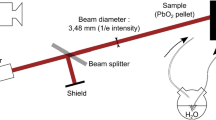
Abstract
Lead white, a mixture of cerussite (PbCO3) and hydrocerussite (2PbCO3·Pb(OH)2), is the most ancient and common white pigment used in mural paintings. However, it tends to blacken with time due to its oxidation to plattnerite (β-PbO2). Chemical treatments were used but they can put the pictorial layers supports at risks. Hereby, we address the possibility of thermally reconverting black plattnerite to white lead carbonates via a massicot (β-PbO) intermediate. We first investigated the conditions (temperature, time, and environment) in which pure powders react, before studying mural painting samples. Experiments were made in ovens and thermogravimetric analysis (TGA), X-ray diffraction (XRD) and scanning electron microscopy (SEM) characterisation were achieved. Litharge (α-PbO) and massicot were obtained from plattnerite, respectively, between 564 and 567 °C and at 650 °C. Lead carbonates, namely cerussite, hydrocerussite and plumbonacrite (3PbCO3·Pb(OH)2·PbO) formed from massicot in wet CO2 below 100 °C in a few hours. Lastly, when heating plattnerite-based mural painting samples, lead species reacted with binders and mortar, yielding massicot, plumbonacrite but also lead silicate and calcium lead oxides. This demonstrates the viability of thermal reconversion of darkened lead in mural, while raising concerns about the formation of several lead species by reaction with mural painting constituents.




Similar content being viewed by others
Notes
Reconversion is hereby used in the sense of a chemical reaction (conversion) taking place backward. Here the conversion is the oxidation to plattnerite; the reconversion is thus the formation of cerussite or hydrocerussite (white) or minium (red) from plattnerite (black) [5].
References
E. W. Fitzhugh, ‘Red Lead and Minium’, in Artists’ Pigments: A Handbook of Their History and Characteristics, vol. 1, R. L. Feller, Ed. Washington: National Gallery of Art, 1986, pp. 109–139. [Online]. Available: https://www.nga.gov/content/dam/ngaweb/research/publications/pdfs/artists-pigments-vol1.pdf
R. J. Gettens, H. Kühn, and W. T. Chase, ‘Lead White’, in Artists’ Pigments: A Handbook of Their History and Characteristics, vol. 2, A. Roy, Ed. Washington: National Gallery of Art, 1993, pp. 67–81. [Online]. Available: https://www.nga.gov/content/dam/ngaweb/research/publications/pdfs/artists-pigments-vol2.pdf
C. d’Andrea di Cennini, ‘On the Character of White Lead. Chapter LVIIII’, in The Craftsman’s Handbook. The Italian ‘Il Libro dell’ Arte.’ Translated by Daniel V. Thompson, Jr, New York: Dover Publications, Inc, 1933, p. 34. [Online]. Available: http://www.noteaccess.com/Texts/Cennini/2.htm
F. Baldinucci, ‘Biacca’, Vocabolario Toscano dell’Arte del Disegno. Firenze, p. 21, 1681. [Online]. Available: ark:/12148/bpt6k9762233v
S.M. Lussier, G.D. Smith, A review of the phenomenon of lead white darkening and its conversion treatment. Stud. Conserv. 52(sup1), 41–53 (2007). https://doi.org/10.1179/sic.2007.52.Supplement-1.41
E. Kotulanová, P. Bezdička, D. Hradil, J. Hradilová, S. Švarcová, T. Grygar, Degradation of lead-based pigments by salt solutions. J. Cult. Herit. 10(3), 367–378 (2009). https://doi.org/10.1016/j.culher.2008.11.001
J.P. Petushkova, N.N. Lyalikova, Microbiological degradation of lead-containing pigments in mural paintings. Stud. Conserv. 31(2), 65 (1986). https://doi.org/10.2307/1506003
M. Vagnini et al., Investigation on the process of lead white blackening by Raman spectroscopy, XRD and other methods: Study of Cimabue’s paintings in Assisi. Vib. Spectrosc. 98, 41–49 (2018). https://doi.org/10.1016/j.vibspec.2018.07.006
M. Vagnini, R. Vivani, A. Sgamellotti, C. Miliani, Blackening of lead white: Study of model paintings. J. Raman Spectrosc. (2020). https://doi.org/10.1002/jrs.5879
C. Aibéo, E. M. Castellucci, M. Matteini, B. Sacchi, A. Zoppi, and C. Lofrumento, ‘A micro-Raman spectroscopy study of the formation of lead dioxide from lead white’, in Art Technology: Sources and Methods, London, 2008, pp. 138–140.
S. Aze, J.-M. Vallet, M. Pomey, A. Baronnet, O. Grauby, Red lead darkening in wall paintings: natural ageing of experimental wall paintings versus artificial ageing tests. Eur. J. Mineral. 19(6), 883–890 (2007). https://doi.org/10.1127/0935-1221/2007/0019-1771
C. Prasartset, ‘Materials and techniques of Thai wall paintings: a comparative study of late 19th century murals and early-period murals’, in ICOM Committee for Conservation, 11th triennial meeting, Edinburgh, Scotland, 1-6 September 1996 : preprints, Edinburgh, 1996, pp. 430–434.
D. Saunders, M. Spring, and C. Higgitt, ‘Colour change in red lead-containing paint films’, in ICOM Committee for Conservation, ICOM-CC : 13th Triennial Meeting, Rio de Janeiro, 22-27 September 2002 : preprints, London, 2002, pp. 455–463.
S. Giovannoni, M. Matteini, A. Moles, Studies and developments concerning the problem of altered lead pigments in wall painting. Stud. Conserv. 35(1), 21–25 (1990). https://doi.org/10.1179/sic.1990.35.1.21
M. Matteini and A. Moles, ‘Recupero di un pigmento modificato, la bianca di piombo, mediante un trattamento chemico’, in Metodo e Scienza, Firenze 23 giugno 1982 - 6 gennaio 1983, pp. 253–256.
M. Matteini, ‘Ossidazione della Biacca in pitture murali. Metodi proposti per la riconversione del pigmento nelle pitture di A. Baldovinetti nella Chiesa di S. Miniato (Firenze)’, in Atti del convegno sul restauro delle opere d’arte. Firenze, 2–7 novembre 1976, Firenze, 1976, pp. 257–269; 527–529.
I. Costantini et al., Darkening of lead- and iron-based pigments on late Gothic Italian wall paintings: Energy dispersive X-ray fluorescence, μ-Raman, and powder X-ray diffraction analyses for diagnosis: presence of β-PbO 2 (plattnerite) and α-PbO 2 (scrutinyite). J. Raman Spectrosc. 51(4), 680–692 (2020). https://doi.org/10.1002/jrs.5817
M. Dneprovskaya, Analysis of medieval fresco pigments from the Georgian republic. MRS Proc. 267, 889 (1992). https://doi.org/10.1557/PROC-267-889
M.B. Dneprovskaya, Medieval pigment and plaster technology in the XII-XIII century mural paintings at David-Garedji, Georgia. MRS Proc. 352, 727 (1995). https://doi.org/10.1557/PROC-352-727
L. de Ferri, F. Mazzini, D. Vallotto, G. Pojana, In situ non-invasive characterization of pigments and alteration products on the masonry altar of S. Maria ad Undas (Idro, Italy). Archaeol. Anthropol. Sci. 11(2), 609–625 (2019). https://doi.org/10.1007/s12520-017-0550-1
V. Fassina, M. Mazza, and A. Naccari, ‘Indagini preliminari sulla tecnica pittorica e sullo stato di conservazione dei dipinti murali della Chiesa della Difesa di Vigo di Cadore (BL)’, in Scienza e beni culturali, 21, Bressanone, 2005, pp. 775–786.
L. Chupin, ‘Rôle de l’environnement dans le noircissement des peintures au blanc de plomb’, Master thesis, Centre Interrégional de Conservation et Restauration du Patrimoine, Marseille, 2011.
C. Degrigny et al., Technical study of Germolles’ wall paintings: the inputof imaging technique. Virtual Archaeol. Rev. 7(15), 1 (2016). https://doi.org/10.4995/var.2016.5831
M. Koller, H. Leitner, H. Paschinger, Reconversion of altered lead pigments in alpine mural paintings. Stud. Conserv. 35(1), 15–20 (1990). https://doi.org/10.1179/sic.1990.35.1.15
J. Trovão, F. Gil, L. Catarino, F. Soares, I. Tiago, A. Portugal, Analysis of fungal deterioration phenomena in the first Portuguese King tomb using a multi-analytical approach. Int. Biodeterior. Biodegrad. 149, 104933 (2020). https://doi.org/10.1016/j.ibiod.2020.104933
P. Bøllingtoft and M. C. Christensen, ‘Early Gothic wall paintings: an investigation of painting techniques and materials of 13th-century mural paintings in a Danish village church’, in ICOM Committee for Conservation tenth triennial meeting, Washington, DC, 22-27 August 1993: preprints, Paris, 1993, pp. 531–535.
R. H. Brill, C. Felker-Dennis, H. Shirahata, and E. C. Joel, ‘Lead Isotope Analyses of Some Chinese and Central Asian Pigments’, in Conservation of Ancient Sites on the Silk Road Los Angeles, Los Angeles, 1997, pp. 369–378. [Online]. Available: https://www.cmog.org/sites/default/files/collections/9D/9DB363B9-B3B8-4C29-8529-A3D12D6B3775.pdf
S. Daniilia et al., Panselinos’ Byzantine wall paintings in the Protaton Church, Mount Athos, Greece: a technical examination. J. Cult. Herit. 1(2), 91–110 (2000). https://doi.org/10.1016/S1296-2074(00)00164-3
M. Matteini and A. Moles, ‘The Reconversion of Oxidised White Lead in Mural Paintings: A Control after a Five Year Period’, in Preprints, Ottawa, 1981, vol. 1, p. 81/15/ 1–8.
M. Matteini, A. Moles, and S. Giovannoni, ‘The reconversion of Oxidised white lead in the paintings by Signorelli in the Abbey of Monteoliveto Maggiore. Study of the phenomenon and preliminary applications’, in Scientific methodologies applied to works of art, Florence, 1986, pp. 113–115.
S. Aze, P. Delaporte, J.-M. Vallet, V. Detalle, O. Grauby, A. Baronnet, Towards the restoration of darkened red lead containing mural paintings: A preliminary study of the β-PbO2 to Pb3O4 reversion by laser irradiation. Proc. Int. Conf. LACONA VII Madrid 21(09), 11–13 (2007). https://doi.org/10.1201/9780203882085
S. Aze, J.-M. Vallet, V. Detalle, and O. Grauby, ‘Reversion of darkened red lead-containing wall paintings by means of cw-laser irradiation: In situ tests and first application’, Lasers Conserv. Artworks VIII, p. 129, 2010.
T. de Seauve et al., Continuous wave laser thermal restoration of Oxidised lead-based pigments in mural paintings. Appl. Phys. B 127(12), 162 (2021). https://doi.org/10.1007/s00340-021-07702-w
P. Bromblet, M. Labouré, G. Orial, Diversity of the cleaning procedures including laser for the restoration of carved portals in France over the last 10 years. J. Cult. Herit. 4, 17–26 (2003). https://doi.org/10.1016/S1296-2074(02)01222-0
C. Rodriguez-Navarro et al., Laser cleaning of stone materials: an overview of current research. Stud. Conserv. 48(sup1), 65–82 (2003). https://doi.org/10.1179/sic.2003.48.Supplement-1.65
S. Siano et al., Laser cleaning in conservation of stone, metal, and painted artifacts: state of the art and new insights on the use of the Nd:YAG lasers. Appl. Phys. A 106(2), 419–446 (2012). https://doi.org/10.1007/s00339-011-6690-8
S. Aze, J.-M. Vallet, A. Baronnet, O. Grauby, The fading of red lead pigment in wall paintings: tracking the physico-chemical transformations by means of complementary micro-analysis techniques. Eur. J. Mineral. 18(6), 835–843 (2006). https://doi.org/10.1127/0935-1221/2006/0018-0835
P. Taylor, V.J. Lopata, Stability and solubility relationships between some solids in the system PbO–CO2–H2O. Can. J. Chem. 62(3), 395–402 (1984). https://doi.org/10.1139/v84-070
J.R. Clarke, J.E. Greene, Reactively evaporated photoconductive PbO: phase transformations induced by water vapor. Thin Solid Films 66(3), 339–349 (1980). https://doi.org/10.1016/0040-6090(80)90387-9
W. Mu et al., Adsorption of CO2 on PbO at ambient temperature. Appl. Surf. Sci. 258(2), 950–954 (2011). https://doi.org/10.1016/j.apsusc.2011.09.034
M.I. Cooper, P.S. Fowles, C.C. Tang, Analysis of the laser-induced discoloration of lead white pigment. Appl. Surf. Sci. 201(1–4), 75–84 (2002). https://doi.org/10.1016/S0169-4332(02)00499-3
R. Bruder, D. L’Hermite, A. Semerok, L. Salmon, V. Detalle, Near-crater discoloration of white lead in wall paintings during laser induced breakdown spectroscopy analysis. Spectrochim. Acta Part B At. Spectrosc. 62(12), 1590–1596 (2007). https://doi.org/10.1016/j.sab.2007.10.031
T. Stratoudaki, A. Manousaki, K. Melesanaki, V. Zafiropulos, G. Orial, Study on the discolouration of pigments induced by laser irradiation. Rev. Métallurgie 98(9), 795–801 (2001). https://doi.org/10.1051/metal:2001125
V. Zafiropulos, T. Stratoudaki, A. Manousaki, K. Melesanaki, G. Orial, Discoloration of pigments induced by laser irradiation. Surf. Eng. 17(3), 249–253 (2001). https://doi.org/10.1179/026708401101517773
P. Pouli, D.C. Emmony, C.E. Madden, I. Sutherland, Analysis of the laser-induced reduction mechanisms of medieval pigments. Appl. Surf. Sci. 173(3–4), 252–261 (2001). https://doi.org/10.1016/S0169-4332(00)00909-0
M. Chappé, J. Hildenhagen, K. Dickmann, M. Bredol, Laser irradiation of medieval pigments at IR, VIS and UV wavelengths. J. Cult. Herit. 4, 264–270 (2003). https://doi.org/10.1016/S1296-2074(02)01206-2
G.L. Clark, N.C. Schieltz, T.T. Quirke, A new study of the preparation and properties of the higher oxides of lead. J. Am. Chem. Soc. 59(11), 2305–2308 (1937). https://doi.org/10.1021/ja01290a063
E.M. Otto, Equilibrium pressures of oxygen over oxides of lead at various temperatures. J. Electrochem. Soc. 113(6), 525 (1966). https://doi.org/10.1149/1.2424016
S. Kumar, M. Sharon, S.R. Jawalekar, Preparation of a thin film of Pb3O4 by thermal treatment of PbO2 film. Thin Solid Films 195(1–2), 273–278 (1991). https://doi.org/10.1016/0040-6090(91)90278-6
D.A. Ciomartan, R.J.H. Clark, L.J. McDonald, M. Odlyha, Studies on the thermal decomposition of basic lead(II) carbonate by Fourier-transform Raman spectroscopy, X-ray diffraction and thermal analysis. J. Chem. Soc. Dalton Trans. 18, 3639–3645 (1996). https://doi.org/10.1039/dt9960003639
V.V. Aleksandrov, V.V. Boldyrev, V.V. Marusin, V.G. Morozov, V.S. Solovjev, T.M. Rozhentseva, Effect of heating rate on the thermal decomposition of lead dioxide. J. Therm. Anal. 13(2), 205–212 (1978). https://doi.org/10.1007/BF01912292
H.A. Wriedt, The O−Pb (Oxygen-Lead) system. J. Phase Equilibria 9(2), 106–127 (1988). https://doi.org/10.1007/BF02890543
K. Gavrichev, A. Bolshakov, D. Kondakov, A. Khoroshilov, S. Denisov, Thermal transformations of lead oxides. J. Therm. Anal. Calorim. 92(3), 857–863 (2008). https://doi.org/10.1007/s10973-007-8590-x
D. Risold, J.-I. Nagata, R.O. Suzuki, Thermodynamic description of the Pb-O system. J. Phase Equilibria 19(3), 213–233 (1998). https://doi.org/10.1361/105497198770342238
C. Real, M. Alcala, J. Criado, Correlation between the structural defects induced by ball-milling of Pb3O4 and the structure of PbO yielded from its thermal decomposition. Solid State Ion. 63–65, 702–706 (1993). https://doi.org/10.1016/0167-2738(93)90183-4
P. Boher, P. Garnier, J.R. Gavarri, A.W. Hewat, Monoxyde quadratique PbOα(I): description de la transition structurale ferroélastique. J. Solid State Chem. 57(3), 343–350 (1985). https://doi.org/10.1016/0022-4596(85)90197-5
R.J. Hill, Refinement of the structure of orthorhombic PbO (massicot) by Rietveld analysis of neutron powder diffraction data. Acta Crystallogr. C 41(9), 1281–1284 (1985). https://doi.org/10.1107/S0108270185007454
F. Platel, AntiSecos. MetGen, 2014.
T. Mendivil-Reynoso et al., Synthetic plumbonacrite thin films grown by chemical bath deposition technique. Chalcogenide Lett. 10(1), 11–17 (2013)
T. Mendivil-Reynoso, L.P. Ramírez-Rodríguez, R. Ochoa-Landín, R. Ramirez-Bon, S.J. Castillo, Synthesis and thermal annealing of plumbonacrite layers deposited by chemical bath technique. Mater. Today Commun. 25, 101676 (2020). https://doi.org/10.1016/j.mtcomm.2020.101676
F. Vanmeert, G. Van der Snickt, K. Janssens, Plumbonacrite identified by X-ray powder diffraction tomography as a missing link during degradation of red lead in a Van Gogh painting. Angew. Chem. 127(12), 3678–3681 (2015). https://doi.org/10.1002/ange.201411691
V. Gonzalez et al., Unraveling the composition of rembrandt’s impasto through the identification of unusual plumbonacrite by multimodal X-ray diffraction analysis. Angew. Chem. Int. Ed. 58(17), 5619–5622 (2019). https://doi.org/10.1002/anie.201813105
D.F. Haacke, P.A. Williams, Stability of plumbonacrite. J. Inorg. Nucl. Chem. 43(2), 406 (1981). https://doi.org/10.1016/0022-1902(81)90036-1
A. Mendoza-Flores, M. Villalobos, T. Pi-Puig, N.V. Martínez-Villegas, Revised aqueous solubility product constants and a simple laboratory synthesis of the Pb(II) hydroxycarbonates: plumbonacrite and hydrocerussite. Geochem. J. 51(4), 315–328 (2017). https://doi.org/10.2343/geochemj.2.0471
Acknowledgements
This work benefited from a state fund, managed by the french ANR as part of the France 2030 investements (ANR-17-EURE-0021 – École Universitaire de Recherche Paris Seine Humanités, Création, Patrimoine – Fondation des sciences du patrimoine). The authors wish to acknowledge the French Ministry of Culture and Communication for financial support and the French Fondation des Sciences du Patrimoine for their financial support to the RECONVERT 2 project. The authors would also like to thank Kevin Ginestar from SCCME (CEA Saclay) for the help in the TGA and oven trials, Vasile Heresanu from CINaM (Aix-Marseille Université) for the µ-XRD experiments, Sébastien Aze (Sinopia) for helping in the preparation of mural painting samples, and Xueshi Bai for the discussion and advises throughout the RECONVERT project.
Author information
Authors and Affiliations
Contributions
TS wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Seauve, T., Bosonnet, S., Grauby, O. et al. Thermal reconversion of oxidised lead white in mural paintings via a massicot intermediate. Appl. Phys. B 129, 137 (2023). https://doi.org/10.1007/s00340-023-08060-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-023-08060-5