Abstract
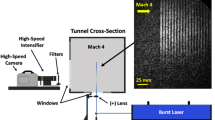
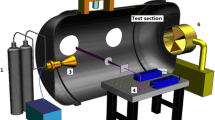
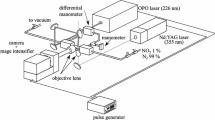
A new seeded velocity measurement technique, N2O molecular tagging velocimetry (MTV), is developed to measure velocity in wind tunnels by photochemically creating an NO tag line. Nitrous oxide “laughing gas” is seeded into the air flow. A 193 nm ArF excimer laser dissociates the N2O to O(1D) that subsequently reacts with N2O to form NO. O2 fluorescence induced by the ArF laser “writes” the original position of the NO line. After a time delay, the shifted NO line is “read” by a 226-nm laser sheet and the velocity is determined by time-of-flight. At standard atmospheric conditions with 4% N2O in air, ∼1000 ppm of NO is photochemically created in an air jet based on experiment and simulation. Chemical kinetic simulations predict 800–1200 ppm of NO for 190–750 K at 1 atm and 850–1000 ppm of NO for 0.25–1 atm at 190 K. Decreasing the gas pressure (or increasing the temperature) increases the NO ppm level. The presence of humid air has no significant effect on NO formation. The very short NO formation time (<10 ns) makes the N2O MTV method amenable to low- and high-speed air flow measurements. The N2O MTV technique is demonstrated in air jet to measure its velocity profile. The N2O MTV method should work in other gas flows as well (e.g., helium) since the NO tag line is created by chemical reaction of N2O with O(1D) from N2O photodissociation and thus does not depend on the bulk gas composition.











Similar content being viewed by others
Notes
k=AT nexp(−E/RT), reaction rates for A are in [cm3-mol-sec-K] units, T [K], E [cal/mol].
NO formation time shown in Fig. 6c is consistent with an estimate based on the (R2) rate constant of k 2=7.2×10−11 cm3/molecules [34] under standard conditions. With this rate constant, the time of formation of 63% of the steady-state NO concentration (at STP) is \(1/(2k_{2}n_{\mathrm{N}_{2}\mathrm{O}}) \approx 10\ \mbox{ns}\) where \(n_{\mathrm{N}_{2}\mathrm{O}}\) is the N2O number density after dissociation.
References
R.J. Adrian, J. Westerweel, Particle Image Velocimetry (Cambridge University Press, New York, 2010)
S. Koike, H. Takahashi, K. Tanaka, M. Hirota, K. Takita, G. Masuya, AIAA J. 45, 2770 (2007)
R.B. Miles, W.R. Lempert, J.N. Forkey, Meas. Sci. Technol. 12, R33 (2001)
M. Zimmermann, R.B. Miles, Appl. Phys. Lett. 37, 885 (1980)
J.C. McDaniel, B. Hiller, R.K. Hanson, Opt. Lett. 8, 51 (1983)
W.J. Marinelli, W.J. Kessler, M.G. Allen, S.J. Davis, S. Arepalli, C.D. Scott, in 29th AIAA Aerospace Sciences Meeting (1991), paper 91-0358
P.H. Paul, M.P. Lee, R.K. Hanson, Opt. Lett. 14, 417 (1989)
M. Allen, S. Davis, W. Kessler, H. Legner, K. McManus, P. Mulhall, T. Parker, D. Sonnenfroh, AIAA J. 32, 1676 (1994)
K.G. Klavuhn, G. Gauba, J.C. McDaniel, J. Propuls. Power 10, 787 (1994)
R.G. Seasholtz, F.J. Zupanc, S.J. Schneider, J. Propuls. Power 8, 935 (1992)
J.N. Forkey, N.D. Finkelstein, W.R. Lempert, R.B. Miles, AIAA J. 34, 442 (1996)
R.B. Miles, W. Lempert, B. Zhang, Fluid Dyn. Res. 8, 9 (1991)
R.W. Pitz, T.M. Brown, S.P. Nandula, P.A. Skaggs, P.A. DeBarber, M.S. Brown, J. Segall, Opt. Lett. 21, 755 (1996)
L.A. Ribarov, J.A. Wehrmeyer, F. Batliwala, R.W. Pitz, P.A. DeBarber, AIAA J. 37, 708 (1999)
R.W. Pitz, J.A. Wehrmeyer, L.A. Ribarov, D.A. Oguss, F. Batliwala, P.A. DeBarber, S. Deusch, P.E. Dimotakis, Meas. Sci. Technol. 11, 1259 (2000)
L.R. Boedecker, Opt. Lett. 14, 473 (1989)
D.F. Davidson, A.Y. Chang, M.D. DiRosa, R.K. Hanson, Appl. Opt. 30, 2598 (1991)
J.A. Wehrmeyer, L.A. Ribarov, D.A. Oguss, R.W. Pitz, Appl. Opt. 38, 6912 (1999)
L.A. Ribarov, J.A. Wehrmeyer, R.W. Pitz, R.A. Yetter, Appl. Phys. B 74, 175 (2002)
R.W. Pitz, M.D. Lahr, Z.W. Douglas, J.A. Wehrmeyer, S. Hu, C.D. Carter, K.Y. Hsu, C. Lum, M.M. Koochesfahani, Appl. Opt. 44, 6692 (2005)
M.D. Lahr, R.W. Pitz, Z.W. Douglas, C.D. Carter, J. Propuls. Power 26, 790 (2010)
M.C. Ramsey, R.W. Pitz, T.P. Jenkins, Y. Matsutomi, C. Yoon, W.E. Anderson, Shock Waves 22, 39 (2011)
A.F.P. Houwing, D.R. Smith, J.S. Fox, P.M. Danehy, N.R. Mudford, Shock Waves 11, 31 (2001)
P.M. Danehy, S. O’Byrne, A.F.P. Houwing, J.S. Fox, D.R. Smith, AIAA J. 41, 263 (2003)
A.G. Hsu, R. Srinivasan, R.D.W. Bowersox, S.W. North, Appl. Opt. 48, 4414 (2009)
N. Dam, R.J.H. Klein-Douwel, N.M. Sijtsema, J.J. ter Meulen, Opt. Lett. 26, 36 (2001)
C. Orlemann, C. Schulz, J. Wolfrum, Chem. Phys. Lett. 307, 15 (1999)
S. Nakaya, M. Kasahara, M. Tsue, M. Kono, Heat Transf. Asian Res. 34, 40 (2005)
A.G. Hsu, R. Srinivasan, R.D.W. Bowersox, S.W. North, AIAA J. 47, 2597 (2009)
N. Jiang, M. Nishihara, W.R. Lempert, Appl. Phys. Lett. 97, 221103 (2010)
S. Krüger, G. Grünefeld, Appl. Phys. B 69, 509 (1999)
U. Westblom, M. Aldén, Appl. Opt. 29, 4844 (1990)
C.D. Carter, R.S. Barlow, Opt. Lett. 19, 299 (1994)
M. Tsuji, J. Kumagae, T. Tsuji, T. Hamagami, J. Hazard. Mater. B 108, 189 (2004)
G. Selwyn, J. Podolske, H.S. Johnston, Geophys. Res. Lett. 4, 427 (1977)
G. Laufer, R.L. McKenzie, W.M. Huo, Opt. Lett. 13, 99 (1988)
A.M. Wodtke, L. Huwel, H. Schluter, G. Meijer, P. Andresen, H. Voges, Opt. Lett. 13, 910 (1988)
M.C. Ramsey, R.W. Pitz, Exp. Fluids 51, 811 (2011)
R. Atkinson, D.L. Baulch, R.A. Cox, R.F. Hampson Jr., J.A. Kerr, M.J. Rossi, J. Troe, J. Phys. Chem. Ref. Data 26, 1329 (1997)
R.F. Heidner III, D. Husain, Int. J. Chem. Kinet. 5, 819 (1973)
V.M. Doroshenko, N.N. Kudryavtsev, V.V. Smetanin, High Energy Chem. 26, 227 (1992)
A.A. Borisov, V.M. Zamanskii, G.I. Skachkov, Kinet. Catal. 19, 26 (1978)
W. Tsang, J.T. Herron, J. Phys. Chem. Ref. Data 20, 609 (1991)
M.S. van Hemert, R. van Harrevelt, private communication, Leiden Institute of Chemistry, Gorlaeus Laboratories, Leiden, The Netherlands (1999)
Acknowledgements
Dr. Ayman M. ElBaz is grateful to the Fulbright Egyptian Scholar Program for supporting his research experience at Vanderbilt University. RWP acknowledges support by the Air Force Office of Scientific Research, Combustion and Diagnostics Program. The authors thank Dr. Campbell Carter at the Air Force Research Laboratories for the suggesting photodissociation of N2O as a possible MTV method. We also thank Mr. Marc Ramsey at Vanderbilt University for technical advice and help in this effort.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
ElBaz, A.M., Pitz, R.W. N2O molecular tagging velocimetry. Appl. Phys. B 106, 961–969 (2012). https://doi.org/10.1007/s00340-012-4872-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-012-4872-5