Abstract
Photocatalytic membranes (PMR) have significant potential for utilization in energy-efficient water purification and wastewater treatment. The integration of membrane filtration's physical separation with photocatalysis's organic degradation is facilitated by their respective capabilities. In the present study, a more advanced graphene oxide (GO) membrane with improved photocatalytic properties was developed. This was achieved by incorporating tin dioxide (SnO2) and titanium dioxide (TiO2) nanoparticles (NPs) into a polyvinyl chloride (PVC) matrix, resulting in the fabrication of a microfiltration flat sheet membrane. The hydrophilicity of the membrane surface was investigated. The existence of NPs on membrane surfaces was demonstrated by FESEM images, Raman spectra, and FT-IR measurements. The porosity was affected by the addition of NPs; it increased from 59 to 76, and 92 for GO/TiO2, and GO/SnO2 respectively. The relationship between photocatalysis and filtration was investigated. Each nanocomposite membrane displayed a greater water flux and removal efficiency than a blank PVC membrane. Whereas the water flux enhanced from 1.3 to 17.6, and 20.5 for GO/TiO2, and GO/SnO2 respectively. Sunlight improves water flow and rejection compared to darkness. This research provides an alternative and highly efficient photocatalytic membrane for removing organic compounds from water, as the GO/SnO2 nanocomposites membrane exhibits the highest photocatalytic degradation up to a rejection rate of 98% when compared to an unmodified membrane.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Currently, membranes and membrane-based processes are used in both cleaning technology and the environmental field [1, 2]. New membrane materials with characteristics suitable for their applications are now required due to the effectiveness and utility of membrane applications. Therefore, the qualities of the membrane are determined by the type of material employed and the method of production. Membrane fouling, which can reduce filtration efficiency and membrane life, presents a significant financial and technical challenge to expanding applications [3, 4]. Ultrafiltration membranes require polymeric membranes as a fundamental component due to their low cost, high efficiency, and mobility. However, their hydrophobic nature makes them susceptible to membrane fouling [5, 6].
Consequently, a number of techniques, such as surface and blending modification, have been used to reduce or minimize membrane fouling [7,8,9,10]. Recent innovations in polymer-blended modifications have focused on the incorporation of inorganic nanoparticles (NPs) with casting solutions to produce organic–inorganic hybrid membranes [11]. To modify polymeric membranes, NPs, such as ZnO [12], SnO2 [13], Al2O3 [14, 15], ZrO2 [16, 17], TiO2 [18,19,20], Fe3O4 [21], and carbon nanotubes [22], are preferred. TiO2 is particularly promising due to its safety, affordability, photocatalytic, and superhydrophilicity properties [23].
The use of photocatalytic membrane reactors, which contain thin layers of immobilized photocatalysts on membranes, is an alternative [24,25,26]. PMR has a higher surface-to-volume ratio than standard thin-film photocatalysts, because the coating permeates the pores [27,28,29,30]. The potential resolution of the construction paradigm for photocatalytic water treatment systems can be achieved through the utilization of a PMR [31, 32]. Titanium dioxide (TiO2), a desirable material due to its low toxicity, low cost, and oxidative solid power, has been the focus of most research on photocatalytic degradation of microscopic pollutants [33,34,35]. Polyethersulfone (PES) [36], polysulfone (PSF) [37], polyvinylidene fluoride (PVDF) [38,39,40], polyacrylonitrile (PAN) [41] PVC [42], and cellulose acetate (CA) [43] have all utilized in the fabrication of membranes for PMR using the phase inversion method.
TiO2 is essential for customizing permeability, promoting surface hydrophilicity, increasing pollutant removal using ultraviolet (UV) light, and enhancing membranes' ability to self-clean and resist fouling [26, 44, 45]. However, the high surface energy of TiO2 and its strong propensity to aggregate in the polymer matrix [46], as well as the low photocatalytic effectiveness caused by the fast rate of charge recombination inside TiO2 NPs [47], are significant issues that preclude its practical use in ultrafiltration technology. As a result, numerous approaches have been developed to address these issues, including the incorporation of metal or nonmetal doping [48, 49], linking with other semiconductors [50], and integration with carbon materials [51,52,53,54,55]. Due to their unique characteristics, graphene oxide (GO) or else reduced graphene oxide (RGO) when used in combination with TiO2, is thought to be an efficient solution to the issues [56, 57]. The newly developed GO [58, 59] is another potential match for polymeric membrane modification. Furthermore, GO is the perfect nanomaterial for adhering to TiO2 and enhancing TiO2's photocatalytic effectiveness because of its wide surface area and strong charge carrier efficiency [60, 61].
New technological possibilities in the water filtration process have been made possible by graphene–metal nanoparticle composites or graphene–semiconductor materials. Recent studies have shown that heterogeneous photocatalysis is crucial to increasing the effectiveness of the photocatalytic process. One effective approach to improve photocatalytic activity and stability involves the combination of graphene with metal oxide semiconductors and other NPs. The e-/h+ recombination mechanism, which lowers photocatalytic efficiency, limits the uses. Different semiconductors have been coupled, outperforming charge separation and boosting photocatalytic effectiveness [62]. The effectiveness of the membrane is increased when GO or RGO is added. Because GO's carbonyl, hydroxyl, and carboxylic functional groups increase the membrane's hydrophilicity and enhance its mechanical properties, adding GO to the photocatalytic membrane is essential to avoid membrane fouling.
The daily use of photocatalysts is enhanced by graphene's increased surface area, improved mechanical strength, decreased aggregation, corrosion inhibition, leakage, electron scavengers, increased photosensitivity, and other properties [63]. Adding an additional band between the two levels transforms the semiconductor from a semiconductor with a wide bandgap to a semiconductor with a confined bandgap, which also increases UV absorption in the visible spectrum. Incorporating graphene into semiconductors appears to be the solution for increasing the photoactivity of nanocomposites. For the transport of the photogenerated e-/h+ pair between the semiconductor and graphene, the interfacial connection, or connection between the linked semiconductors, is required [64, 65].
Due to the well-established coupling of GO and TiO2 in the published literature [66, 67], GO/TiO2 nanocomposites provide numerous advantages. There are two key factors that contribute to the exceptional nature of GO as a platform for anchoring TiO2 and facilitating a closer and longer lasting interaction between TiO2 and pollutants. This interaction has the potential to reduce accumulation and enhance the photocatalytic efficiency of GO/TiO2 nanocomposites [68] and improve the photocatalytic efficiency of GO/TiO2 nanocomposites. First, the electronic characteristics of GO may encourage electron transfer and reduce the carrier recombination process [69, 70].
To gain insight into the practical applications of nanoparticles and composites, it is imperative to devise straightforward, distinctive, environmentally sustainable, and cost-effective methods for producing high-performance nanoparticles. This objective can be readily achieved by effectively controlling the shape, dimension, dispersity, and distribution of nanoparticles/nanocomposites [71].
Various studies have been conducted to create GO nanocomposite membranes for PMR. Yan et al. mix GO on polyacrylonitrile support and connect GO nanosheets to produce GO/TiO2 photocatalytic membrane via polyethyleneimine [72]. Chen et al. used a polysulfone membrane and N-TiO2/graphene to create a photocatalytic membrane [73]. Gao et al. 2014 used TiO2/GO with polysulfone to improve their photocatalytic activities [74]. Xua et al. 2017 created a novel photocatalytic membrane using nitrogen-doped graphene/TiO2 nanocomposites with polysulfone (PSF) [74]. Xua et al. 2016 supplied GO/TiO2 nanocomposites into the PVDF matrix [70]. Vatanpour et al. 2021 used polyethersulfone to create N-doped porous graphene oxide (N-PGO) membranes [75]. Croitoruo et al. fabricated graphene oxide (GO) and chitosan (CS) composite membranes [76]. Mahlangu et al. prepared the GO–ZnO/PES mixed matrix PMR with various GO–ZnO loadings using a unique and straightforward double-casting phase inversion approach [77]. Liu et al. 2023 created a photocatalytic self-cleaning graphene oxide (GO) membrane using g-C3N4 nanofillers and polydopamine (PDA) [78]. Tran et al. 2020 developed the nanocomposite titania- graphene oxide (TiO2–GO) hybrids after immobilizing them on the surface of hydrophobic microporous poly (vinylidene fluoride) (PVDF) membranes to produce photocatalysis synergy [79].
The novelty of this study lies in the development of a high-performance photocatalytic membrane that exhibits superior selectivity and permeate flux. This achievement was made possible through the utilization of a green synthesis method to prepare highly efficient, eco-friendly, and cost-effective photocatalyst nanoparticles. In this research, the synthesis of SnO2 and TiO2 NPs was conducted utilizing Seder leaves and pomegranate peels. These two types were chosen for specific reasons, the most important of which is that they contain many important compounds (reducing agents) such as flavonoids responsible for NPS reduction and their imprisonment provides these materials in abundance in the environment surrounding us, as well as because they are considered waste and thus have a low cost, in addition to being considered a way to recycle plant waste. Additionally, the preparation of graphene oxide (GO) nanosheets using a modified Hummer approach involved the utilization of pomegranate peel extract. The prepared NPs were dispersed in a PVC casting solution that was diluted to a relatively low concentration. This resulted in the formation of GO/TiO2 and GO/SnO2 PVC composite membranes.
2 Materials and methods
2.1 Chemicals and materials
Poly(vinyl chloride) (PVC, high molecular weight), polyvinylpyrrolidone (K25) N, and N-dimethylacetamide (DMAc, 99%), were obtained from Qualikem Fine Chem Pvt. Ltd. (India). Humic acid (60%), titanium tetrachloride (98%) (TiCl4), Stannous chloride SnCl4, Potassium Permanganate (KMnO4), graphite, Sulphuric acid (H2SO4 (99%), and phosphoric acid (H3PO4 (99%), were obtained from Loba (India), Seder leaves, Pomegranate peels.
2.2 Preparation of NPS
2.2.1 Seder extract
A total of 140 g of freshly picked Seder leaves were thoroughly rinsed under running water to remove any dust. The leaves were subsequently subjected to multiple DW washes. The Seder leaves were then added to 700 mL of DW, which was heated and refluxed for 2 h to create a light-yellow solution. Previously, the solution was allowed to cool while being stored at room temperature. After cooling, Whatman filter sheets were used to remove the desired extract from the extract.
2.2.2 Pomegranate extract
The pomegranates were repeatedly washed under running water to remove dust. After that, DW was used multiple times to wash the pomegranate. Fresh pomegranate peels weighing 240 g were cut into small pieces, heated in 1200 mL DW until boiling, and then cooled at room temperature. After cooling, the extract was filtered through Whatman filter papers to obtain the necessary extract.
2.2.3 Preparation of NPs
2.2.3.1 Preparation of TiO2 NPs
A volume of 500 mL of pomegranate extract was subjected to agitation for a duration of 30 min, followed by refrigeration. The beaker, which held the plant extract, was incrementally filled with a moderate quantity of TiCl4 (100 mL). The reaction was submerged in an ice bath for a full hour. Subsequently, the temperature was raised to 80 °C and maintained at this level for 2 h. Before drying at 70 °C, the TiO2 NPs were washed several times with DW. The powders were then calcined for 3 h at 450 °C [80].
2.2.3.2 Preparation of SnO2 NPs
After stirring 500 ml of Seder extract for 30 min, 20 g from SnCl2·2H2O were added gradually. Reaction kept for 30 min in the same conditions. After that, the temperature was raised to 80 °C. The solution was kept in these conditions for 2 h. The particle was cleaned with DW multiple times and then dried at 80 °C. Finally, it was calcined at 450 °C for 3 h, as demonstrated in Fig. 1.
2.2.3.3 GO preparation
A modified version of the Hummers method was used to produce GO. Afterward, 40 ml H3Po4 and 360 ml H2SO4 were combined in an ice bath using a stirrer. After 15 min, 3 g of graphite powder were added gradually, followed by 18 g of potassium permanganate. The reaction was kept for 72 h at room temperature. Hydrogen peroxide was added drop by drop until a yellowish-brown color appeared, after which GO nanosheets were produced. The obtained NPs were washed with HCl and DW multiple times before being dried at 50 ◦C for 24 h [81].
2.3 Preparation of photocatalytic nanocomposite membranes
Photocatalytic flat sheet membranes were created using phase inversion. 2 g of NPs were initially ultrasonically dispersed for 1 h in 84 g of DMAc. After adding 2 g of PVP, 12 g of PVC was dissolved in the solution with vigorous stirring to produce a homogenous casting solution. Subsequently, the degassed mixture is poured onto a glass plate, utilizing a casting knife with a thickness of 200 µm, which is carefully drawn across a nonwoven fabric. To complete the solvent/nonsolvent separation, the cast films were immersed in a coagulation bath of distilled water without the solvent evaporating. The prepared flat sheet membrane was thoroughly coagulated, rinsed multiple times in DW to remove residual solvent, and stored in clean water for additional testing.
2.4 Characterization
XRD diffractometer A D8-Advance Bruker AXS diffractometer was used to identify the structure and crystallinity of the synthesized NPs.
The high-resolution transmission electron microscopy (HRTEM) was used to describe their morphology with an accelerating voltage of 100 kV, TEM-1230 (JEOL Co., Japan) performed transmission electron microscopy.
Field emission scanning electron microscope (FESEM) Field emission scanning electron microscope, model JSM-T20 JEOL, Japan, was used to describe the cross-sectional, and surface morphologies of the prepared membranes.
The surface roughness: topography and roughness Using an atomic force microscope (AFM) (Agilent Technologies, 5600 LS), the roughness of the top membrane surfaces was examined.
A contact angle goniometer (Attention, Theta, by Biolin Scientific) outfitted with image-processing software was used to calculate the membrane's contact angle. This instrument was used to test how hydrophilic the additional NPs was.
FT-IR measurement The chemical components of the manufactured membrane's surface were discovered using Fourier transform infrared spectroscopy (FT-IR, PE-100, US). All of the spectra were recorded in wavenumber between 400 and 4000 cm−1 with the samples mounted on a sample holder.
Raman analyses A Confocal Raman Microscope (Witec, 300alpaR, made in Germany) was used to conduct Raman studies. A laser (785 nm) was used to excite the samples, and measurements were taken with the microscope's beam path adjusted to 50X.
2.5 Mechanical characterization
An electric elastic yarn strength analyzer was used to assess the membranes' mechanical characteristics, such as their tensile strength and elongation (YG020B, Nantong Sansi Co. Ltd., China). The measurements were performed at room temperature with a constant crosshead speed of 2 mm/min.
2.6 Porosity, pore radius, and pure water flux of membranes
Porosity using the following equation Eq. (1) [82], water uptake tests were used to calculate the overall porosity (\(\varepsilon\) %) of membranes as a function of membrane weight:
where the weights of the membrane in its wet and dry conditions are m1 and m2, respectively. A stands for the evaluated membrane sample's effective area, d for the membrane's thickness, and ρ for pure water's density. The synthetic membranes were submerged in DW for 24 h before the measurement to achieve the equilibrium swelling state.
The total volume of the porous membrane divided by the volume of the holes is known as the membrane porosity. The membrane will be weighed, dried in an oven for 24 h at 100 °C, soaked overnight in deionized water at room temperature, and wiped dry reweighing after wiping away any surface moisture with absorbent paper. The mean membrane pore radius was calculated using the Guerout–Elford–Ferry equation (Eq. 2) [83,84,85,86]
where L is the membrane thickness (m), Q is the permeate water volume per unit time (m3 s−1),), η is the water viscosity (8.9 × 10–4 Pa s), and ΔP is the load pressure (Pa). The generated membranes' water flux J (L m2 h−1) was determined using Eq. (3) [87]
where L stands for permeate, A for the effective membrane area (m), and V for permeate per unit time (h).
2.7 Photocatalytic performance tests
The performance of the membranes as prepared for photocatalysis was tested using degradation systems with dark and sunlight. For the dynamic system, the transmembrane pressure was fixed at 1.65 MPa, and the humic acid concentration in the feed water was 500 mg L−1, with pH 9.5 and temperature 25 °C. Samples of the reaction solution collected at predetermined intervals during visible-light irradiation. The humic acid concentration was measured using a Cary UV-2450 spectrophotometer at 254 nm [88]. The following equation describes the degradation efficiency of humic acid Eq. (4), represents the photocatalytic activity, Degradation of humic acid (η%), C0, and C are the concentration of humic acid before and after treatment [Eq. (4)] [89, 90]
3 Results and discussion
3.1 NPs’ characterization
3.1.1 XRD
XRD spectrum was used to identify the crystal structure of the produced NPs and the crystallite size. The XRD configurations of the prepared GO, TiO2, and SnO2 NPs, as shown in Fig. 2. The typical XRD configuration of the prepared SnO2 NPs demonstrated numerous well-defined diffraction reflections. The appearance of diffraction reflections at ((110) (101), (200), (211), (220), (002), (310), (112), (301), (202), (222), and (321) for SnO2 with a tetragonal structure Casseriet phase [File Card No:01-070-4176]. The typical XRD configuration of the prepared TiO2 NPs demonstrated numerous well-defined diffraction reflections. The appearance of diffraction reflections at (101), (112), (200), (213), (116), and (215) with a tetragonal structure anatase phase [File Card No: 04-014-0493] and characteristic peaks at (101), (112) (200), (211), (220), (002), (320), (301), and (310) with a tetragonal structure rutile phase [File Card No: 04-012-7240] for TiO2 NPs. Furthermore, characteristic peaks were indicated at 9.5, 17.8, 25.1, 26.3, 28.2, 34.1, 42.2, (001), and (202) for GO sheets. The sharpness and strong diffraction reflections in the XRD peaks confirmed that the prepared NPs have good crystallinity. No characteristic reflection allied to the impurities was detected in the pattern. Crystallite size was calculated according to sheerer equation [Eq. (5)] [91]. It was 8 and 25.3 nm for SnO2 and TiO2, respectively
3.1.2 FT-IR
FT-IR analysis for the extracts: The stretching frequency corresponding to the hydroxyl group (O–H) appeared at 3434 and 3446 cm−1 (alcohols-phenols) for Pom and Seder extract, respectively, and the stretching frequency corresponding to the alkane group (C–H aliphatic) appeared at 2930 cm−1 for two extracts. The functional group O–H bending appeared at 1647 cm−1 for Pom, and the asymmetric stretching frequency of the Alkene group (C=C) emanated at 1630 for Seder extract, and functional group N–H bending appeared at 1545 cm−1 and 1540 cm−1 are corresponding to Pom. In addition, the C–C-stretching or O–H-bending peaks that appeared at 1462 cm−1 and 1450 cm−1 correspond to both Pom and Seder extract. Furthermore, the observed S=O bond at 1390 cm−1 is indicated for Seder extract only. Conversely, the C=S peak at 1340 cm−1 is indicated for Pom, but at 1390 cm−1, it is for Seder. Consequently, the C–O peak at 1226 cm−1 corresponds solely to Pom, whereas the S=O bond at 1060 cm1 and 1061 cm−1 corresponds to both Pom and Seder. The C–H bend appeared at 620 cm−1 for Seder extract, whereas, as shown in figure, this study revealed the presence of numerous functional groups that may be responsible for the reduction and stability of NPs. This study revealed the presence of many functional groups that may be responsible for the reduction and stability of NPs, as depicted in Fig. 3A [92]. The FT-IR spectrum of GO, SnO2, and TiO2 NP samples is displayed in Fig. 3B. The stretching vibrations of C=O were identified in the GO sample's absorption bands at 1723 cm−1. The C=C-stretching vibration (sp2-hybridized carbon atoms) was identified as the source of the absorption band at 1613 cm−1 [93]. Epoxy groups were detected in the symmetric stretching, asymmetric stretching, and deformation vibrations at 1087, 1042, 578, and 498 cm−1. The O–H-stretching vibration of the absorbed water molecules over the synthesized materials was confirmed by the broad absorption band in the range of 1913–3675 cm−1 [94, 95]. The peak at 420 and 431 cm−1 indicates the presence of TiO2 NPs [96, 97]. Peaks near 596 cm−1 and 465 cm−1 are attributed to the O–Sn–O vibration mode, showing that the products were transferred to SnO2 NPs with excellent crystallization [98].
3.1.3 HRTEM of the prepared NPs
TEM images depict TiO2 NPs as having a spherical form and a range of nanometric sizes [80, 99]. The SnO2 NPs are spherical and uniform-sized particles. The mutual influence between the particles, resulting from various factors such as van der Walls, capillary, and electrostatic forces, causes the particles to agglomerate to a high degree [98, 100], as demonstrated by the GO TEM images. It exhibits the classic characteristics of GO, including wrinkling and a thin, crumpled structure with numerous folds [101, 102]. The brilliant circular rings shown in the selected area electron diffraction (SAED) pattern indicate that the prepared NPs have a crystalline structure. A diffused ring and a brilliant spot in the SAED pattern indicate that the NPs well crystallized in nature [103], as shown in Fig. 4.
3.2 Membranes’ characterization
3.2.1 The cross-sectional FESEM of the prepared membranes
The cross-sectional FESEM images of the (blank, GO, GO/TiO2, and GO/SnO2) membranes are displayed in Fig. 5, respectively. With a thick skin layer and soft finger-like sublayers, all membranes have an asymmetrical porous structure. The system's kinetic and thermodynamic characteristics are changed by adding NPs to the PVC casting solution. Due to the NPs' hydrophilic characteristics, the coagulation bath's solvent (DMAc)–nonsolvent (water) exchange rate was accelerated or the diffusion of water to the polymeric film was improved. Furthermore, the membranes were neither weakened nor made brittle by the integration of NPs, because there were no obvious fractures on their surface. The white points on the surface of the membranes are NPs (inside the red circle) that have aggregated and are linked together. Because of the NPs' low concentration and good distribution in the nanocomposite membranes solution, there was no evidence of NP aggregation, indicating that the NPs were well dispersed in the casting solution and membrane matrix [82, 104,105,106,107].
3.2.2 FT-IR, and Raman spectrum of the prepared membranes
FT-IR was used to analyze the pure PVC and nanocomposite membranes' chemical structure (Fig. 6). All pure PVC signature peaks were visible in the composite membranes. This indicates that the entrapped of NPs did not disrupt the polymer structure. Specific bands and their assignments are summarized as: C–H-bond stretching vibrations were detected at 2910 cm−1. Peaks at 1436 cm−1 and 1667 cm−1 conform to the stretching of the C=C and carbonyl (C=O) groups, respectively. The combined stretching vibration of C–H causes the absorbing band at 1330 cm−1. Furthermore, the peak at 600 cm−1 was caused by the stretching vibration of C–Cl bond on the PVC.
The nucleophilic substitution of the PVC membrane, which resulted in some of the -Cl being present, was the origin of all the additional peaks listed above. FT-IR spectra have validated certain hydrophilic groups that may interact with polymer chains. The exchange rate of solvent/nonsolvent during the phase separation process improves as the hydrophilicity of the dope solution increases with the addition of the NPs. Absorbent bands at 968 cm−1 and 1245 cm−1 indicate bending vibration that occurs either inside or outside the C–H-bonding plate.
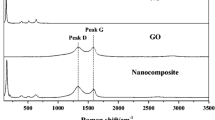
Raman spectra All blank and nanocomposite membranes were analyzed using Raman spectroscopy, as depicted in Fig. 7. Due to the C–Cl-stretching vibration, PVC exhibits two prominent characteristic peaks at 636 and 693 cm−1 [108,109,110,111]. All pure PVC signature peaks were visible in the composite membranes. Surprisingly, when GO, SnO2, and TiO2 NPs were added, the intensities of the peaks for PVC polymer were affected, confirming NP intercalation into the PVC matrix. The first-order E2g mode from sp2 carbon domains (G-band) and disorder mode (D-band), respectively, were visible in the Raman spectrum of GO at 1580–1620 and 1340–1365 cm−1, respectively [112, 113]. Two broad peaks at (1306 and 1585 cm−1) were observed in all GO nanocomposite membranes. There was an interface interaction force between the NPs and the polymer, as evidenced by the G-bands of all the membranes shifting in the high-frequency direction. This result demonstrates that the composite membranes' functional layer contained NPs [114]. The relative intensity ratio (ID/IG) of these bands is affected by several variables, such as faults and disorders (vacancies and grain boundaries), as well as by the amount of amorphous carbon in the graphite layer [94, 115]. The ID/IG intensity ratio shows the sp3-to-sp2 hybridization ratio [116]. The ratios were found to be 1.15 to GO and 1.14 to GO/TiO2 and GO/SnO2, respectively. It showed that the addition of TiO2 and SnO2 NPs increased the degree of disorder, which is consistent with a decrease in the crystal structure. According to factor group analysis, anatase has six Raman active modes (A1g + 2B1g + 3Eg). The six permitted modes, according to Ohsaka's analysis of the Raman spectra of an anatase single crystal, can be found at 144 cm−1 (Eg), 197 cm−1 (Eg), 399 cm−1 (B1g), 513 cm−1 (A1g), 519 cm−1 (B1g), and 639 cm−1 (Eg) [117,118,119]. The shift at 144 cm−1 is the strongest. This intense peak indicates that the TiO2 NPs in the membrane layer have some long-range order [120].
3.2.3 The water contact angle and the membrane porosity
Table 1 shows the produced membranes’ total porosity data. The porosity measurements showed that the produced membranes all had good porosity in the range of (59–75%). This table illustrates how the kind of NPs impacted the porosity of membranes. Thus, using NPs improves the membrane porosity's internal structure, which enhances the membrane characteristics and could lead to a rise in lateral flow rates through the membranes. Blank PVC membrane provides the lowest porosity and GO/TiO2 nanocomposite membrane was the greatest one. The pore radius also affected by the type of NPs. All prepared membranes have pore radius in the range of (100–1000) nm; this means that all membranes were considered as microfiltration membranes.
The hydrophilicity of PVC membrane surfaces was assessed using the water contact angle. The rule states that samples with a contact angle more than 90 degrees are hydrophobic and have poor wetting, whereas samples with a contact angle less than 90 degrees are hydrophilic. They were less than 90 degrees; the addition of NPs effects on the contact angle of the samples which effects on the water flux tests [121]. The modified PVC with GO/TiO2 membrane provides the lowest water contact angle of 60.5°, while modified PVC with GO membranes exhibits the greatest water contact angles (77.6), indicating that the hydrophilicity of the membrane surface was affected and Fig. 8 illustrates the contact angle of the (blank, GO, GO/TiO2, and GO/SnO2) membranes. Other studies have found that adding inorganic nanoparticles to polymeric membranes reduces the contact angle [122,123,124,125], and the same results have been reported in inorganic/PVC composite membranes [126, 127]. In general, increasing membrane hydrophilicity improves fouling resistance due to high absorption of water through the hydrophilic membranes surface than hydrophobic membranes [127, 128].
3.2.4 Mechanical properties
The mechanical properties of created membranes were tested to indicate the effect of the NPs. Tensile strength, and mechanical properties of blank, GO, GO/TiO2, and GO/SnO2 membranes are shown in Fig. 9. All modified membranes had higher tensile strength than the blank PVC membrane; the GO nanocomposite membrane being the best. The results showed that adding NPs to membranes increased their tensile strength, even though adding ribbons NPs to the membrane may cause it to lose elasticity. This can be attributed to the positioning of NPs within the membrane in the same direction as well as the positioning of perpendicular to the direction of the applied force. As a result of the reinforcement effect of inorganic NPs, incorporation of inorganic NPs improves mechanical strength of composite membranes.
While the additive of the NPs reduces the breaking elongation due to the high viscosity of the casting solution and the coalescence effect, accordingly, the elongation was reduced. As a result of their high surface area, aspect ratio, and excellent interaction with PVC, NPs reinforce PVC membranes. It can be concluded that the addition of NPs influences the mechanical properties of the membrane [129].
3.2.5 Membrane filtration performance
The effect of NPs on the pure water flux and rejection was investigated after 30 min in the dark, and the results are shown in Fig. 10. The pure water flux of the blank (PVC) membrane was 3.3 L/(m2 h) with an efficiency of 77.7%. The flux was reduced to 1.3 L/(m2 h) with the addition of GO, and the efficiency was enhanced to 97%, because it has the lowest porosity and high contact angle. The flux was increased to [16.7 and 20.5 L/(m2 h)] after using a couple of nanocomposite membranes (GO/TiO2 and GO/SnO2), respectively (Table 2). Due to the increase in the porosity of the membranes. The degradation efficiency of all nanocomposite membranes to humic acid wastewater was higher than that of the blank PVC membrane, showing improved rejection of nanocomposite membranes (GO, GO/TiO2, and GO/SnO2) in darkness, which may suggest advantages for NPs in improving the property of the membranes.
Three nanocomposite membranes (GO, GO/TiO2, and GO/SnO2) provide high rejection for humic acid wastewater, exhibiting a higher flux under sunlight irradiation than in the dark for all prepared nanocomposite membranes. In the reaction shown below, organic wastewater is degraded photocatalytically in the presence of metal oxide nanocomposite under sunlight irradiation. The reaction (Eqs. 6–11) that produces the hydroxyl radical (OH·) and superoxide anion (O·2¯), which is the primary catalyst for the breakdown of organic materials, is decreased by the e¯ in the conduction band CB (e¯CB). Following hydroxyl radical (OH·) decomposition, the organic contaminants in wastewater are mineralized to CO2 and H2O [130,131,132]
The flux for the coupled photocatalytic membrane (GO/TiO2 and GO/SnO2) was greater than the single GO photocatalytic membrane. The GO/TiO2 membrane achieves the highest flux under sunlight irradiation. Therefore, the photocatalysts' ability to absorb more visible light and the GO nanosheets' high charge mobility helped in electron transfer and separation. The inclusion of GO might have the effect of a semiconductor photosensitizer, changing the catalyst's Fermi level in a positive direction and improving visible-light absorption. Since GO has electrical solid conductivity, electrons moved through it quickly, which decreased the rate at which electrons and holes recombined [133, 134].
The addition of GO resulted in a significant enhancement of the catalyst's activity through improved effect coupling. This phenomenon occurred due to the substantial enhancement in the generation of photocarriers and the subsequent increase in their spatial separation resulting from the coupling. These mechanisms are depicted in Fig. 11. According to this scheme, when the GO/SnO2, GO/TiO2 nanocomposite is subjected to sunlight, the photoexcited electrons undergo a transfer from the cathodic conduction band (CB) levels of SnO2 or TiO2 to the anodic CB levels of GO. Therefore, the enhancement of interfacial charge migration to adsorbed molecules can be achieved through the extension of charge carrier lifetimes. The results of this study indicate that the ability of the PMR to degrade organic compound wastewater was greatly enhanced through the utilization of photocatalytic NPs. The primary benefit of the current study over previous studies is the adequate sunlight exposure time (30 min), which resulted in the degradation of 90% of organic contaminants. These findings confirmed that the modified PVC membrane's (GO/TiO2) photocatalytic activity in the presence of sunlight was superior to that of other modified membranes both with and without irradiation, showing considerable promise for wastewater decomposition and membrane fouling capability. The photocatalytic activity, efficiency, and flux during sunlight irradiation interval times for GO, GO/TiO2, and GO/SnO2 membranes are shown in Fig. 12. The present study compares the performance of PMR supplemented with inorganic nanomaterial, as reported in previous studies, with that of a SnO2/GO PVC membrane. The results demonstrate that the SnO2/GO PVC membrane exhibits exceptional properties, suggesting that these highly functional modified membranes have the potential to enhance water treatment techniques.
As shown in Table 3, the overall performance of photocatalytic membranes supplemented with inorganic nanomaterial published in the previous reports is compared with SnO2/GO PVC membrane in the work illustrated that SnO2/GO PVC membrane possessed an excellent property, indicating clearly that this high function modified membranes can expand the level of water treatment technique.
4 Conclusion
This study involved the synthesis of Green prepared TiO2 and SnO2 NPs using extracts derived from Pomegranate and Seder plants. Additionally, graphene oxide (GO) nanosheets were synthesized using a modified Hummer method. The prepared nanoparticle suspensions were utilized in the production of PVC nanocomposite membranes through the phase inversion method, with the aim of treating humic acid wastewater. The nanocomposite membrane exhibited notable alterations in surface morphology and surface roughness. In the interim, the incorporation of NPs serves to augment the porosity and hydrophilicity of the membrane, resulting in increased flux of pure water and improved rejection of humic acid. The nanocomposite membranes demonstrated photocatalytic activity when exposed to sunlight, as evidenced by the photodegradation and permeation of humic acid. The water flux and rejection rates were found to be enhanced under sunlight exposure, particularly for the GO/TiO2 and GO/SnO2 couples. This enhancement can be attributed to the efficient separation of photogenerated carriers and the subsequent increase in carrier lifetime, resulting in an overall increase in water flux. The GO/TiO2 composite exhibited superior performance compared to the other samples. These advantages demonstrated the promising potential of high-performance PMR for water filtration applications, such as GO/TiO2 and GO/SnO2 membranes.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
L. Yang, B. Tang, P. Wu, J. Mater. Chem. A Mater. 2, 18562 (2014)
F. Qu, H. Liang, J. Zhou, J. Nan, S. Shao, J. Zhang, G. Li, J. Membr. Sci.Membr. Sci. 449, 58 (2014)
L. Zhang, T.C.A. Ng, X. Liu, Q. Gu, Y. Pang, Z. Zhang, Z. Lyu, Z. He, H.Y. Ng, J. Wang, Appl. Catal. B 264, 118528 (2020)
X. Li, X. Fang, R. Pang, J. Li, X. Sun, J. Shen, W. Han, L. Wang, J. Membr. Sci.Membr. Sci. 467, 226 (2014)
F. Liu, N.A. Hashim, Y. Liu, M.R.M. Abed, K. Li, J. Membr. Sci.Membr. Sci. 375, 1 (2011)
B.S. Lalia, V. Kochkodan, R. Hashaikeh, N. Hilal, Desalination 326, 77 (2013)
T. Noeiaghaei, J.-O. Kim, S.-R. Chae, Curr. Org. Chem. 18, 2381 (2014)
L.Y. Ng, A.W. Mohammad, C.P. Leo, N. Hilal, Desalination 308, 15 (2013)
M. Hadidi, A.L. Zydney, J. Membr. Sci.Membr. Sci. 452, 97 (2014)
D. Rana, T. Matsuura, Chem. Rev. 110, 2448 (2010)
P.S. Goh, B.C. Ng, W.J. Lau, A.F. Ismail, Sep. Purif. Rev. 44, 216 (2015)
J. Hong, Y. He, Desalination 332, 67 (2014)
S. Zhang, R. Wang, S. Zhang, G. Li, Y. Zhang, Desalination 332, 109 (2014)
J. Garcia-Ivars, M.-I. Alcaina-Miranda, M.-I. Iborra-Clar, J.-A. Mendoza-Roca, L. Pastor-Alcañiz, Sep. Purif. Technol. 128, 45 (2014)
F. Liu, M.R.M. Abed, K. Li, J. Membr. Sci.Membr. Sci. 366, 97 (2011)
K. De Sitter, C. Dotremont, I. Genné, L. Stoops, J. Membr. Sci.Membr. Sci. 471, 168 (2014)
R. Pang, X. Li, J. Li, Z. Lu, X. Sun, L. Wang, Desalination 332, 60 (2014)
J.-P. Méricq, J. Mendret, S. Brosillon, C. Faur, Chem. Eng. Sci. 123, 283 (2015)
H. Song, J. Shao, Y. He, B. Liu, X. Zhong, J. Membr. Sci.Membr. Sci. 405, 48 (2012)
H.P. Ngang, B.S. Ooi, A.L. Ahmad, S.O. Lai, Chem. Eng. J. 197, 359 (2012)
Z.-Q. Huang, F. Zheng, Z. Zhang, H.-T. Xu, K.-M. Zhou, Desalination 292, 64 (2012)
E.J. Sisay, G. Veréb, Z. Pap, T. Gyulavári, Á. Ágoston, J. Kopniczky, C. Hodúr, G. Arthanareeswaran, G.K.S. Arumugam, Z. László, Chemosphere 307, 135589 (2022)
X. Liao, H.-T. Ren, B. Shen, J.-H. Lin, C.-W. Lou, T.-T. Li, Chemosphere 313, 137195 (2023)
O. Iglesias, M.J. Rivero, A.M. Urtiaga, I. Ortiz, Chem. Eng. J. 305, 136 (2016)
C.P. Athanasekou, N.G. Moustakas, S. Morales-Torres, L.M. Pastrana-Martínez, J.L. Figueiredo, J.L. Faria, A.M.T. Silva, J.M. Dona-Rodriguez, G.E.M. Romanos, P. Falaras, Appl. Catal. B 178, 12 (2015)
X. Zhang, T. Zhang, J. Ng, D.D. Sun, Adv. Funct. Mater. 19, 3731 (2009)
N. Wang, X. Zhang, Y. Wang, W. Yu, H.L.W. Chan, Lab Chip 14, 1074 (2014)
K. Li, Y. Zhang, L. Xu, L. Liu, Z. Wang, D. Hou, Y. Wang, J. Wang, Appl. Catal. B 264, 118512 (2020)
L. Paredes, S. Murgolo, H. Dzinun, M.H.D. Othman, A.F. Ismail, M. Carballa, G. Mascolo, Appl. Catal. B 240, 9 (2019)
C.R. Second, E. Edition, G. Ertl, H. Knözinger, F. Schüth, J. Weitkamp, W.-V. V. G. C. KGaA (2008)
E.L. Cates (2017)
S.K. Loeb, P.J.J. Alvarez, J.A. Brame, E.L. Cates, W. Choi, J. Crittenden, D.D. Dionysiou, Q. Li, G. Li-Puma, X. Quan (2018)
K. Nakata, A. Fujishima, J. Photochem. Photobiol. C 13, 169 (2012)
M.N. Chong, B. Jin, C.W.K. Chow, C. Saint, Water Res. 44, 2997 (2010)
R. Lyubimenko, O.I.G. Cardenas, A. Turshatov, B.S. Richards, A.I. Schäfer, Appl. Catal. B 291, 120097 (2021)
M. Peyravi, A. Rahimpour, M. Jahanshahi, A. Javadi, A. Shockravi, Microporous Mesoporous Mater. 160, 114 (2012)
A.T. Kuvarega, N. Khumalo, D. Dlamini, B.B. Mamba, Sep. Purif. Technol. 191, 122 (2018)
J. Yin, M. Roso, C. Boaretti, A. Lorenzetti, A. Martucci, M. Modesti, J. Environ. Chem. Eng. 9, 106250 (2021)
E. Erusappan, S. Thiripuranthagan, R. Radhakrishnan, M. Durai, S. Kumaravel, T. Vembuli, N.J. Kaleekkal, J. Environ. Chem. Eng. 9, 105776 (2021)
A. Yadav, P. Sharma, A.B. Panda, V.K. Shahi, J. Environ. Chem. Eng. 9, 105904 (2021)
S. Salehian, M.H. Mehdipour, F. Fotovat, S.A. Mousavi, Chemosphere 302, 134893 (2022)
X. Xu, Y. Wang, D. Zhang, J. Wang, Z. Yang, Chem. Eng. J. 429, 131058 (2022)
L. Kong, Q. Wang, Y. Wang, Q. Yan, W. Qiu, C. Zheng, Sustain. Horizons 5, 100047 (2023)
R.A. Damodar, S.-J. You, H.-H. Chou, J. Hazard. Mater. 172, 1321 (2009)
S. Leong, A. Razmjou, K. Wang, K. Hapgood, X. Zhang, H. Wang, J. Membr. Sci.Membr. Sci. 472, 167 (2014)
Y.H. Teow, A.L. Ahmad, J.K. Lim, B.S. Ooi, Desalination 295, 61 (2012)
S. Anandan, T. Narasinga Rao, M. Sathish, D. Rangappa, I. Honma, M. Miyauchi, ACS Appl. Mater. Interfaces 5, 207 (2013)
Y. Chen, W. Huang, D. He, Y. Situ, H. Huang, ACS Appl. Mater. Interfaces 6, 14405 (2014)
G. Wu, M.A. Nelson, N.H. Mack, S. Ma, P. Sekhar, F.H. Garzon, P. Zelenay, Chem. Commun. 46, 7489 (2010)
Y. Li, L. Zhu, Y. Guo, H. Song, Z. Lou, Z. Ye, J. Mater. Chem. A Mater. 2, 14245 (2014)
S. Linley, Y. Liu, C.J. Ptacek, D.W. Blowes, F.X. Gu, ACS Appl. Mater. Interfaces 6, 4658 (2014)
Y. Jiang, W.-N. Wang, P. Biswas, J.D. Fortner, ACS Appl. Mater. Interfaces 6, 11766 (2014)
G. Wu, K.L. More, P. Xu, H.-L. Wang, M. Ferrandon, A.J. Kropf, D.J. Myers, S. Ma, C.M. Johnston, P. Zelenay, Chem. Commun. 49, 3291 (2013)
J. Wang, Y. Chen, Y. Zhang, M.I. Ionescu, R. Li, X. Sun, S. Ye, S. Knights, J. Mater. Chem. 21, 18195 (2011)
J. Wang, G. Yin, Y. Shao, Z. Wang, Y. Gao, J. Phys. Chem. C 112, 5784 (2008)
X. Huang, X. Qi, F. Boey, H. Zhang, Chem. Soc. Rev. 41, 666 (2012)
Q. Xiang, J. Yu, M. Jaroniec, Chem. Soc. Rev. 41, 782 (2012)
C. Zhao, X. Xu, J. Chen, F. Yang, J. Environ. Chem. Eng. 1, 349 (2013)
Z. Wang, H. Yu, J. Xia, F. Zhang, F. Li, Y. Xia, Y. Li, Desalination 299, 50 (2012)
J. Wang, Y. Li, X. Sun, Nano Energy 2, 443 (2013)
Z. Jiang, J. Wang, L. Meng, Y. Huang, L. Liu, Chem. Commun. 47, 6350 (2011)
H. Derikvandi, A. Nezamzadeh-Ejhieh, J. Mol. Catal. A Chem. 426, 158 (2017)
P. Singh, P. Shandilya, P. Raizada, A. Sudhaik, A. Rahmani-Sani, A. Hosseini-Bandegharaei, Arab. J. Chem. 13, 3498 (2020)
S. Sakthivel, H. Kisch, Angew. Chem. Int. Ed. 42, 4908 (2003)
S.A. Mousa, S.Z. Noby, A.E. Shalan, in Advances in Nanocomposite Materials for Environmental and Energy Harvesting Applications (Springer, 2022), pp. 95–128
T.N. Lambert, C.A. Chavez, B. Hernandez-Sanchez, P. Lu, N.S. Bell, A. Ambrosini, T. Friedman, T.J. Boyle, D.R. Wheeler, D.L. Huber, J. Phys. Chem. C 113, 19812 (2009)
S. Morales-Torres, L.M. Pastrana-Martínez, J.L. Figueiredo, J.L. Faria, A.M.T. Silva, Environ. Sci. Pollut. Res. 19, 3676 (2012)
M. Safarpour, A. Khataee, V. Vatanpour, Sep. Purif. Technol. 140, 32 (2015)
P. Gao, D.D. Sun, Chem. Asian J. 8, 2779 (2013)
Z. Xu, T. Wu, J. Shi, K. Teng, W. Wang, M. Ma, J. Li, X. Qian, C. Li, J. Fan, J. Membr. Sci.Membr. Sci. 520, 281 (2016)
T. Iqbal, K. Munir, S. Afsheen, M. Zafar, M. Abrar, M.T. Qureshi, M. Al Elaimi, R.A. Hameed, R. Chand, G. Yunus, J. Inorg. Organomet. Polym. Mater.Inorg. Organomet. Polym. Mater. 32, 2221 (2022)
X. Yan, L. Huo, C. Ma, J. Lu, Process. Saf. Environ. Prot. 130, 257 (2019)
W. Chen, T. Ye, H. Xu, T. Chen, N. Geng, X. Gao, RSC Adv. 7, 9880 (2017)
H. Xu, M. Ding, S. Liu, Y. Li, Z. Shen, K. Wang, Polymer (Guildf) 117, 198 (2017)
V. Vatanpour, S.S.M. Khadem, A. Dehqan, M.A. Al-Naqshabandi, M.R. Ganjali, S.S. Hassani, M.R. Rashid, M.R. Saeb, N. Dizge, Chemosphere 263, 127892 (2021)
A.-M. Croitoru, A. Ficai, D. Ficai, R. Trusca, G. Dolete, E. Andronescu, S.C. Turculet, Materials 13, 1687 (2020)
O.T. Mahlangu, G. Mamba, B.B. Mamba, J. Environ. Chem. Eng. 11, 110065 (2023)
Y. Liu, M. Liao, D. Gan, M. Chen, L. Ma, B. Yang, L. Li, W. Tu, M. Zhu, S. Liu, Colloids Surf A Physicochem Eng AspPhysicochem. Eng. Asp. 663, 131080 (2023)
M.L. Tran, C.-C. Fu, L.-Y. Chiang, C.-T. Hsieh, S.-H. Liu, R.-S. Juang, J. Environ. Chem. Eng. 8, 104422 (2020)
S.A. Mousa, A.E. Shalan, H.H. Hassan, A.A. Ebnawaled, S.A. Khairy, J. Mol. Struct. 1250, 131912 (2022)
M.J.-Y. Tai, W.-W. Liu, C.-S. Khe, N.M.S. Hidayah, Y.-P. Teoh, C.H. Voon, H.C. Lee, P.Y.P. Adelyn, in AIP Conference Proceedings (AIP Publishing, 2018)
V. Vatanpour, S.S.M. Khadem, M. Masteri-Farahani, N. Mosleh, M.R. Ganjali, A. Badiei, E. Pourbashir, A.H. Mashhadzadeh, M.T. Munir, G. Mahmodi, J. Water Process Eng. 38, 101652 (2020)
N.A.A. Hamid, A.F. Ismail, T. Matsuura, A.W. Zularisam, W.J. Lau, E. Yuliwati, M.S. Abdullah, Desalination 273, 85 (2011)
T. Wu, B. Zhou, T. Zhu, J. Shi, Z. Xu, C. Hu, J. Wang, RSC Adv. 5, 7880 (2015)
J.-F. Li, Z.-L. Xu, H. Yang, L.-Y. Yu, M. Liu, Appl. Surf. Sci. 255, 4725 (2009)
S.P. Kusumocahyo, S.K. Ambani, S. Kusumadewi, H. Sutanto, D.I. Widiputri, I.S. Kartawiria, J. Environ. Chem. Eng. 8, 104381 (2020)
R.L. Ramos, L.B. Grossi, B.C. Ricci, M.C.S. Amaral, J. Environ. Chem. Eng. 8, 104056 (2020)
C.S. Uyguner, M. Bekbolet, Catal. Today 101, 267 (2005)
Y. Lee, T. Kim, B. Kim, S. Choi, K. Kim, J. Environ. Chem. Eng. 11, 109266 (2023)
M. Ahmad, M.T. Qureshi, W. Rehman, N.H. Alotaibi, A. Gul, R.S. Abdel Hameed, M. Al Elaimi, M.F.H. Abd el-kader, M. Nawaz, R. Ullah, J. Alloys Compd. 895 (2022)
N. Ullah, M.T. Qureshi, A.M. Toufiq, F. Ullah, M. Al Elaimi, R.S.A. Hameed, A. Khan, H.M.E. Ragab, Appl. Phys. A Mater. Sci. Process. 127 (2021)
M.S. Othman, S.T. Obeidat, G.M. Aleid, A.H. Al-Bagawi, M.A. Fareid, R.A. Hameed, K.M. Mohamed, M.S. Abdelfattah, A. Fehaid, M.M. Hussein, Appl. Sci. 13, 1050 (2023)
T.F. Emiru, D.W. Ayele, Egypt. J. Basic Appl. Sci. 4, 74 (2017)
R. Shyamala, L.G. Devi, Chem. Phys. Lett. 748, 137385 (2020)
A. Abd El-Fattah, H. Youssef, M.A.H. Gepreel, R. Abbas, S. Kandil, Polymers (Basel) 13, 3006 (2021)
R. Dobrucka, Iran. J. Pharm. Res. 16, 756 (2017)
S.S. Mathew, N.E. Sunny, V. Shanmugam, Inorg. Chem. Commun. 126, 108485 (2021)
A. Gaber, A.Y. Abdel-Latief, M.A. Abdel-Rahim, M.N. Abdel-Salam, Mater. Sci. Semicond. Process. 16, 1784 (2013)
D.A.A. Fadeel, M.S. Hanafy, N.A. Kelany, M.A. Elywa, Heliyon 7 (2021)
E. Gomathi, M. Jayapriya, M. Arulmozhi, Inorg. Chem. Commun. 130, 108670 (2021)
F. Du, B. Yang, X. Zuo, G. Li, Mater. Lett. 158, 424 (2015)
S. Karthick, H.-S. Lee, S.-J. Kwon, R. Natarajan, V. Saraswathy, Sensors 16, 2079 (2016)
D. Anbumani, K. vizhi Dhandapani, J. Manoharan, R. Babujanarthanam, A.K.H. Bashir, K. Muthusamy, A. Alfarhan, K. Kanimozhi, J. King Saud Univ. Sci. 34, 101896 (2022)
H. Rabiee, M.H.D.A. Farahani, V. Vatanpour, J. Membr. Sci.Membr. Sci. 472, 185 (2014)
M.H.D.A. Farahani, H. Rabiee, V. Vatanpour, J. Water Process Eng. 27, 47 (2019)
Q.F. Alsalhy, F.H. Al-Ani, A.E. Al-Najar, S.I.A. Jabuk, Chem. Eng. Process. Process Intensif. 130, 262 (2018)
M. Farjami, A. Moghadassi, V. Vatanpour, S.M. Hosseini, F. Parvizian, J. Ind. Eng. Chem. 72, 144 (2019)
R.W. Berg, in 2. Dansk Kemiingeniørkonference (Institut fpr Kemiteknik, DTU, 2006), pp. 136–137
S. Khakpour, Y. Jafarzadeh, R. Yegani, Chem. Eng. Res. Des. 152, 60 (2019)
V.S. Solodovnichenko, Y.G. Kryazhev, A.B. Arbuzov, V.P. Talzi, N.V. Antonicheva, V.A. Drozdov, E.S. Zapevalova, V.A. Likholobov, Russ. Chem. Bull. 65, 2712 (2016)
H.-H. Pham, S.-J. You, Y.-F. Wang, M.T. Cao, V.-V. Pham, Sustain. Chem. Pharm. 19, 100367 (2021)
E.J. Ekoi, A. Gowen, R. Dorrepaal, D.P. Dowling, Results Phys. 12, 1574 (2019)
D.V. Mashtalyar, I.M. Imshinetskiy, K.V. Nadaraia, A.S. Gnedenkov, S.N. Suchkov, D.P. Opra, E.V. Pustovalov, A.Y. Ustinov, S.L. Sinebryukhov, S.V. Gnedenkov, J. Magn. Alloys 11, 735 (2023)
H. Liu, C. Wang, Y. Qin, Y. Huang, C. Xiao, J. Taiwan Inst. Chem. Eng. 120, 278 (2021)
Y. Yang, L. Qu, L. Dai, T. Kang, M. Durstock, Adv. Mater. 19, 1239 (2007)
G. Williams, B. Seger, P.V. Kamat, ACS Nano 2, 1487 (2008)
T. Ohsaka, J. Phys. Soc. Jpn.Jpn. 48, 1661 (1980)
H.C. Choi, Y.M. Jung, S. Bin Kim, Vib. Spectrosc.Spectrosc. 37, 33 (2005)
M.C. Mathpal, A.K. Tripathi, M.K. Singh, S.P. Gairola, S.N. Pandey, A. Agarwal, Chem. Phys. Lett. 555, 182 (2013)
S. Mamedov, MRS Online Proc. Libr.Libr. 1806, 1 (2015)
A. Colburn, N. Wanninayake, D.Y. Kim, D. Bhattacharyya, J. Membr. Sci.Membr. Sci. 556, 293 (2018)
S. Balta, A. Sotto, P. Luis, L. Benea, B. Van der Bruggen, J. Kim, J. Membr. Sci.Membr. Sci. 389, 155 (2012)
J. Liang, Y. Jiao, M. Jaroniec, S.Z. Qiao, Angew. Chem. 124, 11664 (2012)
V. Vatanpour, S.S. Madaeni, R. Moradian, S. Zinadini, B. Astinchap, Sep. Purif. Technol. 90, 69 (2012)
G.M. Wu, S.J. Lin, J.H. You, C.C. Yang, Mater. Chem. Phys. 112, 798 (2008)
Z. Yu, X. Liu, F. Zhao, X. Liang, Y. Tian, J. Appl. Polym. Sci. 132 (2015)
H. Rabiee, V. Vatanpour, M.H.D.A. Farahani, H. Zarrabi, Sep. Purif. Technol. 156, 299 (2015)
E. Yuliwati, A.F. Ismail, Desalination 273, 226 (2011)
A. Behboudi, Y. Jafarzadeh, R. Yegani, Chem. Eng. Res. Des. 114, 96 (2016)
T. Liu, L. Wang, X. Liu, C. Sun, Y. Lv, R. Miao, X. Wang, Chem. Eng. J. 379 (2020)
R. Singh, V.S.K. Yadav, M.K. Purkait, Sep. Purif. Technol. 212, 191 (2019)
M.N. Subramaniam, P.S. Goh, W.J. Lau, A.F. Ismail, Sep. Purif. Technol. 275 (2021)
M. Safarpour, A. Khataee, V. Vatanpour, J. Membr. Sci.Membr. Sci. 489, 43 (2015)
T. Li, Y. Gao, J. Zhou, M. Zhang, X. Fu, F. Liu, Appl. Sci. 9, 855 (2019)
H. Xu, M. Ding, W. Chen, Y. Li, K. Wang, Sep. Purif. Technol. 195, 70 (2018)
L.A. Shah, T. Malik, M. Siddiq, A. Haleem, M. Sayed, A. Naeem, J. Environ. Chem. Eng. 7, 103291 (2019)
S.F. Zakeritabar, M. Jahanshahi, M. Peyravi, Catal. Lett.. Lett. 148, 882 (2018)
J. Pan, D. Hua, Y. Hong, X. Cheng, F. Guo, K.B. Tan, Z. Zhong, G. Zhan, Chem. Eng. J. 466, 143164 (2023)
T.D. Kusworo, A.C. Kumoro, N. Aryanti, H. Hasbullah, D.R.S. Chaesarifa, M.D. Fauzan, F. Dalanta, J. Environ. Chem. Eng. 11, 109449 (2023)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
SAM: conceptualization, investigation, writing—original draft, visualization, and writing—review and editing; HA: conceptualization, investigation, and writing—review and editing; SSI: review and editing; SAK: investigation and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mousa, S.A., Abdallah, H., Ibrahim, S.S. et al. Enhanced photocatalytic properties of graphene oxide/polyvinylchloride membranes by incorporation with green prepared SnO2 and TiO2 nanocomposite for water treatment. Appl. Phys. A 129, 831 (2023). https://doi.org/10.1007/s00339-023-07117-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-07117-8