Abstract
La substituted M-type Barium hexaferrite, Ba(1−x)La(x)Fe12O19, (x = 0.25 and 0.30) were synthesized using ball milling followed by heat treatment at 1150˚C and 1300˚C and the influence of sintering temperature as well as La doping were presented. X-ray diffraction, FESEM, dielectric spectroscopy, and ferroelectric measurements were performed to study the structural, morphological, dielectric, and ferroelectric properties of the prepared samples. Rietveld refinement on the X-ray diffraction measurement reveals the formation of a phase magnetoplumbite structure of barium hexaferrite only for the samples sintered at 1300˚C. The secondary phase, along with the major magnetoplumbite structure, was observed for the sample sintered at 1150˚C. Hexagonal plate-like grains of different sizes were observed, and samples sintered at 1300˚C showed large-size grains. Porosity was observed to decrease with La doping. Impedance spectroscopy reveals that Maxwell-Wagner-type polarisation occurs with the relaxation of mobile charges having long-range hopping. However, the ferroelectric property was suppressed for samples sintered at 1300˚C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
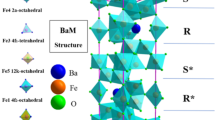
Recently, there has been considerable attraction towards hexaferrites due to their multifunctional properties, such as magnetic, dielectric, ferroelectric, microwave absorption, and optical properties. They were discovered in Phillip’s laboratories in the 1950s as an alternative material for permanent magnets [1] and are still interesting as they possess large magnetocrystalline anisotropy, high curie temperature, high saturation magnetization, large coercivity, high resistivity, good chemical, and thermal stability. Hence, they are widely used in permanent magnets, memory elements, sensors, actuators, transducers, EM shielding, optoelectronic devices, etc. [2,3,4,5,6,7]. Hexaferrites, also known as hexagonal ferrites, possess hexagonal symmetry [8]. There are different types of hexaferrites, viz., M-type, Y-type, W-type, Z-type, U-type, and X-type [9]. M-type barium hexaferrites are the most common and famous among them. Barium hexaferrites are represented by the chemical formula BaFe12O19. Two chemical formula units are required to obtain a unit cell. The lattice comprises ten layers of oxygen anions [10]. All the 12 Fe3+ ions reside in the five different crystallographic positions, viz., 12k, 4f2, 4f1, 2a, and 2b sites which are either tetrahedral, octahedral as well as trigonal bipyramidal sites created by oxygen anions. Six of twelve ferric ions are in 12k, two in 4f1 and 4f2, and one in 2a and 2b sites, respectively [11, 12].
M-type hexaferrites are known for their magnetic and multifunctional properties [9, 13,14,15]. The physical and chemical properties of M-type hexaferrite could be tuned in various ways, viz., synthesis method, synthesis parameters, as well as by substitution either to Ba2+(or Sr) or Fe3+ sites by metal cations such as transition metals, rare earths, or diamagnetic substitution [13, 14]. Rare earth elements are substituted for Ba (or Sr) in M-type hexaferrite at low concentrations, showing good magnetic properties [13, 16]. Comparable ionic radii of La3+ with Ba2+, the substitution of La has created a lot of interest among the scientific community [16]. The influence of La3+ substitution on various properties, such as structural, magnetic, optical, and electrical properties synthesized by different methods, has been studied by various groups [12, 15, 16]. However, achieving a single-phase magnetoplumbite structure(M-phase) of rare earth substituted barium hexaferrite at higher doping concentration(x > 0.20) is challenging. Many groups trying to achieve a single phase were either restricted to their work at low concentrations or not successful completely (beyond x = 0.20). For example, Ounnunkad et al. [17] prepared single phase Ba(1−x)La(x)Fe12O19, x = 0-0.20 via the citrate combustion process and studied its structural and magnetic properties. They showed magnetic properties decreased for x > 0.15. Verma et al. [18] worked on Ba(1−x)La(x)Fe12O19, x = 0-0.20, prepared by solid-state method, and they observed the secondary phase along with the major magnetoplumbite structure for La substituted compositions x > 0. Sozeri et al. [19] synthesized La substituted barium hexaferrite from x = 0.0–1.0 using ammonium nitrate melt with varying Fe/(Ba + La) ratios between 12 and 2. They couldn’t achieve the Barium hexaferrite phase above x = 0.6. However, multiple phases, along with major Barium hexaferrite phases, were observed below x = 0.6. They also observed a decrease in magnetization and increased coercivity with La substitution. Maximum coercivity was observed for the x = 0.30 concentration. Similarly, we can observe many groups, such as Liu et al. [16], synthesized Sr(1−x)La(x)Fe12O19 from x = 0.0-0.6 and achieved a single phase up to La concentration x = 0.30 and noticed different secondary phases above x = 0.30. In addition, Mossbauer spectroscopic results revealed that substituting La3+ with the Ba2+ site results in a valence change of Fe3+ from Fe2+ at 4f2 or 2a sites. Taryana et al. [20] synthesized La3+ doped barium hexaferrite(x = 0.0-0.7) using ball milling followed by solid-state reaction method, sintered at 1200˚C and studied microwave absorption efficiency and achieved single phase barium hexaferrite structure only upto La concentration x = 0.1. However, they did not analyze the effect of secondary phases or sintering temperature effects on the properties they studied. Many groups tried different synthesis methods to achieve a single-phase La substituted Barium hexaferrite at higher concentrations of La and studied the magnetic and dielectric properties [13, 21, 22]. Kumar et al. [13] synthesized La-doped barium hexaferrite, where La content, x = 0-0.20 by the sol-gel technique, and observed lattice strain-mediated magnetic and dielectric properties. They achieved a single-phase magnetoplumbite structure, but they observed that La3+ substituted the Fe3+ ions for Ba2+ ions above x > 0.10. They achieved optimal magnetic properties for the x = 0.10 sample, and dielectric permittivity increased with La substitution. Lou et al. [21] prepared Ba(1−x)La(x)Fe12O19 (x = 0, 0.2, 0.4, 0.6) hollow microspheres prepared via quenching and heat treatment method. They found that saturation magnetisation Ms increases to x = 0.20 and dielectric loss increases to x = 0.40 concentration. Mari˜no-Castellanos et al. [22] examined the effect of La3+ solubility on structural, magnetic, and dielectric properties of barium hexaferrite (BHF) for the La concentrations, x = 0, 0.1, 0.6. They observed multiple phases along with a major BaFe12O19 phase for x > 0. Magnetic properties decreased for x > 0, and electrical properties showed dependence on both La content and secondary phases.
These results reveal that the formation of La-substituted barium hexaferrite at higher substitution levels is not easy, and the effect of La and sintering temperature on its physical and electrical properties was not understood well. Sintering temperature and duration generally aid in forming the phase, grain growth, and microstructure, which affects magnetic, optical, and dielectric properties [23]. A systematic study on the variation of formation of a single phase and the effect of La3+ substitution at higher concentrations on different properties, such as electrical, optical, and dielectric properties with sintering temperature, is lacking in the present literature. Therefore, in the present work, a comparative study on structural, electrical, and ferroelectric properties of highly substituted Ba(1−x)La(x)Fe12O19 (x = 0.25 & 0.30) is carried out for two different sintering temperatures.
2 Experimental methodology
La substituted Barium hexaferrite, Ba(1−x)La(x)Fe12O19 (x = 0.25 & 0.30), was synthesized using ball milling followed by a solid-state reaction method. The lower concentrations, i.e., x = 0-0.20, were reported elsewhere [24]. The stoichiometric amounts of precursor powders, BaCO3, Fe2O3, and preheated La2O3 were weighed carefully and ground for about 3 hours using agate mortar and pestle. Mixed powders were ball milled for 8 hours using a planetary ball mill (Retsch 100) in an acetone media at a rotation speed of 250 rpm. The ball-to-powder mass ratio was kept at 8:1. Milled powders were dried, pressed into pellets, and sintered at 1150℃. The sintered pellets were crushed, ground into fine powders, and pressed into pellets. Pressed pellets were sintered at two different temperatures to study the effect of sintering temperature. Batch ‘A’ and B’ were sintered at 1150℃ and 1300℃, respectively.
The powder X-ray diffraction (XRD) measurements were carried out to study the structure and phase of all the prepared samples in the 2θ range 20°-80° at a step size of 0.020. Morphological studies (of the prepared samples) were carried out using Field Emission Scanning Electron Microscopy (FESEM) (Zeiss, Oxford Instrument) at 40kX magnification. Optical properties of the prepared samples were carried out using UV-Visible-NIR spectroscopy in diffused reflectance mode (DRS) in the wavelength range from 200 nm to 850 nm. Dielectric spectroscopy was carried out using an Impedance analyzer (E4990A, Keysight Technologies, USA) in the frequency range of 20 Hz to 20 MHz with 50mV AC voltage at room temperature. Capacitance Cp and tanδ were measured, and dielectric permittivity, ac conductivity, impedance, and electric modulus values were obtained. Ferroelectric measurements were carried out using a P-E loop tracer (Radiant Technologies).
3 Results and discussions
3.1 Structural studies
3.1.1 X-ray diffraction
X-ray diffraction patterns of all synthesized La substituted barium hexaferrite, Ba(1−x)La(x)Fe12O19, (x = 0.25 and 0.30) samples sintered at 1150℃ and 1300℃ (Batch ‘A’ and ‘B’) were Rietveld refined using ‘FullProf Suite’ software and were shown in Fig. 1. From the refinement, unindexed peaks at 31.1̊ belong to α-Fe2O3 phase were observed along with major magnetoplumbite structure in batch A samples with different phase fraction for x = 0.25 and 0.30. The α-Fe2O3 phase fraction increased with La doping. However, the same has vanished for batch B samples, which are sintered at 1300℃. The batch B samples (x = 0.25 and 0.30) show a single-phase magnetoplumbite structure of barium hexaferrite, crystallizes in a hexagonal crystal structure, and belongs to the P63/mmc space group. La has less diffusivity than Ba when La is substituted to the ‘Ba’ site. Hence, it needs more energy to diffuse and form a required phase. In the ‘A’ batch, the α-Fe2O3, an unreacted phase, is observed when La is not completely diffuse and the reaction is incomplete. When the sintering temperature increases, La gets more thermal energy to diffuse, completes the reaction, and forms the required phase. So, high-sintered samples (batch B) form a single phase.
The lattice parameters, phase fraction, and refinement parameters are listed in Table 1. The lattice parameters ‘a’ and ‘c’ and cell volume ‘V’ decreased slightly with La doping, possibly due to the smaller radii of La3+(1.36Ǻ) than Ba2+(1.61 Ǻ) at 12 coordinated 2d site [25]. The average crystallite size ‘D’ is calculated using Sherrer’s formula [26],
Where ‘K’ is a constant, ‘β’ is full-width half maximum, ‘λ’ is the wavelength of the source, and ‘θ’ is the angle. The average crystallite size decreases slightly with La doping for batch ‘A,’ whereas it increases for batch ‘B’(listed in Table 1). However, it decreases with the sintering temperature as well. Two main possibilities involve getting smaller crystallite sizes at high sintering temperatures. First, the thermal stresses generated during the process of sintering at high temperature(1300˚C). This induces plastic deformation and a dynamic recrystallization process, resulting in a smaller crystallite size. Second, the nucleation rate increased significantly at high sintering temperatures, resulting in highly dense but smaller crystallite sizes[27]. The ionic radii mismatch, lattice mismatch, and structural disorder were calculated and given in Tables 1 and found to increase with La doping. The structural disorder is a combination of lattice strain, microstrain, and displacement. The microstrain was calculated using the W-H method. The plots of the W-H method were given in the supplementary file. Both the ionic radii mismatch and structural disorder caused by La doping significantly influence the optical and electrical properties of the synthesized material. The decrease in the distortion of Fe-O5 polyhedral was observed with sintering temperature. Higher sintering temperature can enhance atomic mobility, grain growth (hence fewer defects/dislocations), stress relief mechanism, and drive towards thermodynamical stability (more energetically favourable state). These processes lead to a more symmetrical crystal structure and reduce distortions [27, 28].
3.2 Morphological studies: FESEM
Morphological studies were carried out to study the sintering effect using FESEM, and images at 40kX magnification were shown in Fig. 2. The observed images show irregular-sized, porous, hexagonal plate-like grains for Batch ‘A’ samples. Meanwhile, elongated hexagonal rod-like grains were observed for batch B samples. Average grain size is calculated using ‘ImageJ’ software. The Average grain size of lower sintered samples was 0.3 and 0.4 μm for x = 0.25 and x = 0.30, respectively. Whereas it increases to 3.5 μm and 3.2 μm, respectively, when sintered at 1300˚C. Larger grains were observed for Batch B samples. This may be due to the sintering temperature. An increase in the sintering temperature aids grain growth and a change in the microstructure of the material. As the temperature increased, the small grains were combined to grow large grains, and some of the pores were trapped inside the large grains due to grain growth. As a result of grain growth, high-density material is produced. This shows that the sintering temperature was insufficient to form highly dense/compact material in the earlier case (Batch A) [29, 30]. The slight variation in the average grain size with the La substitution for both batches could be due to the grain growth inhibition nature of La [31].
Interestingly, grain size increases, but crystallite size decreases with sintering temperature. Average grain size increases with an increase in sintering temperature because higher temperature enhances atomic diffusion, promotes grain coalescence, leads to better densification, and results in fewer but larger grains. However, average crystallite size decreases due to the inhibitory nature of La3+, formation of subgrains, dynamic recrystallization process due to stress, and nucleation processes that prevent the crystallites from growing large at higher temperatures. In summary, the complex interplay between the atomic diffusion, nucleation process, grain boundary dynamics, dopant effects, and internal stress relaxation mechanism at higher sintering temperatures can result in larger grains but smaller crystallites within the grain [27, 28].
3.3 Optical studies
To measure the band gap variation with sintering temperature and La dopant levels, the optical properties of the prepared samples were studied using UV-Vis-NIR spectroscopy in diffused reflectance mode. The variation of diffused reflectance spectra with wavelengths ranging from 200 nm to 850 nm is shown in Fig. 3. The absorption of incident light was observed in the wavelength range 250 nm to 600 nm. Above 600 nm, reflection increased for all the samples. The Kubelka-Munk function was used to calculate the absorption coefficient ‘α’ and is given by
Where ‘R’ is the diffused reflectance. Further, optical band gap energy Eg is calculated using the Tauc relation [31, 32],
Where ‘A’ is a constant, ‘h’ is Plank’s constant, ‘ν’ is frequency, and ‘n’ is the absorption characterized index and equals ‘0.5’ for direct and ‘2’ for indirect band gap material. A linear interpolation of a part of (αhν)2 in a plot of (αhν)2 Vs hν gives the optical band gap energy [33], and the plot is shown in Fig. 4. The variation of band gap energy Eg with sintering temperature and La doping is listed in Table 2. With the increase in the sintering temperature, the band gap decreased significantly. However, band gap energy, Eg, increased for La doping in samples sintered at 1300˚C. The variation in the band gap energy could be correlated to variation in the crystallite size and Fe-O5 polyhedra distortion, where sub-energy levels are created due to La doping [26, 34]. The change or modification in the electronic band structure of the prepared material depends highly on the crystallite size and Fe-O5 polyhedra distortion. Smaller crystallites have a high surface-to-volume ratio, leading to more surface states and defects, which creates mid-gap states within the band gap. This results in a decrease in the band gap. In addition to this, Fe-O5 polyhedra distortion causes local strain, which alters the electronic band structure. Strain pushes the energy levels apart and increases the separation between the valance and conduction bands, increasing the band gap. So, the optical band gap variation with La doping level and sintering temperature is complex [27, 28].
3.4 Dielectric studies: impedance spectroscopy
The Dielectric properties of Ba(1−x)La(x)Fe12O19 (x = 0.25 and 0.30) sintered at different sintering temperatures were studied using an Impedance Analyzer. The dielectric constant and tanδ were calculated using the equations [35],
where ε΄ is the real part of the complex dielectric function or permittivity, also known as ‘dielectric constant,’ ‘Cp’ is the pellet’s capacitance, ‘d’ and ‘A’ are the thickness and area of the pellet, respectively, tanδ is tan loss, and \({\varepsilon }^{{\prime }{\prime }}\) is the imaginary part of the complex dielectric function, represents the dielectric loss in the sample.
The frequency response of dielectric constant/permittivity is plotted in Fig. 5. Initially, the dielectric constant is very high at low frequencies, then it decreases at mid-frequencies and remains constant at high frequencies. The plot shows the typical behavior of Maxwell-Wagner polarisation [36, 37]. According to this model, heterogenous materials, like hexaferrites, are made up of conducting grains separated by insulating grain boundaries [38]. When the electric field is applied, the charge carriers inside the grains move and accumulate at grain boundaries. This creates a build-up of charge carriers and leads to space-charge polarisation. According to Koop [39], grain boundaries dominate at low frequencies as they can follow the field, and grains at high frequencies (as they cannot follow the field). So, a large value of space charge polarisation gives rise to a large dielectric constant value at low frequencies. Whereas, at high frequencies, charge carriers cannot follow the field, the accumulation of charge carriers decreases at the grain boundaries, and hence, the dielectric constant value decreases.
Figure 5 shows that the permittivity value increased with the sintering temperature increase. The increase in the sintering temperature aids an increase in grain size, leading to an increase in the accumulation of dipoles/charge carriers within the grain, which increases the space-charge polarisation value and, hence, the greater permittivity/dielectric constant value. Higher sintering temperatures may also create conversion of Fe3+/Fe2+, or oxygen vacancies or defects lead to further increase in the charge carriers, which again increases permittivity [40]. The permittivity decreased with La doping in batch ‘A’ samples, whereas it increased with La doping in batch ‘B’ samples. The observed behavior is due to the combined effect of ionic radii mismatch, lattice strain, and structural distortion. The large mismatch of ionic radii and lattice distortion creates more dipoles, which leads to large polarizability, resulting in the increased value of the dielectric constant. However, an increase in structural disorder weakens the polarizability by disrupting the long-range order necessary for large polarisation. The less porous and closely packed grain boundaries help in increasing the polarizability in batch ‘B’ samples, whereas porous structure or less packing in batch ‘A’ samples aids in the disruption of the long-range order and, hence, polarisation resulting in a reduction in the dielectric constant of BLFO30_A [27, 41, 42].
The loss factor tanδ measures the energy loss within the material (here ferrites). The frequency response of tanδ is plotted in Fig. 6. This shows the variation of dielectric loss with frequency. The relaxation peak in all except BLFO25_A was asymmetrically broadened(shown as inset figure), which indicates the non-Debye relaxation. Hence, all the plots are fitted using the generalized non-Debye model, Hevriliak-Nagami (HN) function, which is a combination of the Cole-Cole and Cole-Davidson model [43] and reads as,
where, \(^{{\prime }}{\epsilon }_{HN}^{*}\left(\omega \right)^{{\prime }}\) is the complex dielectric function, ‘∆ε’ is the strength of the dielectric relaxation, ‘ω’ is the angular frequency, ‘\({\tau }_{HN}\)’ is the relaxation time, ‘β’ and ‘γ’ are the shape parameters, which describe symmetric and asymmetric broadening parameters, respectively, and the condition 0 < β and βγ ≤ 1 holds.
The relaxation parameters are listed in Table 3. The values of β < 1 confirm the relaxation is non-Debye type. In general, there are two kinds of energy losses: conduction loss and dielectric loss. Conduction loss occurs due to the flow of charge carriers, and dielectric loss arises when polarisation lags behind the applied alternating field. In ferrites, conduction is mainly due to the hopping of electrons between Fe3+ ions and Fe2+ ions via oxygen. The dielectric loss is significant in the low-frequency region and decreases in the high-frequency region. This is because in low-frequency regions, where non-conducting grain boundaries predominate, more energy is needed for electron exchange between Fe3+/Fe2+. In the high-frequency range, conducting grains predominate; hence, less energy is sufficient for hopping. The peak in the frequency response of the tanδ plot is due to the various relaxation processes, viz., Maxwell-Wagner-Sillar type relaxation, dipolar relaxation, and intrinsic dipolar relaxation [43]. Relaxation peaks were broadened with La doping due to structural disorder . Relaxation frequency shifts towards the right (towards higher frequency) with La concentration and with sintering temperature, indicating shorter relaxation time is due to the collective contribution of greater structural disorder (as increased strain typically leads to higher dielectric losses due to enhanced scattering and trapping of charge carriers), increased grain size and decreased grain boundary resistance, and increased defect states or oxygen vacancies formed due to high sintering. This shows the importance of precise control over doping levels and sintering conditions to tailor the dielectric properties [43, 44].
3.4.1 AC conductivity:
The AC conductivity is calculated using the formula [35, 45],
The frequency response of AC conductivity is shown in Fig. 7. The AC conductivity plot follows the Jonscher power law [35, 46,47,48],
where σ0 is DC conductivity, ‘A’ is constant, ‘ω’ is the angular frequency, and ‘s’(0 < s ≤ 1) is the exponent. The power law fitted parameters are listed in Table 4. In ferrites, the conduction mechanism is the same as dielectric polarisation [49, 50]. From the table, s ≤ 1 confirms the Maxwell-Wagner polarisation [35]. Due to the grain and grain boundary effect, the conduction mechanism is very low at low frequencies. It is almost independent of frequencies, which could be extrapolated to get DC conductivity σ0. Further, from Table 2, a significant increase in the dc conductivity is observed for x = 0.30 sintered at high temperature. It is due to increased grain growth as well as an increase in the number of charge carriers created during the sintering process, which shows the influence of sintering temperature [51]. Due to conducting grains at high frequencies, the hopping of charge carriers increases, increasing the ac conductivity σac. The variation in the ac conductivity at high frequencies with La doping and sintering temperature is complex. The observed behavior is due to increased disorder and strain, typically leading to enhanced ac conductivity due to increased hopping conduction of charge carriers between localized states. This enhances the AC conductivity at high frequencies. However, ionic radii mismatch and lattice strain could create potential wells and barriers affecting the ease of charge transport [41, 42].
3.4.2 Impedance analysis
Impedance analysis in the frequency range 20Hz to 20 MHz was carried out to understand the impedance behavior of the prepared material with the AC signal and plots of the frequency response of real(Z´) and imaginary(Z´´) parts of complex impedance function Z*(ω) were shown in Figs. 8 and 9. The frequency response of the real part shows a dispersion followed by a plateau at a low-frequency region and becomes independent at high frequencies. The behavior shows increased conduction with frequency, doping, and sintering temperature. The merging of all curves at high frequencies shows that releasing space charges leads to decreased space charge polarisation (by faster recombination at higher frequencies) [52]. The inset figure shows the frequency response of the BLFO30_A sample, where Z´ values are one order higher. This could be due to a secondary phase (α-Fe2O3, ~ 10%), where the electron hopping process varies in the interface of grains of the secondary phase and barium hexaferrite phase, increasing the grain boundary resistance [53]. The structural disorder of both phases could contribute to an increase in resistive components, particularly at low frequencies, due to the scattering of charge carriers [42].
The imaginary part of the complex impedance function Z´´ represents the capacitor nature of the material. (Z*=Z´+iZ´´). Z´´ curves provide information regarding the contribution of grain, grain boundary, and electrode effects. From Fig. 9, the appearance of the peak in the Z´´ plot is at a higher frequency region, and it shifts towards the right (towards high frequency), suggesting the contribution of relaxation of highly mobile charge carriers in the grain. This could be correlated to the observed dielectric loss (tanδ) behavior variation. The lattice strain introduced by the La substitution can create defects in the crystal structure, such as vacancies, interstitials, and dislocations. These defects can trap charge carriers and enhance the scattering in the grain, resulting in various types of relaxation processes [42], which can be observed as the frequency response of Z´´. The strain can lead to the shift in the peak position of Z´´, due to changes in the relaxation dynamics [35, 54].
The Nyquist plots of Z´ and Z´´ were plotted in Fig. 10. They were fitted using ‘EIS’ software with its equivalent circuit, and the equivalent circuit parameters are tabulated in Table 5. The barium hexaferrite samples with La concentration, x = 0.25 (batch ‘A’) fit with two parallel RC circuits connected in series. Similarly, for La content, x = 0.25(batch ‘A’) and x = 0.30(both the batches), a capacitor connected in series with two R‖C circuits in series. The ‘Constant Phase Element (CPE)’ (since the semicircles are not perfect) is substituted for the capacitor. The CPE is defined in the admittance format by,
The radius of the downward semicircle calculates the resistance. The resistance could be from grain and grain boundaries. Two semicircles are possible, one for grain boundary and another representing grain. The semicircle on the high-frequency side shows low resistance of grains, and the one on the low-frequency side shows high resistance of grain boundaries. In Fig. 10, only one semicircle appears, representing the contribution from the grains and given by R1 in the equivalent circuit. The contribution from the grain boundary is given by the equivalent resistor R2, which is out of scale and fails to show one more semicircle. C1 and C2 gave the capacitance values for La content x = 0.25(for Batch A and B) and represented the capacitance of grain and grain boundaries, respectively. For La content, x = 0.30 (for Batch A and B) fits with two resistors and three capacitors representing the resistance of grain(R1) and grain boundary(R2), Capacitance of grain(C1), grain boundary(C2), and the electrode-surface interface(C3). The ‘n’ values represent the type of capacitor. For n = 0, the CPE represents a resistance with R = Y0 − 1, where Y is the admittance. For n = 1, a capacitance with C = Y0; for n = 0.5, a Warburg capacitor; for n=-1, inductance L = Y0 − 1 [55, 56] (See Table 5).
3.4.3 Electric modulus
The complex electric modulus function is given by [31], \({M}^{*}\left(\omega \right)={M}^{{\prime }}+i{M}^{{\prime }{\prime }}=\frac{1}{{\varepsilon }^{*}}\) ,
where M’ is the real part and M" is the imaginary part of the electric modulus.
The frequency response of the real part and imaginary part of the electric modulus is shown in Figs. 11 and 12, respectively. The frequency response of M’ shows small values at low frequencies and dispersion when the frequency increases, indicating the long-range hopping of charge carriers [57]. The frequency response of M" presents a single peak at high frequencies in all the prepared samples. Also, peaks shift towards the right (higher frequencies) with La doping and sintering temperature. The observed peak corresponds to the relaxation in conduction processes [58, 59] caused by lattice strain and structural disorder.
Further, the plots Z´´ and M" plotted with frequency and plotted in Fig. 13 show peaks of Z´´ and M" almost coincide at almost the same frequency, which suggests the long-range movement of charge carriers is dominant in the relaxation process [60]. So, we can conclude that long-range movement (hopping) with high mobility charge carriers dominates the relaxation process.
Overall, we can observe the overlapping of different dielectric behavior, which can be explained as follows.
3.4.4 Lower frequency range
At the lower frequency range, interfacial polarisation is dominant due to the charge accumulation at the insulating grain boundaries. La doping increases the ionic radii mismatch and distortion, increasing polarisation. Meanwhile, there is an increase in disorder with La doping, which induces defects/charges trapping centers and disrupts the polarization due to the scattering of charge carriers, causing the polarization to decrease and create the resonance, leading to maximum dielectric loss and dielectric relaxation (i.e., interfacial polarisation relaxation). This also explains the relaxation peak in the impedance as well as the electric modulus. Because charge carriers accumulate at the insulating grain boundary, the mobility of the charge carriers is very low at low frequencies. Hence, ac conductivity is almost independent of the frequency at low frequencies.
3.4.5 Mid-frequency range
In the mid-frequency range, polarisation starts to decrease due to the conducting grain and insulating grain boundary effect (because of the competitive behavior of grain and grain boundaries, charges could not accumulate much at insulating grain boundaries, and polarisation decreases). In the mid-frequency range, dipolar relaxation (both dipolar and intrinsic dipolar) predominates as the dipole created due to ionic mismatch and local distortion. La doping and sintering effects cause the shift in the relaxation frequency and shorter relaxation time due to enhanced defect mobility and decreased grain boundary effects.
3.4.6 High-frequency range
At high frequencies, the effect of conducting grain dominates, and the charge carriers cannot accumulate at the grain boundaries and stop following the field-applied polarization; hence, the dielectric constant decreases and becomes independent of frequencies. Meanwhile, due to the same effect, the mobility of the charge carriers increases, leading to a sudden increase in the AC conductivity. So, the combined effect of La doping and sintering, which has an impact on these parameters, either by microstructural changes (such as ionic mismatch, lattice distortion, structural disorder, strain, grain growth, etc.) or polarization on the overlapping dielectric behavior [28, 31, 43].
3.5 Ferroelectric studies
P-E loop measurements were carried out to determine the effect of sintering temperature and La doping on the ferroelectric properties of the synthesized samples (BLFO25 and BLFO30, both batches). The P-E loop of BLFO25_A and BLFO30_A for different DC voltages were plotted in Fig. 14. The observed P-E loops of batch A samples were discontinued ellipse-shaped loops indicating a P-E loop of resistor kind of circuit leading to high leakage current [61], which may be due to the conduction caused by defects or oxygen vacancies created with La substitution. For BLFO25_A, the P-E loop was observed only at 100 V. Whereas for BLFO30_A, we could observe a loop up to 250 V. So, the ability to show the P-E loop behavior has increased with the increase in La concentration. The list of P-E loop parameters was tabulated in Table 6. The maximum Pr and Ec va were observed for La content x = 0.30(batch A). The samples BLFO25_B and BLFO30_B were very leaky, and P-E loops were not observed. The increased sintering temperature may cause oxygen defects, leading to heavy leakage current, suppressing the ferroelectric nature.
4 Conclusions
La substituted Barium hexaferrite, Ba(1−x)La(x)Fe12O19, (x = 0.25 and 0.30) was synthesized using ball milling followed by a solid-state reaction method. The samples were sintered at two different temperatures, viz., 1150˚C and 1300˚C. The effect of sintering temperature and La substitution were studied. Rietveld refinement on the X-ray diffraction patterns shows the formation of single-phase magnetoplumbite structure of barium hexaferrite only for high sintered (at 1300˚C) samples, which belongs to the P63/mmc space group. The sample sintered at 1150˚C shows the α-Fe2O3 phase along with the major magnetoplumbite phase. Morphological studies using FESEM show larger grains for samples sintered at 1300˚C. Dielectric studies using impedance analyzer show Maxwell- Wagner polarisation for all the samples. It also indicates non-Debye relaxation in dielectric loss spectra, which could be fitted using the HN function. AC conductivity plots follow the Jonscher power law. DC conductivity increases for BLFO30_B confirm the increase in the number of charge carriers (or oxygen vacancies/defects) with the increase in sintering temperature. The frequency response of impedance spectra reveals the relaxation of mobile charges with long-range hopping. From Nyquist plots of impedance spectra, grain, grain boundary, and electrode effects are successfully calculated using corresponding equivalent circuits. Ferroelectric studies using a P-E loop tracer show suppression of ferroelectric properties for samples sintered at 1300˚C due to a large leakage current.
Data availability
To access any data set, anyone could contact/write to either the first author or the communicating author.
References
J.J. Went, Phil Tech. Rev. 13 (1952)
C. de Julián Fernández, C. Sangregorio, J. de la Figuera, B. Belec, D. Makovec, A. Quesada, J. Phys. D: Appl. Phys. (2021). https://doi.org/10.1088/1361-6463/abd272
J.M.D. Coey, Engineering (2020). https://doi.org/10.1016/j.eng.2018.11.034
S. Hirosawa, M. Nishino, S. Miyashita, Adv. Nat. Sci: Nanosci. Nanotechnol (2017). https://doi.org/10.1088/2043-6254/aa597c
M. Kumar, S. Shankar, A. Kumar, A. Anshul, M. Jayasimhadri, O.P. Thakur, J. Mater. Sci: Mater. Electron. (2020). https://doi.org/10.1007/s10854-020-04574-2
H. Stablein, Handbook of Magnetic Materials, vol. 3, ed. by E.P. Wohlfarth (North-Holland, Amsterdam, 1982), p. 448
M. Rani, K.S. Bhatia, H. Singh, H. Kaur, N. Gupta, SN Appl. Sci. (2020). https://doi.org/10.1007/s42452-020-03786-9
H. Kojima, Ferromagnetic Materials: A Handbook on the Properties of Magnetically Ordered Substances, ed. by E.P. Wohlfarth (North-Holland, Amsterdam, 1982), p. 303
R.C. Pullar, Prog Mater. Sci. (2012). https://doi.org/10.1016/j.pmatsci.2012.04.001
A. Saxena, A. Planes, Mesoscopic Phenomena in Multifunctional Materials, Springer series in Materials Science, Vol. 198 (Springer, Berlin, Heidelberg, 2014)
G. Albanese, J. phys. Colloq. (1977). https://doi.org/10.1051/jphyscol:1977117
J.G. Rensen, J.S. Van Wieringen, Solid State Commun. (1969). https://doi.org/10.1016/0038-1098(69)90502-X
S. Kumar, M.K. Manglam, S. Supriya, H.K. Satyapal, R.K. Singh, M. Kar, J. Magn. Magn. Mater. (2019). https://doi.org/10.1016/j.jmmm.2018.10.085
G. Tan, X. Chen, J. Magn Magn Mater, (2013). https://doi.org/10.1016/j.jmmm.2012.09.047
V.G. Kostishyn, L.V. Panina, A.V. Timofeev, L.V. Kozhitov, A.N. Kovalev, A.K. Zyuzin, J. Magn. Magn. Mater. (2016). https://doi.org/10.1016/j.jmmm.2015.09.011
X. Liu, W. Zhong, S. Yang, Z. Yu, B. Gu, Y. Du, Phys. Status Solidi. (2002). https://doi.org/10.1002/1521-396X(200209)193:2%3C314::AID-PSSA314%3E3.0.CO;2-W
S. Ounnunkad, Solid State Commun. (2006). https://doi.org/10.1016/j.ssc.2006.03.020
S. Verma, P. Sharma, O.P. Pandey, A. Paesano, A.C. Sun, IEEE Trans. Magn. (2014). https://doi.org/10.1109/TMAG.2013.2278373
H. Sözeri, I.L.K.E.R. Küçük, H. Özkan, J. Magn. Magn. Mater. (2011). https://doi.org/10.1016/j.jmmm.2011.02.012
Y. Taryana, Y. Sarwanto, W.A. Adi, J M A. (2021). https://doi.org/10.53533/JMA.v1i1.2
H. Lou, X. Lu, Y. Pan, X. Wang, J. Wang, S. Sun, Q. Song, J. Mater. Sci: Mater. Electron. (2016). https://doi.org/10.1007/s10854-016-5243-5
P. Mariño-Castellanos, F. Guerrero, Y. Romaguera-Barcelay, E. Goveia-Alcaide, E.A. Cotta, Y. Leyet, R. Peña-Garcia, Ceram. Int. (2021). https://doi.org/10.1016/j.ceramint.2020.11.183
X. Wang, H. Zhang, J. Li, K. Liu, L. Jin, L. Cheng, L. Jia, Y. Liao, D. Zhang, Ceram. Int. (2022). https://doi.org/10.1016/j.ceramint.2022.06.071
K.R. Nishkala, R.R. Rao, S. Mutalik, M.S. Murari, M.D. Daivajna, Phys. B: Condens. Matter. (2023). https://doi.org/10.1016/j.physb.2023.414954
R.D. Shannon, Acta Crystallogr., Sect. A. (1976) https://doi.org/10.1107/S0567739476001551
D. Brandon, W.D. Kaplan, Microstructural Characterisation of Materials, second edn. (Wiley, 2008)
W.D. Kingery, H.K. Bowen, D.R. Uhlmann, Introduction to ceramics, Vol. 17, (John Wiley & Sons, 1976)
C.B. Carter, M.G. Norton, Ceramic Materials: Science and Engineering, vol. 716 (Springer, New York, 2007)
M.S. Alkathy, A. Hezam, K.S.D. Manoja, J. Wang, C. Cheng, K. Byrappa, K.J. Raju, J. Alloys Compd. (2018). https://doi.org/10.1016/j.jallcom.2018.05.138
J.J. Xu, C.M. Yang, H.F. Zou, Y.H. Song, G.M. Gao, B.C. An, S.C. Gan, J. Magn. Magn. Mater. (2009). https://doi.org/10.1016/j.jmmm.2009.05.039
M. Suthar, P.K. Roy, Mater. Sci. Eng. B (2022). https://doi.org/10.1016/j.mseb.2022.115801
J. Tauc, R. Grigorovici, A. Vancu, Phys. Status Solidi B (1966). https://doi.org/10.1002/pssb.19660150224
T. Kaur, J. Sharma, S. Kumar, A.K. Srivastava, Cryst. Res. Technol. (2017). https://doi.org/10.1002/crat.201700098
P. Makuła, M. Pacia, W.J. Macyk, Phys. Chem. Lett. (2018). https://doi.org/10.1021/acs.jpclett.8b02892
F. Kremer, A. Schönhals, Broadband Dielectric Spectroscopy (Springer Science & Business Media, 2002)
J.C. Maxwell, Electricity and magnetism, Vol. 2 (New York, Dover, 1954)
K.W. Wagner, Ann. Phys. (1913) 817
C.G. Koops, Phys. Rev. (1951). https://doi.org/10.1103/PhysRev.83.121
J. Smit, H.P.J. Wijn, Advances in Electronics and Electron Physics, vol. 6 (Academic, 1954), pp. 69–136
S. Kumar, S. Supriya, L.K. Pradhan, R. Pandey, M. Kar, Phys. B: Condens. Matter. (2020). https://doi.org/10.1016/j.physb.2019.411908
K. Alamelu Mangai, K. Tamizh Selvi, M. Priya, M. Rathnakumari, P. Sureshkumar, S. Sagadevan, J. Mater. Sci. : Mater. Electron. (2017). https://doi.org/10.1007/s10854-016-5876-4
A. Gholizadeh, V. Banihashemi, J. Am. Ceram. Soc. (2023). https://doi.org/10.1111/jace.19191
A.K. Jonscher, Dielectric Relaxation in Solids (Chelsea Dielectrics Press Ltd., 1983)
A.K. Jonscher, J. Phys. D: Appl. Phys. (1999). https://doi.org/10.1088/0022-3727/32/14/201
T. Winie, A.K. Arof, S. Thomas (eds.), Polymer Electrolytes: Characterization Techniques and Energy Applications (Wiley, 2019)
A.K. Jonscher, Nature. (1977). https://doi.org/10.1038/267673a0
A.K. Jonscher, J. Phys, D: Appl Phys. (1999). https://doi.org/10.1088/0022-3727/32/14/201
A.A. Lukichev, J. Non-Cryst, Solids. (2016). https://doi.org/10.1016/j.jnoncrysol.2016.02.027
N. Singh, A. Agarwal, S. Sanghi, Curr. Appl. Phys. (2011). https://doi.org/10.1016/j.cap.2010.11.073
M.A. El Hiti, J. Magn Magn Mater. (1999) https://doi.org/10.1016/S0304-8853(98)00356-4
A. Simchi, H. Danninger, B. Weiss, Powder Metall. (2000). https://doi.org/10.1179/003258900665970
M.K. Anupama, B. Rudraswamy, N. Dhananjaya, J. Alloys Compd. (2017). https://doi.org/10.1016/j.jallcom.2017.02.241
K.C. Verma, K.N. Goyal, M. Singh, M. Singh, R.K. Kotnala, Results Phys. (2019) https://doi.org/10.1016/j.rinp.2019.102212
A.J. Moulson, J.M. Herbert, Electroceramics: Materials, Properties, Applications (Wiley, 2003)
B.A. Boukamp, Equivalent Circuit (Equivcrt. pas) user Manual, 2nd edn. (University of Twente, The Netherlands, 1989)
G.G. Kumar, N. Munichandraiah, J. Power Sources. (2001) https://doi.org/10.1016/S0378-7753(01)00772-8
M.B. Hossen, A.K.M.A. Hossain, J. Adv. Ceram. (2015). https://doi.org/10.1007/s40145-015-0152-2
A. Radon, D. Łukowiec, M. Kremzer, J. Mikuła, P. Włodarczyk, Materials. (2018) https://doi.org/10.3390/ma11050735
W. Chen, W. Zhu, O.K. Tan, X.F. Chen, J. Appl. Phys. (2010). https://doi.org/10.1063/1.3457217
R.K. Panda, R. Muduli, S.K. Kar, D. Behera, J. Alloys Compd. (2014). https://doi.org/10.1016/j.jallcom.2014.07.031
F. L.Jin, J. Am. Ceram. Soc. (2014). https://doi.org/10.1142/9789811210433_0002
Acknowledgements
The author (Nishkala K.R.) is very grateful to Manipal Academy of Higher Education, Manipal, for financial assistance through the Dr. T.M.A. Pai fellowship; Dr. S. Rayaprol, UGC-DAE CSR Centre, Mumbai, for X-ray Diffraction Measurements; Dr. V.R. Reddy, UGC-DAE CSR Centre, Indore for ferroelectric measurements.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal
Author information
Authors and Affiliations
Contributions
Nishkala K.R.: Conceptualisation, Writing-original draft. Mamatha D. Daivajna: Supervision. Srinivas Mutalik: Resources (providing Ball mill Facility). Rajat Radhakrishna Rao: Resources (Helping to use Ball Mill Facility). M.S. Murari: Investigation.
Corresponding author
Ethics declarations
Novelty statement
The present work reports the study of the effect of sintering temperature on the structural, morphological, dielectric, and ferroelectric properties of La substituted polycrystalline barium hexaferrite(x=0.25&0.30) synthesized by ball milling using planetary ball mill followed by high-temperature sintering at 1150°C and 1300°C. The single-phase magnetoplumbite structure of barium hexaferrite was formed for samples sintered at 1300°C. Maxwell-Wagner type polarisation with non-Debey kind of relaxation and long-range hopping of mobile charge carriers was observed. The ferroelectric nature was suppressed in the samples sintered at 1300°C.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nishkala, K.R., Rao, R.R., Mutalik, S. et al. Effect of sintering temperature on the structural, morphological, optical and electrical properties of Ba1 − xLaxFe12O19. Appl. Phys. A 130, 542 (2024). https://doi.org/10.1007/s00339-024-07696-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-024-07696-0