Abstract
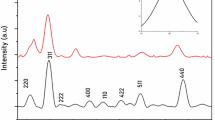
With the rapid development of population and technology, many pollutants are released into the environment. It is very crucial to protect the environment as well as human health. In this work, Iron oxide nanoparticles (IONPs) were synthesized by hydrothermal method and loaded into the chitosan salicylamide copper complex (CSC), the final sample was characterized by XRD, UV–Vis, FTIR, and SEM–EDX techniques. From the XRD analysis, the crystal structure was studied and the average particle size of the IONP was measured to be 18.79 nm. The UV–Vis was used to determine the presence of IONP by appearing corresponding intense peaks, it was also used to determine the energy gap value 2.1 eV for the IONP by the Tauc’s relation. FTIR spectrum determines the presence of Fe–O bond by appearing stretching frequencies at 538 cm−1 and 627 cm−1 and also confirms the presence of CSC by appearing relative stretching frequencies. The SEM graph has shown spherical morphology and uniform distribution of IONPs. The experimental study of photocatalytic degradation of nitrophenols was repeated within the time interval of 60 min at different light sources (UV-254 nm, UV-365 nm, UV-395 nm, Visible light, and Sunlight), at different pH values (2.5, 7 and 10.5) and different AOP techniques. Here, the AOP techniques are photocatalytic experiments with prepared photocatalyst (PC), photocatalyst combined with Fenton’s reagent (PFC) and, only with Fenton’s reagent (F). It is observed that the photocatalytic degradation of three nitrophenols reached 100% within 30 min under sunlight with PFC at a pH value of 2.5.













Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to privacy or ethical restrictions but are available from the corresponding author on reasonable request.
Abbreviations
- IONP:
-
Iron oxide nanoparticle or Fe3O4 nanoparticle
- CS:
-
Chitosan salicylamide ligand
- CSC:
-
Chitosan salicylamide copper complex
- PC:
-
Prepared photocatalyst
- PFC:
-
Photocatalyst combined with Fenton’s reagent
- MNP:
-
Mono-nitrophenol
- DNP:
-
Di-nitrophenol
- TNP:
-
Tri-nitrophenol
- CB:
-
Conduction band
- VB:
-
Valance band
References
H. Yu, H. Lin, Y. Xie et al., MUC1 vaccines using β-cyclodextrin grafted chitosan (CS-g-CD) as carrier via host–guest interaction elicit robust immune responses. Chin. Chem. Lett. 33, 4882–4885 (2022). https://doi.org/10.1016/j.cclet.2022.02.072
N. Yousefi, M. Emtyazjoo, M.N. Sepehr et al., Jou rna lP. Environ. Technol. Innov. (2020). https://doi.org/10.1016/j.eti.2020.101071
Z. Huang, J. Ding, X. Yang et al., Highly efficient oxidation of propane at low temperature over a Pt-based catalyst by optimization support. Environ. Sci. Technol. 56, 17278–17287 (2022). https://doi.org/10.1021/acs.est.2c05599
S.J. Jung, J.S. Mehta, L. Tong, Effects of environment pollution on the ocular surface. Ocul. Surf. 16, 198–205 (2018). https://doi.org/10.1016/j.jtos.2018.03.001
R. Bavandi, M. Emtyazjoo, H. Nasrollahzadeh et al., Study of capability of nanostructured zero-valent iron and graphene oxide for bioremoval of trinitrophenol from wastewater in a bubble column bioreactor. Electron. J. Biotechnol. 39, 8–14 (2019). https://doi.org/10.1016/j.ejbt.2019.02.003
A. Balakrishnan, G.J. Gaware, M. Chinthala, Chemosphere heterojunction photocatalysts for the removal of nitrophenol: a systematic review. Chemosphere 310, 136853 (2023). https://doi.org/10.1016/j.chemosphere.2022.136853
G. Li, S. Huang, K. Li et al., Near-infrared responsive Z-scheme heterojunction with strong stability and ultra-high quantum efficiency constructed by lanthanide-doped glass. Appl. Catal. B Environ. 311, 121363 (2022). https://doi.org/10.1016/j.apcatb.2022.121363
B. Usharani, G. Murugadoss, M.R. Kumar, S.G. Peera, Reduced graphene oxide–metal oxide nanocomposites (ZrO2 and Y2O3: fabrication and characterization for the photocatalytic degradation of picric acid. Catalysts 12(10), 1249 (2022). https://doi.org/10.3390/catal12101249
D. Chen, T. Savidge, Comment on “Extreme electric fields power catalysis in the active site of ketosteroid isomerase.” Science (80-) 349, 936b (2015). https://doi.org/10.1126/science.aab0095
R. Fatima, M.N. Afridi, V. Kumar et al., Photocatalytic degradation performance of various types of modified TiO2 against nitrophenols in aqueous systems. J. Clean. Prod. 231, 899–912 (2019). https://doi.org/10.1016/J.JCLEPRO.2019.05.292
M. Ismail, M.I. Khan, S.B. Khan et al., Catalytic reduction of picric acid, nitrophenols and organic azo dyes via green synthesized plant supported Ag nanoparticles. J. Mol. Liq. 268, 87–101 (2018). https://doi.org/10.1016/J.MOLLIQ.2018.07.030
Z. Xiong, H. Zhang, W. Zhang et al., Removal of nitrophenols and their derivatives by chemical redox: a review. Chem. Eng. J. 359, 13–31 (2019). https://doi.org/10.1016/J.CEJ.2018.11.111
W. Xie, K. Zhao, L. Xu et al., Oxalic acid cross-linked sodium alginate and carboxymethyl chitosan hydrogel membrane for separation of dye/NaCl at high NaCl concentration. Chin. Chem. Lett. 33, 1951–1955 (2022). https://doi.org/10.1016/j.cclet.2021.11.058
M.J. Muñoz-Batista, R. Luque, Heterogeneous photocatalysis. Chem. Eng. 5(2), 26 (2021). https://doi.org/10.3390/chemengineering5020026
Z. Lei, W. Hengliang, L. Zhang et al., A study on the catalytic performance of the ZrO2@γ-Al2O3 hollow sphere catalyst for COS hydrolysis. New J. Chem. 47, 7070–7083 (2023). https://doi.org/10.1039/D2NJ04970H
P. Xu, C. Ding, Z. Li et al., Photocatalytic degradation of air pollutant by modified nano titanium oxide (TiO2)in a fluidized bed photoreactor: optimizing and kinetic modeling. Chemosphere 319, 137995 (2023). https://doi.org/10.1016/j.chemosphere.2023.137995
W. Somraksa, S. Suwanboon, P. Amornpitoksuk, C. Randorn, Physical and photocatalytic properties of CeO2/ZnO/ZnAl2O4 ternary nanocomposite prepared by co-precipitation method. Mater. Res. (2020). https://doi.org/10.1590/1980-5373-MR-2019-0627
N.P. Ferraz, A.E. Nogueira, F.C.F. Marcos et al., CeO2–Nb2O5 photocatalysts for degradation of organic pollutants in water. Rare Met. (2019). https://doi.org/10.1007/s12598-019-01282-7
B. Fu, Q. Liu, M. Liu et al., Carbon dots enhanced gelatin/chitosan bio-nanocomposite packaging film for perishable foods. Chin. Chem. Lett. 33, 4577–4582 (2022). https://doi.org/10.1016/j.cclet.2022.03.048
A.M. Rao, D. Suresh, R. Sribalan, G. Sandhya, Nano-CeO2-loaded chitosan-bocglycine zinc complex for the photocatalytic degradation of picric acid by the combination of Fenton’s reagent. Appl. Phys. A 128, 742 (2022). https://doi.org/10.1007/s00339-022-05841-1
H.H. Bahjat, R.A. Ismail, G.M. Sulaiman, Photodetection properties of populated Fe3O4@TiO2 core–shell/Si heterojunction prepared by laser ablation in water. Appl. Phys. A Mater. Sci. Process. 128, 1–9 (2022). https://doi.org/10.1007/s00339-021-05139-8
B. Usharani, V. Manivannan, Enhanced photocatalytic activity of reduced graphene oxide-TiO2 nanocomposite for picric acid degradation. Inorg. Chem. Commun. 142, 109660 (2022). https://doi.org/10.1016/J.INOCHE.2022.109660
D. Hariharan, A. Jegatha Christy, J. Mayandi, L.C. Nehru, Visible light active photocatalyst: Hydrothermal green synthesized TiO2 NPs for degradation of picric acid. Mater. Lett. 222, 45–49 (2018). https://doi.org/10.1016/j.matlet.2018.03.109
B. Anusha, M. Anbuchezhiyan, R. Sribalan, Srinivasan alias Arunsankar N, Synergistic effect of TiO2-rGO nanocomposites with Fenton’s reagent for the enhanced photocatalytic degradation of nitrophenols in solar light. Appl. Phys. A Mater. Sci. Process. (2022). https://doi.org/10.1007/S00339-022-05554-5
Y. Li, Y. Zhang, Y. Zhang et al., Tripodal naphthalimide assembled novel AIE supramolecular fluorescent sensor for rapid and selective detection of picric acid. Dye Pigment (2020). https://doi.org/10.1016/j.dyepig.2020.108563
A.G. Ramu, S. Salla, S. Chandrasekaran et al., J. Proof Environ. Pollut. (2020). https://doi.org/10.1016/j.envpol.2020.116063
M.A. Mohd Adnan, L.P. Bao, N. Muhd Julkapli, Mitigation of pollutants by chitosan/metallic oxide photocatalyst: a review. J. Clean. Prod. 261, 121190 (2020)
Z. Li, W. Yang, L. Xie et al., Prominent role of oxygen vacancy for superoxide radical and hydroxyl radical formation to promote electro-Fenton like reaction by W-doped CeO2 composites. Appl. Surf. Sci. 549, 1–11 (2021). https://doi.org/10.1016/j.apsusc.2021.149262
A. Bouafia, S.E. Laouini, A. Khelef et al., Effect of ferric chloride concentration on the type of magnetite (Fe3O4) nanoparticles biosynthesized by aqueous leaves extract of artemisia and assessment of their antioxidant activities. J. Clust. Sci. 32, 1033–1041 (2021). https://doi.org/10.1007/s10876-020-01868-7
S.K. Sen, T.C. Paul, S. Dutta et al., XRD peak profile and optical properties analysis of Ag-doped h-MoO3 nanorods synthesized via hydrothermal method. J. Mater. Sci. Mater. Electron. 31, 1768–1786 (2020). https://doi.org/10.1007/s10854-019-02694-y
A.A. Kabure, B.S. Shirke, S.R. Mane et al., LPG gas sensor activities of CeO2-Fe2O3 nanocomposite thin film at optimum temperature. Appl. Phys. A Mater. Sci. Process. 127, 1–12 (2021). https://doi.org/10.1007/s00339-021-04849-3
A. Radoń, A. Drygała, Ł Hawełek, D. Łukowiec, Structure and optical properties of Fe3O4 nanoparticles synthesized by co-precipitation method with different organic modifiers. Mater. Charact. 131, 148–156 (2017). https://doi.org/10.1016/j.matchar.2017.06.034
T.M. Tiama, A.M. Ismail, H. Elhaes, M.A. Ibrahim, Structural and spectroscopic studies for chitosan/Fe3O4 nanocomposites as glycine biosensors. Biointerface Res. Appl. Chem. 13, 1–13 (2023). https://doi.org/10.33263/BRIAC136.547
A. Ara, R. Khattak, M.S. Khan et al., Synthesis, characterization, and solar photo-activation of chitosan-modified Nickel nagnetite bio-composite for degradation of recalcitrant organic pollutants in water. Catalysts 12(9), 983 (2022). https://doi.org/10.3390/catal12090983
A. Badawi, E.M. Ahmed, N.Y. Mostafa et al., Enhancement of the optical and mechanical properties of chitosan using Fe2O3 nanoparticles. J. Mater. Sci. Mater. Electron. 28, 10877–10884 (2017). https://doi.org/10.1007/s10854-017-6866-x
M. Shafaati, M. Miralinaghi, R.H.S.M. Shirazi, E. Moniri, The use of chitosan/Fe3O4 grafted graphene oxide for effective adsorption of rifampicin from water samples. Res. Chem. Intermed. 46, 5231–5254 (2020). https://doi.org/10.1007/s11164-020-04259-9
N. Kurnaz Yetim, F. Kurşun Baysak, M.M. Koç, D. Nartop, Characterization of magnetic Fe3O4@SiO2 nanoparticles with fluorescent properties for potential multipurpose imaging and theranostic applications. J Mater Sci Mater Electron 31, 18278–18288 (2020). https://doi.org/10.1007/s10854-020-04375-7
T. Kamakshi, G.S. Sundari, H. Erothu, R.S. Singh, Effect of nickel dopant on structural, morphological and optical characteristics of Fe3O4 nanoparticles. Rasayan J. Chem. 12, 531–536 (2019). https://doi.org/10.31788/RJC.2019.1225054
A. Azizi, Green synthesis of Fe3O4 nanoparticles and its application in preparation of Fe3O4/cellulose magnetic nanocomposite: a suitable proposal for drug delivery systems. J. Inorg. Organomet. Polym. Mater. 30, 3552–3561 (2020). https://doi.org/10.1007/s10904-020-01500-1
M. Fuentes-Pérez, M. Sotelo-Lerma, J.L. Fuentes-Ríos et al., Synthesis and study of physicochemical properties of Fe3O4@ZnFe2O4 core/shell nanoparticles. J. Mater. Sci. Mater. Electron. (2021). https://doi.org/10.1007/s10854-021-06236-3
R. Bagheri, P. Ariaii, A. Motamedzadegan, Characterization, antioxidant and antibacterial activities of chitosan nanoparticles loaded with nettle essential oil. J. Food Meas. Charact. 15, 1395–1402 (2021). https://doi.org/10.1007/s11694-020-00738-0
C. Rodrigues, J.M.M. de Mello, F. Dalcanton et al., Mechanical, thermal and antimicrobial properties of chitosan-based-nanocomposite with potential applications for food packaging. J. Polym. Environ. 28, 1216–1236 (2020). https://doi.org/10.1007/s10924-020-01678-y
A. Nithya, K. Jothivenkatachalam, S. Prabhu, K. Jeganathan, Chitosan based nanocomposite materials as photocatalyst—a review. Mater. Sci. Forum 781, 79–94 (2014). https://doi.org/10.4028/www.scientific.net/MSF.781.79
S. Govindan, E.A.K. Nivethaa, R. Saravanan et al., Synthesis and characterization of chitosan–silver nanocomposite. Appl. Nanosci. 2, 299–303 (2012). https://doi.org/10.1007/s13204-012-0109-5
R.P. Senthilkumar, V. Bhuvaneshwari, R. Ranjithkumar et al., Synthesis, characterization and antibacterial activity of hybrid chitosan-cerium oxide nanoparticles: as a bionanomaterials. Int. J. Biol. Macromol. 104, 1746–1752 (2017). https://doi.org/10.1016/j.ijbiomac.2017.03.139
S.B. Aziz, M.A. Brza, H.M. Hamsan et al., Electrochemical characteristics of solid state double-layer capacitor constructed from proton conducting chitosan-based polymer blend electrolytes. Polym. Bull. 78, 3149–3167 (2021). https://doi.org/10.1007/s00289-020-03278-1
W. Jiang, L. Zhang, X. Guo et al., Adsorption of cationic dye from water using an iron oxide/activated carbon magnetic composites prepared from sugarcane bagasse by microwave method. Environ. Technol. (United Kingdom) 42, 337–350 (2021). https://doi.org/10.1080/09593330.2019.1627425
T.M. Laid, K. Abdelhamid, L.S. Eddine, B. Abderrhmane, Optimizing the biosynthesis parameters of iron oxide nanoparticles using central composite design. J. Mol. Struct. 1229, 129497 (2021). https://doi.org/10.1016/j.molstruc.2020.129497
M. Aghazadeh, I. Karimzadeh, M.R. Ganjali, PVP capped Mn2+ doped Fe3O4 nanoparticles: a novel preparation method, surface engineering and characterization. Mater. Lett. 228, 137–140 (2018). https://doi.org/10.1016/j.matlet.2018.05.087
S.M. Mousavi, S.A. Hashemi, M. Zarei et al., Data on cytotoxic and antibacterial activity of synthesized Fe3O4 nanoparticles using Malva sylvestris. Data Br. 28, 104929 (2020). https://doi.org/10.1016/J.DIB.2019.104929
Z. Liu, J. Zheng, W. Liu et al., Identification of the key host phases of Cr in fresh chromite ore processing residue (COPR). Sci. Total Environ. 703, 135075 (2020). https://doi.org/10.1016/j.scitotenv.2019.135075
S.T. Shah, Z.Z. Chowdhury, J.M.R. Bin et al., Surface functionalization of magnetite nanoparticles with multipotent antioxidant as potential magnetic nanoantioxidants and antimicrobial agents. Molecules (2022). https://doi.org/10.3390/molecules27030789
M. Maruthupandy, T. Muneeswaran, M. Anand, F. Quero, Highly efficient multifunctional graphene/chitosan/magnetite nanocomposites for photocatalytic degradation of important dye molecules. Int. J. Biol. Macromol. 153, 736–746 (2020). https://doi.org/10.1016/j.ijbiomac.2020.03.045
P. Mandade, Introduction, basic principles, mechanism, and challenges of photocatalysis. Handb. Nanomater. Wastewater Treat. (2021). https://doi.org/10.1016/b978-0-12-821496-1.00016-7
N. Pani, V. Tejani, T.S. Anantha-Singh, A. Kandya, Simultaneous removal of COD and ammoniacal nitrogen from dye intermediate manufacturing industrial wastewater using Fenton oxidation method. Appl Water Sci 10, 1–7 (2020). https://doi.org/10.1007/s13201-020-1151-1
B. Anusha, M. Anbuchezhiyan, R. Sribalan, An efficient photocatalytic degradation of nitrophenols using TiO2 as a heterogeneous photo-Fenton catalyst. React. Kinet. Mech. Catal. 134, 501–515 (2021). https://doi.org/10.1007/s11144-021-02064-y
S.J. Peighambardoust, R. Foroutan, S.H. Peighambardoust et al., Decoration of Citrus limon wood carbon with Fe3O4 to enhanced Cd2+ removal: a reclaimable and magnetic nanocomposite. Chemosphere 282, 131088 (2021). https://doi.org/10.1016/j.chemosphere.2021.131088
M. Zhao, S. Li, M. Wang et al., Adsorption of water and formic acid molecules on the (104) surface of calcite: a theoretical study by DFT-D3. New J. Chem. 47, 8737–8743 (2023). https://doi.org/10.1039/D3NJ00925D
Z. Jiang, X. Huang, Q. Wu et al., Adsorption of sulfonamides on polyamide microplastics in an aqueous solution: behavior, structural effects, and its mechanism. Chem. Eng. J. 454, 140452 (2023). https://doi.org/10.1016/j.cej.2022.140452
Author information
Authors and Affiliations
Contributions
AMR: synthesis, characterization and manuscript preparation. DS: research work design and scientific discussions. RS: carried out the photocatalytic experiment and helped in manuscript writing. GS: developed the theoretical formalism and performed the analytic calculations.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Ethical approval
All co-authors ensure that accepted principles of ethical and professional conduct have been followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rao, A.M., Suresh, D., Sribalan, R. et al. Nano-Fe3O4-loaded chitosan salicylamide copper complex as a photocatalyst to degrade nitrophenols under the sunlight. Appl. Phys. A 129, 625 (2023). https://doi.org/10.1007/s00339-023-06899-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06899-1