biology and Medicine, 37, 937–945.
Porte, C., & Escartin, E. (1998). Cytochrome P450 system in the hepatopancreas of the red swamp crayfish Procambarus clarkii: a field study. Comparative Biochemistry and Physiology. C, 121, 333–338.
Rivadeneira, P. R., Agrelo, M., Otero, S., & Kristoff, G. (2013). Different effects of subchronic exposure to low concentrations of the organophosphate insecticide chlorpyrifos in a freshwater gastropod. Ecotoxicology and Environmental Safety, 90, 82–88.
Rodrigues Capítulo, A. (1984). Efecto de los detergentes aniónicos sobre la supervivencia y tasa de metabolismo energético de Palaemonetes argentinus Nobili (Decapoda Natantia). Limnobios, 2, 549–555.
Sanchez-Hernandez, J. C. (2007). Ecotoxicological perspectives of B-esterases in the assessment of pesticide contamination. In R. H. Plattenberg (Ed.), Environmental pollution: New research (pp. 1–45). NY: Nova Science Publishers, Inc.
Sanchiricoa, R., Pintob, G., Polliob, A., Cordellac, M., & Cozzanic, V. (2012). Thermal degradation of fenitrothion: identification and eco-toxicity of decomposition products. Journal of Hazardous Material, 200, 390–400.
Sierra, C., Guevara, J., Lascurain, R., Perez, A., Agundis, C., Zenteno, E., & Vazquez, L. (2001). Sialylation is modulated through maturation in hemocytes from Macrobrachium rosenbergii. Comparative Biochemistry and Physiology. C, 130, 179–189.
Sukhotin, A. A., Abele, D., & Pörtner, H. (2002). Growth, metabolism and lipid peroxidation in Mytilus edulis: age and size effects. Marine Ecology Progress Series, 226, 223–234.
Symons, P. E. K. (1976). Dispersal and toxicology of the insecticide fenitrothion predicting hazards of forest spraying. Residue Reviews, 68, 1–36.
Takimoto, Y., Oshima, M., & Miyamoto, J. (1987). Comparative metabolism of fenitrothion in aquatic organisms. III. Metabolism in crustaceans. Daphnia pulex and Palaemon pausidens. Ecotoxicology and Environmental Safety, 13, 126–134.
Vijayavel, K., Gomathi, R. D., Durgabhavani, K., & Balasubramanian, M. P. (2004). Sublethal effect of naphthalene on lipid peroxidation and antioxidant status in the edible marine crab Scylla serrata. Marine Pollution Bulletin, 48, 429–433.
Vioque-Fernández, A., de Almeida, E. A., & López-Barea, J. (2007). Esterases as pesticide biomarkers in crayfish (Procambarus clarkii, Crustacea): tissue distribution, sensitivity to model compounds and recovery from inactivation. Comparative Biochemistry and Physiology. C, 145, 404–12.
Xuereb, B., Noury, P., Felten, V., Garric, J., & Geffard, O. (2007). Cholinesterase activity in Gammarus pulex (Crustacea Amphipoda): characterization and effects of chlorpyrifos. Toxicology, 236, 178–189.
Yaqin, K., & Hansen, P. D. (2010). The use of cholinergic biomarker, cholinesterase activity of blue mussel Mytilus edulis to detect the effects of organophosphorous pesticides. African Journal of Biochemistry Research, l., 4, 265–272.
Zayed, S. M. A. D., & Mahdy, F. (2008). Decomposition of 14C-fenitrothion under the influence of UV and sunlight under tropical and subtropical conditions. Chemosphere, 70, 1653–1659.
Acknowledgments
This work was partially supported by grants from CONICET (PIP 5888 and PIP 075) and Agencia Nacional de Promoción Científica y Técnica, Argentina (PICT 25.794 and PICT 1601), through grants to M. Cunningham and F. Garcia. We are grateful to G. Tarelli from GLEBA for supplying the FS and to Norma Tedesco and M. Caviglia for the language revision. The authors are members of CONICET, Argentina.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lavarías, S., García, C.F. Acute toxicity of organophosphate fenitrothion on biomarkers in prawn Palaemonetes argentinus (Crustacea: Palaemonidae). Environ Monit Assess 187, 65 (2015). https://doi.org/10.1007/s10661-014-4224-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-014-4224-5