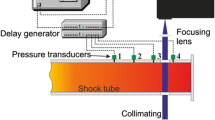
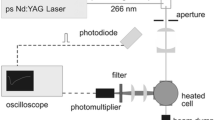
Abstract
1,4-Difluorobenzene (p-DFB) is a promising aromatic tracer for determining concentration, temperature, and O2 partial pressure in mixing gas flows based on laser-induced fluorescence (LIF). Signal quantification requires the knowledge of absorption and fluorescence properties as a function of environmental conditions. We report absorption and fluorescence spectra as well as fluorescence lifetimes of p-DFB in the temperature, pressure, and oxygen partial pressure range that is relevant for many applications including internal combustion engines. The UV absorption cross section, investigated between 296 and 675 K, has a peak value close to 266 nm and decreases with temperature, while still exceeding other single-ring aromatics. Time-resolved fluorescence spectra were recorded after picosecond laser excitation at 266 nm as a function of temperature (296–1180 K), pressure (1–10 bar), and O2 partial pressure (0–210 mbar) using a streak camera (temporal resolution 50 ps) coupled to a spectrometer. The fluorescence spectra red-shift (~2 nm/100 K) and broaden (increase in full width at half maximum by 58% in the investigated temperature range) with temperature. In N2 as bath gas (1 bar), the fluorescence lifetime τ eff decreases with temperature by a factor of about 20 (from 7 ns at 298 K down to 0.32 ns at 1180 K), while at 8 bar the shortest lifetime at 975 K is 0.4 ns. A noticeable pressure dependence (i.e., reduced τ eff) is only visible at 675 K and above. Quenching of p-DFB LIF by O2 (for partial pressures up to 210 mbar) shortens the fluorescence lifetime significantly at room temperature (by a factor of 8), but much less at higher temperatures (by a factor of 1.8 at 970 K). For fixed O2 partial pressures (52 mbar and above), τ eff shows a plateau region with temperature which shifts toward higher temperatures at the higher O2 partial pressures. O2 quenching is less prominent for p-DFB compared to other aromatic compounds investigated so far. The temperature dependence of O2 quenching can be approximately expressed by an exponential function. The influence of temperature, total pressure, and O2 partial pressure on absorption cross sections and fluorescence quantum yields are given as empirical functions that allow for interpolation. For typical applications, p-DFB LIF provides up to three orders of magnitude stronger signal compared to toluene LIF.











Similar content being viewed by others
References
C. Schulz, V. Sick, Prog. Energy Combust. Sci. 31, 75 (2005)
C.S. Burton, W.A. Noyes, J. Chem. Phys. 49, 1705 (1968)
C.G. Hickman, J.R. Gascooke, W.D. Lawrance, J. Chem. Phys. 104, 4887 (1996)
F.P. Zimmermann, W. Koban, C.M. Roth, D.-P. Herten, C. Schulz, Chem. Phys. Lett. 426, 248 (2006)
E. Friesen, C. Gessenhardt, S. Kaiser, T. Dreier, C. Schulz, In-cylinder temperature measurements via fiber-based toluene-LIF time-correlated single-photon counting (LACSEA2012, San Diego, CA, USA, 2012)
M. Luong, R. Zhang, C. Schulz, V. Sick, Appl. Phys. B. 91, 669 (2008)
W. Koban, J.D. Koch, R.K. Hanson, C. Schulz, Phys. Chem. Chem. Phys. 6, 2940 (2004)
S. Faust, T. Dreier, C. Schulz, Chem. Phys. 383, 6 (2011)
W. Koban, J.D. Koch, R.K. Hanson, C. Schulz, Appl. Phys. B 80, 777 (2005)
S. Faust, G. Tea, T. Dreier, C. Schulz, Appl. Phys. B 110, 81 (2013)
W. Koban, C. Schulz: SAE technical paper series 2005-01-2091, 2005 (2005)
L.M. Itani, G. Bruneaux, A. Di Lella, C. Schulz, Proc. Combust. Inst. 35, 2915 (2015)
J.M. Hollas, T. Cvitas, Mol. Phys. 18, 793 (1970)
J.R. Lakowicz, Principles of Fluorescence Spectroscopy (Springer, New York, 2006)
M.G. Rockley, J. Metcalfe, D. Phillips, J. Chem. Soc. Faraday Trans. 2(70), 1660 (1974)
S.A. Kaiser, M.B. Long, Proc. Combust. Inst. 30, 1555 (2005)
M. Orain, P. Baranger, B. Rossow, F. Grisch, Appl. Phys. B 102, 163 (2011)
F. Ossler, T. Metz, M. Aldén, Appl. Phys. B 72, 465 (2001)
W. Koban, J. Schorr, C. Schulz, Appl. Phys. B 74, 111 (2002)
R. Devillers, G. Bruneaux, C. Schulz, Appl. Phys. B 96, 735 (2009)
D. Fuhrmann, T. Benzler, S. Fernando, T. Endres, T. Dreier, S. A. Kaiser, C. Schulz: Proc. Combust. Inst. 36 (2016). doi:10.1016/j.proci.2016.06.045
T.B. Settersten, A. Dreizler, R.L. Farrow, J. Chem. Phys. 117, 3173 (2002)
T.B. Settersten, B.D. Patterson, J.A. Gray, J. Chem. Phys. 124, 234308 (2006)
E.K.C. Lee, L.J. Volk, J. Chem. Phys. 67, 236 (1977)
S. Faust, T. Dreier, C. Schulz, Appl. Phys. B 112, 203 (2013)
T. Benzler, S. Faust, T. Dreier, C. Schulz, Appl. Phys. B 121, 549 (2015)
Y. He, E. Pollak, J. Chem. Phys. 116, 6088 (2002)
H. Wadi, E. Pollak, J. Chem. Phys. 110, 11890 (1999)
R.A. Coveleskie, D.A. Dolson, C.S. Parmenter, J. Phys. Chem. 89, 645 (1985)
Acknowledgements
The authors acknowledge funding of this work by the Deutsche Forschungsgemeinschaft (DFG) within SCHU 1369/28. The authors also acknowledge valuable discussions with Björn Rossow (Université de Rouen) on DFB absorption cross sections and fluorescence spectra.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Benzler, T., Dreier, T. & Schulz, C. UV absorption and fluorescence properties of gas-phase p-difluorobenzene. Appl. Phys. B 123, 39 (2017). https://doi.org/10.1007/s00340-016-6612-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00340-016-6612-8