Abstract
In this work, we report the influence of single-ionized oxygen vacancies (\(V^{\prime}_{{\text{O}}}\)) as a spin ½ system in the ferromagnetic response of undoped and Cr-doped SnO2 nanowires. For this study, undoped and Cr-doped SnO2 nanowires were synthesized by a thermal evaporation method. Raman, Auger, and X-ray photoelectron spectroscopies confirmed the incorporation of Cr3+ ions in the SnO2 lattice. Electron paramagnetic resonance measurements demonstrated the presence of single-ionized oxygen vacancies (\(V^{\prime}_{{\text{O}}}\)) in undoped and Cr-doped nanowires. Complementarily, cathodoluminescence measurements confirmed the presence of VO defects in the samples. Magnetic measurements revealed FM behavior from the undoped SnO2 and Cr-doped SnO2 nanowires, showing magnetization saturation values (MS) of ± 1 × 10–3 and ± 1.6 × 10–3 emu/g, respectively, and magnetic coercivity values (HC) of 180 and 200 Oe. We assign the FM response of nanowires to the presence of single ionized \(V^{\prime}_{{\text{O}}}\) acting as a spin ½ system and to the alignment of magnetic moments of Cr3+ ions, finding that \(V^{\prime}_{{\text{O}}}\) defects dominate in the FM generation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Tin oxide (SnO2) is a semiconductor that has received attention due to its use to fabricate gas sensors, solar cells, optoelectronic devices, and catalysts [1,2,3]. In addition, this semiconductor has also received importance in spintronics because it exhibits ferromagnetism (FM) at room temperature, induced by doping with non-magnetic and magnetic impurities [4,5,6]. SnO2 doped with some transition metals, such as chromium [7,8,9], exhibit this property, however, with an origin not straightforward. Several authors have proposed that FM is due to exchange interactions between magnetic impurities and native point defects type oxygen vacancies (VO) [7, 10]. However, there are reports of first principle calculations predicting that tin-vacancy (VSn) defects generate FM in undoped SnO2 and that oxygen vacancy (VO) does not contribute to it [11, 12]. In addition, other authors have suggested that ferromagnetism is a universal feature of metal oxide nanoparticles, assuming its origin to exchange interactions between unpaired electron spins and surface VO [13]. For SnO2 doped with magnetic impurities, Garcia-Tecedor et al. demonstrated that chromium doping of SnO2 nanostructures promotes rising in the luminescence associated with VO [14]. Furthermore, Duhan et al. recently compared the FM response of SnO2, SnO2:Cr, and SnO2:Fe, Cr, finding that the magnetic saturation increases in co-doped samples assigning this effect to the generation of VO [15]. Similarly, Urs et al. reported FM at room temperature in SnO2 nanoparticles doped with Cr3+ at concentrations lower than 5 at.%, attributing its origin to exchange interaction through F-centers of VO and dopant magnetic impurities, explaining such interaction through the Bound Magnetic Polaron (BMP) model [16]. The BMP model in DMS considers the formation of bound states, known as magnetic polarons, where a charge carrier, such as electrons, becomes trapped near a magnetic impurity, such as chromium [17, 18]. Therefore, a study to identify single electrons trapped in VO and another species present in SnO2:Cr, whose spins participate in the FM generation, can help to understand the FM origin in this semiconductor. In this work, we report the presence of single-ionized oxygen vacancies (\(V^{\prime}_{{\text{O}}}\)) in SnO2 nanowires doped with different concentrations of chromium using the electron paramagnetic resonance (EPR) technique, confirming the presence of VO by the cathodoluminescence (CL) technique.
2 Experimental methodology
SnO2 and chromium-doped SnO2 nanostructures were synthesized by thermal evaporation of SnO2 (Aldrich 99.99%) and CrO3 powders (Aldrich 99.99%) onto SiO2/Si(100) substrates, in a homemade horizontal furnace operated at 130 mTorr and using Ar (Infra 99.999%) as carrier gas. A mechanical pump maintained low pressure in the furnace while Ar flow was regulated by a needle valve and measured by a mass flow meter (Omega type FMA-A2302). For the synthesis of the undoped sample, SnO2 powder was placed in an alumina boat and maintained at 1300 °C. For the synthesis of Cr-doped samples, we prepared two samples, the first by maintaining SnO2 and CrO3 powders at 1300 and 1200 °C (sample 2), respectively; and the second by evaporating both SnO2 and CrO3 powders at 1300 °C (sample 3). This last to vary the evaporation rate of chromium in the furnace by considering that the melting temperature of the CrO3 is 197 °C at atmospheric pressure. The SnO2 and CrO3 vapor diffused and condensed onto the SiO2/Si(100) substrates, placed at the downstream end of the quartz tube furnace at 620 °C. For the formation of Cr3+ ions, we propose the decomposition of CrO3 and SnO2 precursors as follows:
Thus, we propose chromium incorporation in SnO2 following:
Table 1 shows the growth temperature, argon flow, and chemical composition of samples measured by EDS.
The relative atomic composition of the samples was characterized by energy-dispersive spectroscopy (EDS) using an Oxford X-Max analyzed. The elemental quantification was calculated by Inca software (Oxford instruments) using a standard base sequence. The crystal structure was determined with a Phillips X’pert X-ray diffractometer using a CuKα (λ = 0.154 nm) line excitation source. Raman spectroscopy characterization was carried out with a Dimension-P2 λs Raman system using an Nd 532 nm laser. X-ray photoelectron spectroscopy (XPS) and Auger electron spectroscopy (AES) were done in a PHI 535 system using an Al anode. High-resolution spectra were obtained using 500 scans during the XPS and AES measurements. AES measurements used an electron beam with an energy of 2 keV as the excitation source. All XPS spectra were calibrated using the C (1 s) signal (284.8 eV) as a reference and deconvoluted by software CASA XPS. Electronic paramagnetic resonance (EPR) measurements were carried out by using a JEOL, JES-TE300 system, operating at X-Band fashions at 100 kHz modulation frequency with a cylindrical cavity in the TE011 mode. The external calibration of the magnetic field was conducted using a JEOL ES-FC5 precision gaussmeter. The acquisition and manipulation of spectra were performed using ES-IPRITS/TE. EPR spectra were recorded as the first derivative. The experimental acquisition frequency was 9.44 GHz at room temperature and a microwave power of 20 mW. For transmission electron microscopy (TEM) measurements a Jeol JEM-2010 operated at 200 keV was used. Samples morphology was studied using JEOL JSM-7800F. A Mono-CL4 Gatan system adapted to a JEOL FIB-4500 SEM system measured the cathodoluminescence (CL) signal from samples at room temperature and in the UV–Vis spectral range.
3 Results and discussion
Table 1 lists the atomic composition of the samples measured by EDS, revealing that Sn concentration decreased by increasing the Cr amount in them, which suggests a substitutional incorporation of the magnetic impurity in the SnO2 lattice. These results also reveal a notable low chromium concentration in samples, which we assign to their limited solubility in SnO2, as reported by other authors [14, 16]. Furthermore, EDS results also show that the oxygen amount in samples decreased slightly due to Cr doping, corresponding with the absence of chromium oxide compounds and the possible generation of oxygen vacancies.
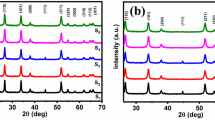
XRD patterns obtained from samples revealed diffraction peaks corresponding with the rutile-type tetragonal structure of SnO2 for all of them, as shown in Fig. 1, according to PDF card #88-0287. Such results did not reveal diffraction peaks associated with chromium oxide compounds, suggesting that Cr impurities were incorporated in the SnO2 lattice. Further, XRD patterns of samples showed that (110), (101), and (211) peaks exhibited higher intensity, in agreement with other reports about the X-ray diffraction of SnO2 nanowires [19, 20]. Figure 1 also shows diffraction peaks corresponding to metallic Sn, which we attribute to the self-catalytic VLS growth mechanism of SnO2 that we reported previously for undoped SnO2 nanowires also obtained by the thermal evaporation method [21].
Figure 2 shows typical SEM images obtained from samples, revealing the formation of large SnO2 nanowires with inhomogeneous diameters lower than 250 nm, besides the presence of irregular nanoparticles adhered to some Cr-doped SnO2 nanowires (Fig. 2b, c). EDS maps obtained from SnO2:Cr nanowires confirmed the presence of oxygen, tin, and chromium along them (Fig. 3). EDS measurements in SnO2:Cr nanoparticles adhered to nanowires revealed the same composition as Cr-doped SnO2 nanowires, finding that their presence only represents a morphological variation of the semiconductor.
Figure 4a shows a typical TEM image obtained from an undoped SnO2 nanowire of sample 1, with a selected area electron diffraction (SAED) pattern corresponding with the [010] zone axis and revealing a growth direction along the [100] in agreement with other reports [19, 22]. In addition, the zoom of the yellow square shown in Fig. 4a revealed that the sample exhibits a separation between the (101) planes of 2.6 Å (Fig. 4b), corresponding with the theoretically expected value for the rutile-type structure of SnO2. Figure 4c shows a typical TEM image of a nanowire of sample 2, also with a SAED corresponding with the [010] zone axis, although with extra diffraction points (arrows in inset) produced possibly by the presence of dislocations as the cause of crystal misorientation. The distance between the (101) planes on this nanowire recorded the same value obtained for sample 1, confirming a growth direction along the [100].
Raman measurements describe the chemical structure of crystals by evaluating their vibrational modes, and for rutile SnO2 with a P42/mnm space group, the group theory indicates that its total vibrational modes at the \(\Gamma\) point of the Brillouin zone are given by [16, 23]:
Among them, only four A1g, B1g, B2g, and Eg modes are Raman active, and only O atoms vibrate while Sn atoms remain at rest. Figure 5, curve 1, shows three fundamental Raman peaks at 474, 632, and 774 cm−1 for the undoped SnO2 sample, corresponding to the Eg, A1g, and B2g vibration modes. The first two modes A1g, B2g, correspond with the O vibration in the perpendicular plane to the c-axis, while the Eg mode arises from O–O vibration parallel to the c-axis [16, 23, 24]. In addition, the Raman spectra of this sample revealed a weak infrared (IR) active mode centered at 695 cm−1, which corresponds to the A2u longitudinal optical (LO) mode of the SnO2 rutile structure [25, 26]. Abello et al. have proposed that such IR mode becomes weakly active due to atomic disorder in the lattice structure [27], apparently by the presence of atomic defects in SnO2. Raman measurements of the chromium-doped samples revealed for sample 2 only the A1g and B2g signals (curve 2 in Fig. 5); in contrast, for sample 3, all vibrational modes found for the undoped SnO2 sample were resolved, besides a peak at 552 cm−1 (curve 3 in Fig. 5). Urs et al. have reported that this mode is generated by the symmetric octahedral vibration Cr–O6 present in the Cr2O3, suggesting that it can be assigned with the A1g mode in the SnO2 by considering the substitution of Sn4+ by Cr3+ ions, which also exhibit octahedral coordination with six oxygen ions in the rutile lattice [16].
Auger spectroscopy allowed us to determine the concentration of elements at the surface of SnO2 nanowires since Auger electrons are generated in this technique via inelastic collision scattering with an average depth of 5.0 nm. Figure 6a shows the Auger spectra obtained from samples with their corresponding derivative curves (Fig. 6b–d). Sample 1 displays Auger peaks at about 421 and 429 eV that correspond to the Sn (MNN) transitions and a peak at 510 eV that corresponds to the O (KVV) transition [28]. Auger spectra of samples 2 and 3 show both Sn (MNN) and O (KVV) transitions besides an Auger peak centered at 528.9 eV corresponding with Cr (L3M23V) transition [29,30,31]. Table 2 shows the composition of samples calculated by AES measurements using the sensitivity factors reported in ref [32], revealing that the Cr-doping of SnO2 nanowires increased its oxygen concentration at their surface. These results also exhibit higher chromium concentrations in samples than measured by the EDS technique, revealing that Cr3+ predominantly incorporates at the surface of SnO2 nanowires.
The chemical state of the elements in the samples was determined by XPS. Figure 7a displays the XPS spectra of the Sn 3d5/2 and 3d3/2 signals from samples centered at 486.3 and 494.7 eV, respectively, revealing an energy difference of 8.4 eV that corresponds with the spin–orbit splitting reported for the bound of Sn4+ ions with O2− ions in the SnO2 lattice [7, 33, 34]. Figure 7a also shows that the Sn 3d5/2 peak of samples exhibits only one symmetrical component, without shoulders, confirming the absence of Sn2+ ions in all samples. Figure 7b shows the XPS spectra of the O 1s signal from samples centered at 531 eV and composed with a shoulder at about 533 eV. The deconvolutions of this XPS signal for each sample are shown in Fig. 8, which were calculated using two Gaussian curves centered at 530.8 ± 0.5, and 532.5 ± 0.5 eV. The component of 530.8 eV corresponds to the O–Sn bonds [21, 34], and the component of 532.5 eV to single bonded oxygen atoms with different hydrocarbon molecules adsorbed onto the SnO2 surface [35, 36]. Figure 9 displays the Cr 2p3/2 and 2p1/2 signals obtained from sample 3 with binding energies of 576.5 and 586.5 eV, respectively, revealing an energy difference of 10 eV that corresponds with the value reported to Cr3+ ions [37,38,39]. To confirm the chemical state 3 + of chromium we calculated the multiplet components of the 2p3/2 signal reported by Biesinger et al. [40], which are shown in Fig. 9b. These components calculated with Gaussian curves were centered at 575.7, 576.7, 577.5, 578.5, and 578.9 obtaining a value for coefficient of determination R2 of 0.9106.
CL measurements allow us to determine the luminescence of the point defects present in SnO2 samples. Figure 11a displays the broad CL spectrum obtained from sample 1, revealing a shoulder at about 2.5 eV that suggests the presence of several components, which we calculated by deconvoluting the spectrum using Gaussian curves centered at 2.11, 2.50, and 2.79 eV with the same FWHM value of 0.5 eV, and values for the coefficient R2 between 0.9983 and 0.9990. For the yellow component (2.11 eV), Zhou et al. reported in a time-resolved X-ray excited optical luminescence study that the SnO2 yellow emission corresponds to radiative transitions involving oxygen vacancy-related states with intrinsic surface states [41]. However, Prades et al. demonstrated by density functional theory (DFT) and CL measurements that the SnO2 yellow emission corresponds with the formation of surface oxygen vacancies 100° coordinated with Sn atoms (bridge oxygen vacancies), also proposing that the SnO2 blue emission (2.79 eV) corresponds with the surface-VO 130° coordinated (in-plane oxygen vacancies) [42]. Similarly, other authors proposed that this blue emission corresponds with the radiative transition between the conduction band and surface states associated with VO [41,42,43,44]. The green component centered at 2.50 eV (Fig. 11a), corresponds with the SnO2 green emission reported by several authors, centered at 2.58 eV, attributing an origin to shallow levels involving surface defects [14, 22, 45]. CL spectra from Cr-doped samples exhibited the same components found for the undoped SnO2 nanowires (Fig. 11b, c), although with a notorious increase in the CL intensity for Cr-doped nanowires of sample 3.
EPR measurements were used to identify the different paramagnetic radicals, or species, associated with point defects in SnO2 and SnO2:Cr samples. We used the EasySpin MATLAB toolbox (supplementary information) to simulate the experimental EPR spectra and to calculate the g tensor values for the paramagnetic species present in samples. Figure 10a shows the EPR spectrum obtained from sample 1 in the range 335–340 mT (curve 1) with its corresponding simulated signal (curve 2) obtained for a g value of 2.0037, as a singlet with a line width peak-to-peak, ∆Hp–p, of 0.34 mT assigned to the presence of S = 1/2, which match with the reported for single ionized oxygen vacancies (\(V^{\prime}_{{\text{O}}}\)) defects in SnO2 [46,47,48]. A single ionized oxygen vacancy is an oxygen vacancy point defect with one trapped electron that acts as a ½ spin system with an associated magnetic moment, \(\mu\), which align along the direction of an external magnetic field, B, minimizing the energy \(E = - \mu \cdot B\). Figure 10b and c show the presence of this same EPR signal for the Cr-doped samples 1 and 2, both with a g value of 2.0035 and ∆Hp–p of 0.32 and 0.35 mT, respectively. This g value of 2.0035 matches with the g value of sample 1, considering that the uncertainty of the EPR system used is ± 0.0003 mT. Additionally, Fig. 10c line 1 reveals a notable decrease in the EPR intensity for sample 3.
Figure 12a shows the magnetization vs. magnetic field (M–H) curve obtained from undoped SnO2 nanowires (sample 1) after subtracting the diamagnetic behavior of the Si substrate [49], revealing a ferromagnetic behavior with magnetization saturation (MS) of ± 1 × 10–3 emu/gr in agreement with other authors [50], besides a small magnetic coercivity (HC) of 180 Oe (inset in Fig. 12a) in agreement with the reported value by Zhang et al. for similar undoped SnO2 nanowires [7]. Some authors have proposed that the intrinsic FM of undoped SnO2 is due to the exchange interaction between unpaired electron spins arising from oxygen vacancies (VO) [9, 13], which we confirmed their presence by EPR as single ionized vacancies \(V^{\prime}_{{\text{O}}}\) (Figs. 10a, 11a). In addition, the M–H curve of SnO2 nanowires doped with chromium at 0.12 at.% (sample 2) also revealed an FM behavior, with MS and HC values of ± 1.6 × 10–3 emu/g and 200 Oe, respectively, resulting be higher than observed to undoped SnO2 (sample 1) due to the chromium incorporation in the rutile structure. The enhancement of magnetic saturation by incorporating magnetic impurities in other DMS has been reported previously [7, 9], and particularly for sample 2, it could be explained in terms of the formation of \(V^{\prime}_{{\text{O}}}\) during chromium incorporation. Therefore, we propose that the formation of this paramagnetic specie is generated by a charge unbalance generated by incorporating Cr3+ ions in the lattice, involving native-neutral oxygen vacancies. Using the Kröger–Vink notation [51, 52], we suggest the following reaction to describe this effect during the substitution of Cr3+ by Sn4+ ions involving an electric charge transfer to neutral VO forming \(V^{\prime}_{{\text{O}}}\):
where × , ·, and ′ refer to neutral, positive, and negatively charged point defects. Thus, we assign the FM in SnO2:Cr to the alignment of the magnetic moment of Cr3+ ions and spin ½ system single ionized \(V^{\prime}_{{\text{O}}}\). Figure 12c shows the M–H curve acquired from sample 3, revealing a quenching for their magnetic coercivity and a decrease in the magnetic saturation to a value of ± 8 × 10–4 emu/g. This effect correlates with the decrease of the EPR signal of \(V^{\prime}_{{\text{O}}}\) defects in sample 3, suggesting that this type of defect is dominant in the FM generation in SnO2:Cr. Furthermore, considering that CL results revealed for this sample an increase in the emissions associated with VO at the SnO2 surface, an electric charge transfer of \(V^{\prime}_{{\text{O}}}\) could occur at the SnO2 surface of sample 3. Sopiha et al. recently demonstrated, using first-principles calculations, ionosorption on the SnO2 surface, reporting the adsorption of superoxide \({\text{O}}_{2}^{ - }\) on the (100) and (101) surface, and doubly ionized \({\text{O}}_{2}^{2 - }\) on the (100) surface [53]. Besides, Mishra et al. demonstrated enhancement of molecular acetone adsorption on SnO2:Cr by increasing the concentration of Cr3+ ions, proposing that such ions favor the adsorption of atmospheric oxygen on the semiconductor generating an electric charge transfer to the acetone molecules [54]. Thus, we propose that the decrease of \(V^{\prime}_{{\text{O}}}\) in sample 3 occurs by the adsorption of atmospheric neutral oxygen molecules, \({\text{O}}_{2}^{ \times }\), on the doped SnO2:Cr surface, improved by the increase of Cr3+ ions in SnO2 following the reaction:
where the hyphen (–) represents the adsorbed state of \({\text{O}}_{2}^{ - }\) molecules on the surface of \({\text{SnO}}_{2} :{\text{Cr}}^{3 + }\).
In summary, we propose that the FM of Cr-doped SnO2 nanowires is influenced by competitive electric charge transfer processes at their surface between oxygen vacancies and adsorbed oxygen, finding that \(V^{\prime}_{{\text{O}}}\) defects, acting as a spin ½ system, dominate the FM generation.
4 Conclusions
Undoped and Cr-doped SnO2 nanowires were synthesized by thermal evaporation method varying the chromium concentration to evaluate the role of point defects in the generation of ferromagnetism (FM). XRD measurements revealed the formation of the rutile-type tetragonal structure in samples. SEM images show the formation of large SnO2 and SnO2:Cr nanowires with diameters lower than 250 nm. TEM images revealed that undoped and doped SnO2 nanowires grew along the [100] direction. Raman spectroscopy revealed the vibrational modes Eg, A1g, A2u (LO), and B2g centered at 474, 632, 695, and 774 cm−1, respectively, corresponding with the SnO2 rutile structure, besides a mode centered at 552 cm−1 associated with the presence of Cr3+ ions with octahedral coordination with oxygen ions in the sample with higher Cr concentration. Auger spectroscopy measurements from samples revealed the Sn (MNN) doublet signal at 421 and 429 eV, and the O (KVV) signal at 510 eV, while the Cr-doped samples the Cr (L3M23V) signal at 528.9 eV. Furthermore, XPS measurements revealed that chromium ions incorporated in the SnO2 structure with chemical state 3 + . Electron paramagnetic resonance (EPR) results revealed a signal associated with a spin ½ system corresponding with single ionized oxygen vacancies (\(V^{\prime}_{{\text{O}}}\)), with g values of 2.0037 and 2.0035 for undoped and Cr-doped samples, respectively. Magnetic measurements revealed FM behavior from the undoped SnO2 (sample 1) and SnO2:Cr doped with 0.12 at.% (sample 2), with magnetization saturation values (MS) of ± 1 × 10–3 and ± 1.6 × 10–3 emu/g and magnetic coercivity values (HC) of 180 and 200 Oe, respectively. We assigned the FM response of SnO2:Cr to the alignment of both the magnetic moment of Cr3+ ions and spin ½ system single ionized \(V^{\prime}_{{\text{O}}}\). Finally, we found a quenching in the FM signal from SnO2:Cr nanowires doped with Cr at 0.52 at.% (sample 3), which correlated with a decrease in the EPR signal of \(V^{\prime}_{{\text{O}}}\) defects, revealing that such spin ½ system in SnO2 dominates the FM generation.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
X.M. Yin, C.C. Li, M. Zhang, Q.Y. Hao, S. Liu, Q.H. Li, L.B. Chen, T.H. Wang, Nanotechnology 20, 455503 (2009)
G. Cannella, F. Principato, M. Foti, J. Appl. Phys 110, 24502 (2011)
S.-G. Zhang, S.-F. Yin, Y.-D. Wei, S.-L. Luo, C.-T. Au, Mater. Lett. 79, 29 (2012)
C.B. Fitzgerald, M. Venkatesan, L.S. Dorneles, R. Gunning, P. Stamenov, J.M.D. Coey, P.A. Stampe, R.J. Kennedy, E.C. Moreira, U.S. Sias, Phys. Rev. B 74, 115307 (2006)
J. Chi, H. Ge, J. Wang, J. Appl. Phys 110, 83907 (2011)
S.K. Srivastava, P. Lejay, B. Barbara, S. Pailhès, V. Madigou, G. Bouzerar, Phys. Rev. B. 82, 193203 (2010)
L. Zhang, S. Ge, Y. Zuo, J. Wang, J. Qi, Scr. Mater. 63, 953 (2010)
J.M.D. Coey, A.P. Douvalis, C.B. Fitzgerald, M. Venkatesan, Appl. Phys. Lett. 84, 1332 (2004)
S.A. Ahmed, Solid State Commun. 150, 2190 (2010)
N.H. Hong, J. Sakai, N.T. Huong, N. Poirot, A. Ruyter, Phys. Rev. B 72, 045336 (2005)
A. Sarkar, D. Sanyal, P. Nath, M. Chakrabarti, S. Pal, S. Chattopadhyay, D. Jana, K. Asokan, RSC Adv. 5, 1148 (2015)
H. Wang, Y. Yan, K. Li, X. Du, Z. Lan, H. Jin, Phys. Status Solidi Basic Res. 247, 444 (2010)
A. Sundaresan, R. Bhargavi, N. Rangarajan, U. Siddesh, C.N.R. Rao, Phys. Rev. B Condens. Matter Mater. Phys. 74, 161306 (2006)
M. García-Tecedor, D. Maestre, A. Cremades, J. Piqueras, J. Phys. Chem. C 120, 22028 (2016)
M. Duhan, N. Kumar, A. Gupta, A. Singh, H. Kaur, Vacuum 181, 109635 (2020)
K. Urs, S.V. Bhat, V. Kamble, J. Appl. Phys. 123, 161518 (2018)
M. Herbich, A. Twardowski, D. Scalbert, Phys. Rev. B Conden. Matter Mater. Phys. 58, 7024 (1998)
P.A. Wolff, J. Warnock, J. Appl. Phys. 55, 2300 (1984)
J.S. Lee, S.K. Sim, B. Min, K. Cho, S.W. Kim, S. Kim, J. Cryst. Growth 267, 145 (2004)
Y. Chen, X. Cui, K. Zhang, D. Pan, S. Zhang, B. Wang, J.G. Hou, Chem. Phys. Lett. 369, 16 (2003)
D. Montalvo, M. Herrera, AIMS Mater. Sci. 3, 525 (2016)
M. Herrera, D. Maestre, A. Cremades, J. Piqueras, J. Phys. Chem. C 117, 8997 (2013)
S.H. Sun, G.W. Meng, G.X. Zhang, T. Gao, B.Y. Geng, L.D. Zhang, J. Zuo, Chem. Phys. Lett. 376, 103 (2003)
S. Mehraj, M.S. Ansari, Thin Solid Films 589, 57 (2015)
A. Diéguez, A. Romano-Rodríguez, A. Vilà, J.R. Morante, J. Appl. Phys. 90, 1550 (2001)
V. Bonu, A. Das, A.K. Sivadasan, A.K. Tyagi, S. Dhara, J. Raman Spectrosc. 46, 1037 (2015)
L. Abello, B. Bochu, A. Gaskov, S. Koudryavtseva, G. Lucazeau, M. Roumyantseva, J. Solid State Chem. 135, 78 (1998)
C. Meier, S. Lüttjohann, V.G. Kravets, H. Nienhaus, A. Lorke, P. Ifeacho, H. Wiggers, C. Schulz, M.K. Kennedy, F.E. Kruis, J. Appl. Phys. 99, 113108 (2006)
I. Grohmann, E. Kemnitz, A. Lippitz, W.E.S. Unger, Surf. Interface Anal. 23, 887 (1995)
U. Rothhaar, H. Oechsner, Thin Solid Films 302, 266 (1997)
E. Kemnitz, A. Kohne, I. Grohmann, A. Lippitz, W.E.S. Unger, J. Catal. 159, 270 (1996)
S. Mroczkowski, D. Lichtman, J. Vac. Sci. Technol. A 3, 1860 (1985)
M. Di Giulio, A. Serra, A. Tepore, R. Rella, P. Siciliano, L. Mirenghi, Mater. Sci. Forum 203, 143 (1996)
C.J.P. Alexander, V. Naumkin, A. Kraut-Vass, S.W. Gaarenstroom, Meas. Serv. Div. Natl. Inst. Stand. Technol. 20899 (2012).
G. Guzmán, M. Herrera, R. Silva, G.C. Vásquez, D. Maestre, Semicond. Sci. Technol. 31, 55006 (2016)
CD Wagner, AV Naumki, A Kraut-Vass, JW Allison, CJ Powell, JR Rumble (2003) Solid State Commun 39:581-585
C. Battistoni, J.L. Dormann, D. Fiorani, E. Paparazzo, S. Viticoli, Solid State Commun. 39, 581 (1981)
G.P. Halada, C.R. Clayton, J. Electrochem Soc. 138, 2921 (1991)
P.G. Harrison, N.C. Lloyd, W. Daniell, J. Phys. Chem. B 102, 10672 (1998)
M.C. Biesinger, B.P. Payne, A.P. Grosvenor, L.W.M. Lau, A.R. Gerson, R.S.C. Smart, Appl. Surf. Sci. 257, 2717 (2011)
X.T. Zhou, F. Heigl, M.W. Murphy, T.K. Sham, T. Regier, I. Coulthard, R.I.R. Blyth, Appl. Phys. Lett. 89, 213109 (2006)
J.D. Prades, J. Arbiol, A. Cirera, J.R. Morante, M. Avella, L. Zanotti, E. Comini, G. Faglia, G. Sberveglieri, Sens. Actuators B Chem. 126, 6 (2007)
J.K. Yang, H.L. Zhao, J. Li, L.P. Zhao, J.J. Chen, B. Yu, Acta Mater. 62, 156 (2014)
S.P. Mondal, S.K. Ray, J. Ravichandran, I. Manna, Bull. Mater. Sci. 33, 357 (2010)
A. Vázquez-López, D. Maestre, J. Ramírez-Castellanos, J.M. González-Calbet, I. Píš, S. Nappini, N. Yuca, A. Cremades, J. Phys. Chem. C 124, 18490 (2020)
S.K. Misra, S.I. Andronenko, D. Tipikin, J.H. Freed, V. Somani, O. Prakash, J. Magn. Magn. Mater. 401, 495 (2016)
D.A. Popescu, J.M. Herrmann, A. Ensuque, F. Bozon-Verduraz, Phys. Chem. Chem. Phys. 3, 2522 (2001)
D. Toloman, O. Pana, M. Stefan, A. Popa, C. Leostean, S. Macavei, D. Silipas, I. Perhaita, M.D. Lazar, L. Barbu-Tudoran, J. Colloid Interface Sci. 542, 296 (2019)
G. Guzmán, D. Maestre, M. Herrera, J. Mater. Sci. Mater. Electron. 110, 15070 (2020)
F. Zhang, Y. Lian, M. Gu, J. Yu, T.B. Tang, J. Sun, W. Zhang, Phys. Lett. Sect. A Gen. At. Solid State Phys. 380, 3138 (2016)
T. Norby, J. Korean Ceram. Soc. 47, 19 (2010)
F. A. Kröger, in (North-Holland, Amsterdam, New York, 1964), p. 1039.
K.V. Sopiha, O.I. Malyi, C. Persson, P. Wu, A.C.S. Appl, Mater. Interfaces 13, 33664 (2021)
R.K. Mishra, A. Kushwaha, P.P. Sahay, J. Exp. Nanosci. 10, 1042 (2015)
Acknowledgements
DM is grateful to CONACYT for a graduate research fellowship (no. CVU 555621). Technical support of E. Aparicio, F. Ruiz, and M. Flores is greatly appreciated.
Funding
The research was financially supported by Conacyt grand No. 284667 and UC-Mexus grand No. CN19-137.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. DM and MH performed material preparation and CL measurements. VG and DM performed EPR measurements and simulations. XPS and Auger spectroscopy studies were performed by DM and WdelaC. SC performed Raman spectroscopy measurements. DM and IR obtained SEM images and EDS data. Magnetic studies were performed by KC, VO, CS, and JM. MH analyzed all experimental results and wrote the first draft of the manuscript. All authors commented on the previous version of the manuscript. Finally, all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Montalvo, D., Gómez, V., de la Cruz, W. et al. Influence of single-ionized oxygen vacancies on the generation of ferromagnetism in SnO2 and SnO2:Cr nanowires. Appl. Phys. A 129, 537 (2023). https://doi.org/10.1007/s00339-023-06790-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06790-z