Abstract
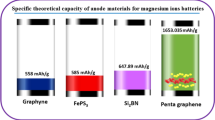
This study explores the potential applications of graphene oxide frameworks (GOFs) in Na-ion batteries using density functional theory calculations. The GOF's graphene layers are linked by benzenediboronic acid pillars. Ab-initio molecular dynamics simulations demonstrate the thermal stability of the structures. The study calculates adsorption and barrier energy, storage capacity, and open-circuit voltage. The results predict the high mobility of Na in GOF due to the low energy barrier. The layered structure of GOF enables the intercalation of Na-ions. GOF has a Na storage capacity of 947 mAh/g in the form of Na21C36, which is higher than the reported values for graphite and some other two-dimensional carbon-based materials. The transition from semiconductor to metal, which is an essential condition for the diffusion of ions within the anode material, occurs after Na adsorption. Therefore, GOFs are a promising anode material with high efficiency for Na-ion batteries.






Similar content being viewed by others
Data availability
Data sharing is not applicable as no new data were generated during this study.
References
J.B. Goodenough, K.S. Park, The Li-ion rechargeable battery: a perspective. J. Am. Chem. Soc. 135, 1167–1176 (2013). https://doi.org/10.1021/JA3091438/ASSET/IMAGES/MEDIUM/JA-2012-091438_0009.GIF
N.A. Kaskhedikar, J. Maier, Lithium storage in carbon nanostructures. Adv. Mater. 21, 2664–2680 (2009). https://doi.org/10.1002/ADMA.200901079
T. Insinna, E.N. Bassey, K. Märker, A. Collauto, A.L. Barra, C.P. Grey, Graphite anodes for Li-Ion batteries: an electron paramagnetic resonance investigation. Chem. Mater. 35, 5497–5511 (2023). https://doi.org/10.1021/ACS.CHEMMATER.3C00860/ASSET/IMAGES/LARGE/CM3C00860_0008.JPEG
K. Sawai, Y. Iwakoshi, T. Ohzuku, Carbon materials for lithium-ion (shuttlecock) cells. Solid State Ion. 69, 273–283 (1994). https://doi.org/10.1016/0167-2738(94)90416-2
E.J. Yoo, J. Kim, E. Hosono, H.S. Zhou, T. Kudo, I. Honma, Large reversible Li storage of graphene nanosheet families for use in rechargeable lithium ion batteries. Nano Lett. 8, 2277–2282 (2008). https://doi.org/10.1021/NL800957B/SUPPL_FILE/NL800957B-FILE003.PDF
D. Sui, L. Si, C. Li, Y. Yang, Y. Zhang, W. Yan, A comprehensive review of graphene-based anode materials for lithium-ion capacitors. Chemistry 3, 1215–1246 (2021). https://doi.org/10.3390/CHEMISTRY3040089
X. Zhang, L. Jin, X. Dai, G. Chen, G. Liu, A record-high ion storage capacity of T-graphene as two-dimensional anode material for Li-ion and Na-ion batteries. Appl. Surf. Sci. 527, 146849 (2020). https://doi.org/10.1016/J.APSUSC.2020.146849
H. Lee, B. Jang, J. Koo, M. Park, H. Lee, J. Nam, Y. Kwon, Graphdiyne as a high-capacity lithium ion battery anode material. Appl. Phys. Lett. 103, 263904 (2013). https://doi.org/10.1063/1.4850236
H. Dua, J. Deb, D. Paul, U. Sarkar, Twin-graphene as a Promising Anode Material for Na-Ion Rechargeable Batteries. ACS Appl Nano Mater. 4, 4912–4918 (2021). https://doi.org/10.1021/ACSANM.1C00460/SUPPL_FILE/AN1C00460_SI_001.PDF
H.J. Hwang, J. Koo, M. Park, N. Park, Y. Kwon, H. Lee, Multilayer graphynes for lithium ion battery anode. J. Phys. Chem. C 117, 6919–6923 (2013). https://doi.org/10.1021/JP3105198/ASSET/IMAGES/MEDIUM/JP-2012-105198_0005.GIF
C. Vaalma, D. Buchholz, M. Weil, S. Passerini, A cost and resource analysis of sodium-ion batteries, Nature Reviews Materials 2018 3:4. 3 (2018) 1–11. https://doi.org/10.1038/natrevmats.2018.13.
M. Binder, M. Mandl, S. Zaubitzer, M. Wohlfahrt-Mehrens, S. Passerini, O. Böse, M.A. Danzer, M. Marinaro, Cover feature: sodium cyclopentadienide as a new type of electrolyte for sodium batteries (ChemElectroChem 2/2021). ChemElectroChem 8, 272–272 (2021). https://doi.org/10.1002/CELC.202001559
E. Goikolea, V. Palomares, S. Wang, I.R. de Larramendi, X. Guo, G. Wang, T. Rojo, Na-Ion Batteries—Approaching Old and New Challenges. Adv. Energy Mater. 10, 2002055 (2020). https://doi.org/10.1002/AENM.202002055
Y. Cao, L. Xiao, M.L. Sushko, W. Wang, B. Schwenzer, J. Xiao, Z. Nie, L.V. Saraf, Z. Yang, J. Liu, Sodium ion insertion in hollow carbon nanowires for battery applications. Nano Lett. 12, 3783–3787 (2012). https://doi.org/10.1021/NL3016957
W. Zhang, F. Zhang, F. Ming, H.N. Alshareef, Sodium-ion battery anodes: status and future trends. EnergyChem. 1, 100012 (2019). https://doi.org/10.1016/J.ENCHEM.2019.100012
Z. Xu, X. Lv, J. Li, J. Chen, Q. Liu, A promising anode material for sodium-ion battery with high capacity and high diffusion ability: graphyne and graphdiyne. RSC Adv. 6, 25594–25600 (2016). https://doi.org/10.1039/C6RA01870J
A.H. Farokh Niaei, T. Hussain, M. Hankel, D.J. Searles, Sodium-intercalated bulk graphdiyne as an anode material for rechargeable batteries, J Power Sources. 343 (2017) 354–363. https://doi.org/10.1016/J.JPOWSOUR.2017.01.027.
J. Deb, R. Ahuja, U. Sarkar, Two-Dimensional Pentagraphyne as a High-Performance Anode Material for Li/Na-Ion Rechargeable Batteries. ACS Appl Nano Mater. 5, 10572–10582 (2022). https://doi.org/10.1021/ACSANM.2C01909/SUPPL_FILE/AN2C01909_SI_001.PDF
R. Majidi, A.I. Ayesh, A density functional theory study of twin T-graphene as an anode material for Na-ion-based batteries. J. Appl. Phys. 132, 194301 (2022). https://doi.org/10.1063/5.0123013
R. Majidi, A.I. Ayesh, Application of T4,4,4-graphyne for anode of Na-ion battery: first principle theoretical study. Mol. Simul. 49, 1044–1050 (2023). https://doi.org/10.1080/08927022.2023.2211170
X. Bai, N. Wu, G. Yu, T. Li, Recent Advances in Anode Materials for Sodium-Ion Batteries, Inorganics 2023, Vol. 11, Page 289. 11 (2023) 289. https://doi.org/10.3390/INORGANICS11070289.
Y. Wen, K. He, Y. Zhu, F. Han, Y. Xu, I. Matsuda, Y. Ishii, J. Cumings, C. Wang, Expanded graphite as superior anode for sodium-ion batteries, Nature Communications 2014 5:1. 5 (2014) 1–10. https://doi.org/10.1038/ncomms5033.
S.U.D. Shamim, M.K. Hossain, S.M. Hasan, A.A. Piya, M.S. Rahman, M.A. Hossain, F. Ahmed, Understanding Na-ion adsorption in nitrogen doped graphene oxide anode for rechargeable sodium ion batteries. Appl. Surf. Sci. 579, 152147 (2022). https://doi.org/10.1016/J.APSUSC.2021.152147
S. Gadipelli, Z.X. Guo, Graphene-based materials: Synthesis and gas sorption, storage and separation. Prog. Mater. Sci. 69, 1–60 (2015). https://doi.org/10.1016/J.PMATSCI.2014.10.004
G. Srinivas, J.W. Burress, J. Ford, T. Yildirim, Porous graphene oxide frameworks: Synthesis and gas sorption properties. J. Mater. Chem. 21, 11323–11329 (2011). https://doi.org/10.1039/C1JM11699A
J.W. Burress, S. Gadipelli, J. Ford, J.M. Simmons, W. Zhou, T. Yildirim, Graphene oxide framework materials: theoretical predictions and experimental results. Angew. Chem. Int. Ed. 49, 8902–8904 (2010). https://doi.org/10.1002/ANIE.201003328
P. Zhu, B.G. Sumpter, V. Meunier, Electronic, thermal, and structural properties of graphene oxide frameworks. J. Phys. Chem. C 117, 8276–8281 (2013). https://doi.org/10.1021/JP401072Z/ASSET/IMAGES/MEDIUM/JP-2013-01072Z_0006.GIF
I. Skarmoutsos, E.N. Koukaras, E. Klontzas, A computational study on phenyldiboronic acid-pillared graphene oxide frameworks for gas storage and separation. ACS Appl Nano Mater. 5, 9286–9297 (2022). https://doi.org/10.1021/ACSANM.2C01617/ASSET/IMAGES/MEDIUM/AN2C01617_0011.GIF
G. Mercier, A. Klechikov, M. Hedenström, D. Johnels, I.A. Baburin, G. Seifert, R. Mysyk, A.V. Talyzin, Porous graphene oxide/diboronic acid materials: structure and hydrogen sorption. J. Phys. Chem. C 119, 27179–27191 (2015). https://doi.org/10.1021/ACS.JPCC.5B06402/ASSET/IMAGES/LARGE/JP-2015-064029_0014.JPEG
Y. Chan, J.M. Hill, Hydrogen storage inside graphene-oxide frameworks. Nanotechnology 22, 305403 (2011). https://doi.org/10.1088/0957-4484/22/30/305403
User’s manual of OpenMX Ver. 3.9, (n.d.). http://www.openmx-square.org/openmx_man3.9/index.html. Accessed 5 Oct 2022
J.P. Perdew, K. Burke, M. Ernzerhof, Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996). https://doi.org/10.1103/PhysRevLett.77.3865
S. Grimme, S. Ehrlich, L. Goerigk, Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011). https://doi.org/10.1002/jcc.21759
S. Grimme, J. Antony, S. Ehrlich, H. Krieg, A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010). https://doi.org/10.1063/1.3382344
G. Henkelman, H. Jónsson, Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113, 9978 (2000). https://doi.org/10.1063/1.1323224
M. Nasrollahpour, M. Vafaee, M.R. Hosseini, H. Iravani, Ab initio study of sodium diffusion and adsorption on boron-doped graphyne as promising anode material in sodium-ion batteries. Phys. Chem. Chem. Phys. 20, 29889–29895 (2018). https://doi.org/10.1039/C8CP04088E
R. Majidi, A. Ramazani, T. Rabczuk, Electronic properties of transition metal embedded twin T-graphene: A density functional theory study. Physica E Low Dimens Syst Nanostruct. 133, 114806 (2021). https://doi.org/10.1016/J.PHYSE.2021.114806
J. Hu, Y. Liu, N. Liu, J. Li, C. Ouyang, Theoretical prediction of T-graphene as a promising alkali-ion battery anode offering ultrahigh capacity. Phys. Chem. Chem. Phys. 22, 3281–3289 (2020). https://doi.org/10.1039/C9CP06099E
C. Kittel, P. McEuen, Introduction to solid state physics, (n.d.) 692. https://www.wiley.com/en-ie/Kittel%27s+Introduction+to+Solid+State+Physics%2C+8th+Edition%2C+Global+Edition-p-9781119454168 (accessed December 1, 2023).
T. Singh, J.R. Choudhuri, M.K. Rana, α-graphyne as a promising anode material for Na-ion batteries: a first-principles study. Nanotechnology 34, 045404 (2022). https://doi.org/10.1088/1361-6528/AC9A54
Author information
Authors and Affiliations
Contributions
F.P. performed the computations and wrote the first draft of the manuscript. R.M., S.I. V, and H.R. S. completed the simulations, discussed the results, and contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peymanirad, F., Majidi, R., Vishkayi, S.I. et al. Exploring the potential of graphene oxide frameworks as anode materials for Na-ion batteries applications: a density functional theory study. Appl. Phys. A 130, 318 (2024). https://doi.org/10.1007/s00339-024-07469-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-024-07469-9