Abstract
The influence of sintering temperature on the thermoelectric (TE) transport of BiCuSeO is reported in the present work, with an aim to optimize the processing conditions for higher TE transport. BiCuSeO samples were synthesized at four different sintering temperatures, viz., 673 K, 773 K, 873 K, and 973 K. A non-degenerate type of conductivity is observed in all the samples at high temperatures, witnessing the thermal activation of the carriers. The Fermi level was positioned below the valence band maximum, thereby exhibiting a p-type degenerate transport in the entire range of temperature. It was observed that the variations of weighted mobility and power factor were found to have identical trends. The highest power factor was noticed at 554 K with a value of 129 μWm−1 K−2 for the sample sintered at 673 K.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
There is a global requirement for cleaner and greener forms of energy as unsustainable amounts of energy are wasted as heat during various types of energy conversion. This not only has major climatic implications, but also results in a scarcity of natural resources [1, 2]. Thus, there is an immediate need for technologies that can reuse the waste heat as an alternate energy source. One such method is by employing thermoelectric (TE) materials that can convert thermal energy into electrical energy and vice versa [3, 4]. The efficiency of a thermoelectric material to convert heat energy into electrical energy and vice versa is governed by a dimensionless entity known as thermoelectric figure of merit which is given by [5]
where S is the Seebeck coefficient, σ is the electrical conductivity, T is the absolute temperature, \({\kappa }_{L}\) is the lattice thermal conductivity, and \({\kappa }_{e}\) is electronic thermal conductivity. Power Factor (PF) of a material is given by the term S2σ which gives the contribution of electronic transport to the thermoelectric performance of a material [2]. Since ZT values are directly related to PF, it is imperative to regulate these transport properties by carefully choosing an ideal combination of thermal and electrical transports to yield maximum ZT value [2, 5].
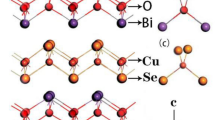
Oxychalcogenides with layered structure have been of great interest in finding efficient thermoelectric materials for nearly 3 decades because of the significant changes observed with respect to electrical and thermal properties by the altering of cation composition [3, 6]. BiCuSeO is part of P4/nmm space group and has layered polyhedral tetragonal structure with fluorite like alternate layers of (Bi2O2)2+, which is the insulating layer, and (Cu2Se2)2− is the conducting layer. The Bi2O2 layers are made up of slightly deformed Bi4O tetrahedra with two different Bi–O–Bi bond angles. The organization of Bi can also be seen as a deformed square antiprism having four O in one of the bases and likewise in the other with four Se. CuSe4 tetrahedra also has a slightly warped structure that share Se-Se edges making up the Cu2Se2 layers. The location of Se atoms in the Cu2Se2 layer is identical to that of Bi atoms in the Bi2O2 layer, whereas the position of O atoms is identical to that of Cu atoms in the Cu2Se2 layer. This suggests that the Cu2Se2 layer is a reversed version of the Bi2O2 layer [7, 8]. BiCuSeO has been studied extensively in the last decade, especially after Zhao et al. [9] have reported intrinsically low thermal conductivity that varies from 0.45 to 0.9 Wm−1 K−1 in a temperature range 300–923 K. It displayed an unparalleled ZT level that could go as high as 1.5 when substituted, far higher than other metal-oxide TE materials [7, 10,11,12].
Pan et al. [13] showed how n-type BiCuSeO can be acquired by replacement of Fe for Cu from pristine BiCuSeO below 500 K. They also observed that co-substitution of Fe for Cu in p-type Bi0.94Pb0.06CuSeO enhanced S and σ which in turn improved the power factor to 0.9 mWm−1 K−2 in the entire experimental temperature range, while \(\kappa\) was low due to surprising enhancement of carrier mobility. A peak ZT of 1.5 and average ZT of 1 was observed for Bi0.94Pb0.06Cu0.99Fe0.01SeO sample. Tang et al. [14] proposed how the carrier mobility and the S of BiCuSeO can be enhanced by doping with light elements like Li. It was also found that by introducing spin entropy, the S was enhanced significantly. A high-power factor resulted due to the high S and enhanced σ. A peak ZT of 0.9 was achieved at 873 K. Feng et al. [15] investigated the effects of doping rare earth element, Sm with BiCuSeO and observed that the 4f orbital of Sm has a strong effect on Valence Band Maximum which resulted in a narrow band gap that increased carrier concentration, in turn, increasing σ. A peak ZT of 0.74 at 873 K was observed for Bi0.94Sm0.06CuSeO sample. To increase the electrical transport of BiCuSeO, several methods of synthesis have been used to improve the efficiency of the materials, viz., two-step solid-state reaction, spark plasma sintering, ball milling, hot-forging, and mechanical alloying processes [8, 15,16,17,18,19]. Zhu et al. [20] have studied the effects of high- and low-pressure sintering and observed a peak ZT of 0.74 at 923 K under high pressure which was almost three times higher than the ZT value obtained at low pressure. Feng et al. [21] prepared samples of BiCuSeO under three different temperatures and were sintered for different durations and found a peak ZT value of 0.62 at 873 K with ball milling duration of 16 h, which increased to 1.15 with the doping of Ca/Pb. Electrical properties of BiCuSeO synthesized via aforementioned methodologies have observed to be considerably different at different temperatures. It is evident from various reports that the synthesis procedure influences the electronic transport substantially as it is directly correlated to the microstructural parameters, such as crystallite size, strain, density etc. [10]. Though, the grain refining can be controlled via extrinsic impurities [22], the extent of the microstructure modulation is relatively small. Hence, precise control of microstructure can only be achieved by varying the growth kinetics which directly reflected in the processing parameters.
Here, we have attempted to optimize the sintering temperature for synthesizing BiCuSeO system using conventional solid-state reaction method. The effect of sintering temperature and experimental conditions are found to influence the grain growth and size which in turn has a major impact on the thermoelectric properties of the material as reported by Feng et al. [21]. Most of the work in the literature has been focused on the effect of doping on the thermoelectric properties. Inadequate work seems to have been done on effect of varying sintering temperature on the TE properties of BiCuSeO system. This motivated us to study the microstructure—TE properties in BiCuSeO via varying the sintering temperature during the synthesis.
2 Experimental details
BiCuSeO samples were prepared at different sintering temperatures using conventional solid-state reaction method. Stoichiometric ratios of highly pure powders of Bi2O3 (3N pure), Bi (3N pure), Cu (3N pure), and Se (3N pure) were weighed and ground using an agate mortar and pestle for 2 h. The finely ground mixture was then subjected to a uniaxial cold press under a load of 6 tons to get pellets of dimensions 6 mm × 11 mm × 1 mm. The pellets obtained were then sealed in a quartz tube at a high vacuum of around 10–5 mbar pressure. The pellets were soaked at 573 K for 8 h in a furnace and further sintered at four different temperatures, viz., 673 K, 773 K, 873 K, and 973 K for 24 h at a heating rate of 1 K/min. The samples were then cooled to room temperature at a cooling rate of 2 K/min. The entire procedure of grinding, palletizing, and vacuum sealing was repeated for the second time with a sintering temperature of 573 K for 12 h. Post second sintering, highly dense and homogenous pellets of BiCuSeO were obtained. Room-temperature X-ray diffraction using Cu-Kα radiation of wavelength 1.5406 Å was carried out for confirming the phase formation of the material using Rigaku Ultima IV. SEM EVO MA-18 with oxford EDAX system was used for topographical and morphological studies. Vicker’s hardness testing was done using the instrument MMTX7 Matsuzawa Co. Ltd. Ecopia HMS-5500 Hall measurement system was used to carry out the Hall coefficient, mobility measurements, and carrier concentration of the samples using Van der Pauw’s method. Linseis LSR-3 system was equipped to conduct the high-temperature electrical transport measurements (300 K–723 K) with linear four-probe technique employed for electrical resistivity measurements and classical method for Seebeck coefficient [23, 24].
3 Results and discussion
3.1 Phase formation and microstructure
X-ray diffraction (XRD) at room temperature is shown in Fig. 1a and the profile of BiCuSeO matches approvingly with JCPDS card #45–0296 that confirm the tetragonal structure with P4/nmm space group and no impurity phases were observed [25, 26]. Rietveld refinement was carried out on the XRD data using FullProf software which employed pseudo-Voigt function, a linear combination of Lorentzian and Gaussian functions, as the peak profile that confirmed the phase formation of BiCuSeO. Linear interpolation between a group of background points that have refinable heights can explain the background data. The crystallographic data obtained post Rietveld refinement are listed in Table 1, and the goodness of fit for these parameters ranged from 1.1 to 1.7. Two different methods were employed to calculate the microstructural data, namely, Scherrer equation and size-strain plot method, which is shown in Fig. 2. [27,28,29,30,31,32]. The crystallite size was found to be in the range of 40–60 nm with an increase till 773 K beyond which there is a decrease in crystalline size. A similar trend of an increase of growth in the crystal domain and the strain was observed as listed in Table 2.
The SEM images of the BiCuSeO sintered at different temperatures, viz., 673 K, 773 K, 873 K, and 973 K, are shown in Fig. 3a–d. An irregular grain growth was observed, but the synthesized pellets showed a fairly dense morphology as seen in Fig. 3. Increase in agglomeration of the grains are evident with increase of sintering temperature, showing up nugget like clusters. This can be connected to the increased hardness of the samples with sintering temperature. The Vicker’s hardness test result of the synthesized BiCuSeO samples is tabulated in Table 2. The hardness of the material increases with increase in sintering temperature with a peak hardness of 1.49 GPa observed for the sample sintered at 873 K [33], above which temperature the hardness is found to be decreasing. The elemental mapping as shown in Fig. 4 illustrate the evenly distributed elements along the surface of the synthesized BiCuSeO system which is the indication of well homogeneous microstructure.
3.2 Electrical transport
Electrical resistivity measurements were conducted at mid temperatures from 300 to 730 K as depicted in Fig. 4a. For the sample sintered at 673 K a typical non-degenerate behavior of decreasing resistivity with increasing temperature was observed. For samples sintered at 773 K, 873 K, and 973 K, there was a small increase in resistivity between 400 and 500 K beyond which the samples exhibited non-degenerate type of electrical transport which agreed well with previously published reports [8, 10]. The small increase in resistivities can be related to the increase in resistivity with increase in temperature until about 430 K due to charge carrier-phonon scattering. When the width of the polaron band is comparable to the energy fluctuation of scattering, band conduction transforms into polaronic conduction. Above a particular temperature, the transition from band conduction to polaronic conduction occurs, which results in an increase in the energy fluctuation caused by scattering. With rising temperatures, this mobility activated activity decreases electrical resistance. This results in the increase of resistivity beyond which it decreases with increase in temperature [8, 34]. To further understand the temperature dependency of resistivity, we have adopted the thermal activation model given by Arrhenius equation [35]
where \({\rho }_{0}\) is the temperature independent constant, \({E}_{A}\) is the activation energy, and \({k}_{B}\) is the Boltzmann constant [17]. The temperature dependency was observed from 434 to 556 K and the resulting fitting matched well with the data. The plot of ln (\(\rho\)) vs 1/T is shown in Fig. 4b and the slope of each gave the activation energy for the corresponding sample. The activation energy is tabulated in Table 3.
Hall measurements were carried out at room temperatures on the BiCuSeO system. It was found to be a p-type semiconductor. At room temperature, the electrical resistivity was found to be decreasing as the sintering temperature was raised from 673 to 773 K, beyond which the resistivity is found to be increasing. We observe an inverse trend in the variations of crystallite size and strain as the samples sintered at 773 K have the highest crystallite size and strain, beyond which both these microstructural parameters are observed to be decreasing. The inverse trend is observed with mobility. This helps in increasing carrier concentration and lowering the electrical resistivity and thereby increasing the electrical transport of the material. Therefore, in the sample sintered at 773 K, the crystallite size growth is observed to be higher and therefore has a direct impact on increasing the electrical transport of the material [8].
The Seebeck coefficient measurements were conducted in the high-temperature range of 300–730 K and were plotted against temperature as shown in Fig. 5a. The Seebeck coefficient was found to be increasing with increase in sintering temperature at higher measuring temperatures. A positive Seebeck coefficient confirms that the material is a p-type semiconductor. Assuming the Single Parabolic Band (SPB) approximation with acoustic phonon scattering, effective mass of the sample was calculated at room temperature using the Mott’s relation which is given by [36]
where \({m}^{*}\) is the effective mass, h is the Planck’s constant, and \({k}_{B}\) is the Boltzmann constant. The effective mass \({m}^{*}\) is used here to fully characterize the data of electronic transport and to examine the variation with respect to sintering temperature which ultimately provides the overall changes in multiple band effects [37]. Effective mass is tabulated in Table 5.3. To have a better assessment on the transport behavior at higher temperatures, Fermi energy is evaluated using an algorithm called SPBcal developed by Chang et al. [38]. The equation used to calculate Fermi energy is given by
where η is the reduced Fermi energy which is connected to Fermi energy EF as EF /kBT. F0 (η) and F1 (η) are Fermi integrals of order n = 0 and 1 respectively. Referring to Fig. 5b, the Fermi level is found to be positioned below the valence band maximum (VBM) in all BiCuSeO samples in the entire temperature range, witnessing the degenerate electrical transport in the material. Lorenz number was obtained from the Seebeck coefficient using the equation
where S is the Seebeck coefficient [39]. Lorenz number versus temperature was plotted, as shown in Fig. 5c. Lorenz number is observed to be varying within the range of 1.51 to 1.59 × 10–8 WΩK−1 indicating the partial degeneracy in the material. The weighted mobility was calculated using the equation given by Snyder et al. [40] to thoroughly evaluate the electrical transport behavior of the material
where h is the Planck’s constant, r is the electrical conductivity, S is the Seebeck coefficient, and kB is the Boltzmann constant. Figure 6a gives the plot of weighted mobility variation with temperature which shows a comprehensible deviation between the weighted mobilities of the samples sintered at different temperatures caused due to the changes induced in scattering mechanisms at different temperatures. The highest weighted mobility value of 18.39 cm2V−1 s−1 was found for the sample sintered at 673 K at a measuring temperature of 467 K.
Zhang et al. [41] have proposed a single material parameter ‘BE’ referred to as ‘electronic quality factor’ which suggest the possible transport phenomena in the material. For a pair of measurements of Seebeck coefficient (S) and electrical conductivity (σ) performed simultaneously at a given temperature, BE is defined as
where \({S}_{r}=\frac{\left|S\right|}{{k}_{B}/e}\). This describes the electronic contribution to the power factor. Increased electronic quality factor is go together with the increase in power factor. Since the rapid surge in temperature-dependent BE values is not observed, the possibility of the band convergence is ruled out. Further, the bipolar contribution to the electronic transport is found to be prompt in the samples sintered at 673 K as there is a decrease in BE values seen beyond 550 K, as shown in Fig. 6b. Power Factor (PF) of the synthesized BiCuSeO samples was obtained in the mid-temperature regime. The plot of PF variation with temperature is shown in Fig. 6c. Highest PF value of 129 μWm−1 K−2 was observed at 554 K for sample sintered at 673 K, but the PF value for the same sample decreased at higher temperatures while an increasing trend of PF was observed for samples sintered at 773 K, 873 K and 973 K at higher measuring temperatures. The trend observed in power factor is reflected in the trend observed in weighted mobility as both are directly related to the variations in electrical conductivity. Highest power factor obtained at 554 K is compared with the pristine BiCuSeO samples with different synthesis methods and previous reports [21, 42,43,44] as shown in Fig. 6d. At 300 K, the samples sintered at 773 K had the highest power factor of the value 44 μWm−1 K−2, while at 730 K, the samples sintered at 973 K had the highest power factor of the value 101 μWm−1 K−2.
4 Conclusions
A detailed study was performed on the effects of sintering temperature on BiCuSeO which was synthesized by the conventional two-step solid-state reaction method. Each of the synthesized samples showed a tetragonal structure with P4/nmm space group with no impurity phase. Seebeck coefficient and Hall measurements conducted confirmed the p-type semi-conductivity of the material. It was observed that the variations of weighted mobility and power factor resembled each other. The highest power factor was noticed at 554 K with a value of 129 μWm−1 K−2 for the sample sintered at 673 K. At 300 K, the samples sintered at 773 K had the highest power factor of the value 44 μWm−1 K−2, while at 730 K, the samples sintered at 973 K had the highest power factor of the value 101 μWm−1 K−2. The sample sintered at 773 K and 973 K found to have better mechanical and electronic properties as compared to the ones sintered at 673 K, suggesting the suitability of the materials in TE device architectures.
Data availability statement
Data will be made available on request.
References
C. Forman, I.K. Muritala, R. Pardemann, B. Meyer, Renew. Sustain. Energy Rev. 57, 1568 (2016)
G. Neeli, D.K. Behara, M.K. Kumar, Int. J. Sci. Res. 5, 1833 (2016)
D. Beretta, N. Neophytou, J. M. Hodges, M. G. Kanatzidis, D. Narducci, M. Martin-Gonzalez, M. Beekman, B. Balke, G. Cerretti, W. Tremel, A. Zevalkink, A. I. Hofmann, C. Müller, B. Dörling, M. Campoy-Quiles, and M. Caironi, Mater. Sci. Eng. R Reports 138, (2019).
J.C. Zheng, Front. Phys. China 3, 269 (2008)
H. Lee, Thermoelectrics: Design and Materials (Wiley, 2016).
L.D. Zhao, J. He, D. Berardan, Y. Lin, J.F. Li, C.W. Nan, N. Dragoe, Energy Environ. Sci. 7, 2900 (2014)
D. Bérardan, L. Zhao, J. He, D. Berardan, Y. Lin, J. Li, Energy Environ. Sci. 7, 2900 (2014)
S. Das, A. Ramakrishnan, M. Rudra, K.H. Chen, T.P. Sinha, D.K. Misra, R.C. Mallik, J. Electron. Mater. 48, 3631 (2019)
L.D. Zhao, D. Berardan, Y.L. Pei, C. Byl, L. Pinsard-Gaudart, N. Dragoe, Appl. Phys. Lett. 97, 95 (2010)
F. Li, J.F. Li, L.D. Zhao, K. Xiang, Y. Liu, B.P. Zhang, Y.H. Lin, C.W. Nan, H.M. Zhu, Energy Environ. Sci. 5, 7188 (2012)
Y. L. Pei, J. He, J. F. Li, Fuli, Q. Liu, W. Pan, C. Barreteau, D. Berardan, N. Dragoe, and L. D. Zhao, NPG Asia Mater. 5, (2013).
Y. Liu, L.D. Zhao, Y. Zhu, Y. Liu, F. Li, M. Yu, D.B. Liu, W. Xu, Y.H. Lin, C.W. Nan, Adv. Energy Mater. 6, 1 (2016)
L. Pan, Y. Lang, L. Zhao, D. Berardan, E. Amzallag, C. Xu, Y. Gu, C. Chen, L.D. Zhao, X. Shen, Y. Lyu, C. Lu, Y. Wang, J. Mater. Chem. A 6, 13340 (2018)
J. Tang, R. Xu, J. Zhang, D. Li, W. Zhou, X. Li, Z. Wang, F. Xu, G. Tang, G. Chen, A.C.S. Appl, Mater. Interfaces 11, 15543 (2019)
B. Feng, X. Jiang, Z. Pan, L. Hu, X. Hu, P. Liu, Y. Ren, G. Li, Y. Li, X. Fan, Mater. Des. 185, 108263 (2020)
S. Das, R. Chetty, K. Wojciechowski, S. Suwas, R.C. Mallik, Appl. Surf. Sci. 418, 238 (2017)
Q. Zhang, C. Chen, N. Li, Q. Huang, Y. He, X. Liu, B. Wang, D. Zhang, D.Y. Kim, Y. Wang, B. Xu, W. Yang, J. Phys. Chem. C 122, 15929 (2018)
J. Sui, J. Li, J. He, Y.L. Pei, D. Berardan, H. Wu, N. Dragoe, W. Cai, L.D. Zhao, Energy Environ. Sci. 6, 2916 (2013)
J. Lei, W. Guan, D. Zhang, Z. Ma, X. Yang, C. Wang, Y. Wang, Appl. Surf. Sci. 473, 985 (2019)
H. Zhu, Y. Li, H. Li, T. Su, C. Pu, Y. Zhao, Y. Ma, P. Zhu, X. Wang, High Press. Res. 37, 36 (2017)
B. Feng, G. Li, Z. Pan, H. Xiaoming, L. Peihai, H. Zhu, L. Yawei, X. Fan, J. Alloys Compd. 754, 131 (2018)
B. Feng, G. Li, X. Hu, P. Liu, R. Li, Y. Zhang, Y. Li, Z. He, X. Fan, J. Mater. Sci. Mater. Electron. 31, 4915 (2020)
R.B. Bhoomika, K. Gurukrishna, N.P. Madhukar, U.D. Shanubhogue, A. Rao, R.Y. Huang, Y.K. Kuo, K.K. Nagaraja, J. Mater. Sci. Mater. Electron. 34, 1 (2023)
K. Gurukrishna, H.R. Nikhita, S.M.M. Swamy, A. Rao, Met. Mater. Int. 28, 2023 (2022)
D.H. Kim, H.Y. Hong, J.K. Lee, S.D. Park, K. Park, J. Mater. Res. Technol. 9, 16202 (2020)
B. Feng, G. Li, Z. Pan, Y. Hou, C. Zhang, C. Jiang, J. Hu, Q. Xiang, Y. Li, Z. He, X. Fan, Mater. Lett. 217, 189 (2018)
U. Holzwarth, N. Gibson, Nat. Nanotechnol. 6, 534 (2011)
A.W. Burton, K. Ong, T. Rea, I.Y. Chan, Microporous Mesoporous Mater. 117, 75 (2009)
N. Rani, S. Chahal, A.S. Chauhan, P. Kumar, R. Shukla, S.K. Singh, Mater. Today Proc. 12, 543 (2019)
M. Khan, A. Mishra, J. Shukla, P. Sharma, A.I.P. Conf, Proc. 2100, 1 (2019)
M. Rabiei, A. Palevicius, A. Monshi, S. Nasiri, A. Vilkauskas, G. Janusas, Nanomaterials 10, 1 (2020)
A. Khorsand Zak, W. H. Abd. Majid, M. E. Abrishami, and R. Yousefi, Solid State Sci. 13, 251 (2011).
B. Feng, G. Li, Z. Pan, X. Hu, P. Liu, Y. Li, Z. He, X. Fan, J. Solid State Chem. 265, 306 (2018)
J. Hoffmann, P. Moschkau, S. Mildner, J. Norpoth, C. Jooss, L. Wu, Y. Zhu, Mater. Res. Express 1, 046403 (2014)
K. Gurukrishna, S. Mangavati, A. Rao, P. Poornesh, V.C. Petwal, V.P. Verma, J. Dwivedi, J. Mater. Sci. Mater. Electron. 33, 22270 (2022)
K. Gurukrishna, A. Rao, Y. Chung, Y. Kuo, J. Solid State Chem. 307, 122755 (2022)
A. Suwardi, D. Bash, H.K. Ng, J.R. Gomez, D.V.M. Repaka, P. Kumar, K. Hippalgaonkar, J. Mater. Chem. A 7, 23762 (2019)
K. Chang, C. Liu, Comput. Phys. Commun. 247, 106875 (2020)
H.S. Kim, Z.M. Gibbs, Y. Tang, H. Wang, G.J. Snyder, APL Mater. 3, 1 (2015)
G.J. Snyder, A.H. Snyder, M. Wood, R. Gurunathan, B.H. Snyder, C. Niu, Adv. Mater. 32, 2001537 (2020)
X. Zhang, Z. Bu, X. Shi, Z. Chen, S. Lin, B. Shan, M. Wood, A.H. Snyder, L. Chen, G.J. Snyder, Y. Pei, Sci. Adv. 6, 6 (2020)
A. Bhaskar, R.T. Lai, K.C. Chang, C.J. Liu, Scr. Mater. 134, 100 (2017)
R. Xu, Z. Chen, Q. Li, X. Yang, H. Wan, M. Kong, W. Bai, N. Zhu, R. Wang, J. Song, Z. Li, C. Xiao, B. Ge, Research 6, 1 (2023)
J. Le Lan, C. Deng, W. Ma, G.K. Ren, Y.H. Lin, X. Yang, J. Alloys Compd. 708, 955 (2017)
Acknowledgements
One of the authors (AR) acknowledges DST-FIST under Grant (SR/FIST/PS-1/2017/8) and Council of Scientific and Industrial Research under Grant [Sanction no: 03(1409)/17/EMR-II] for the financial support required for this work.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal
Author information
Authors and Affiliations
Contributions
Mr. MN: methodology, formal analysis, and writing—original draft. Dr. GK: conceptualization, formal analysis, writing—review & editing, and supervision. Ms. BRB: methodology and formal analysis. Ms. DSU: methodology and formal analysis. Dr. SM: methodology and formal analysis. Dr. AR: project administration, funding acquisition, supervision, resources, and writing—review & editing. Dr. SC: writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Madhukar, N.P., Gurukrishna, K., Bhat, B.R. et al. Role of sintering temperature in modulating the charge transport of BiCuSeO thermoelectric system: correlations to the microstructure. Appl. Phys. A 130, 55 (2024). https://doi.org/10.1007/s00339-023-07218-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-07218-4