Abstract
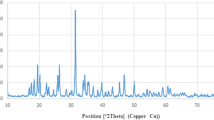
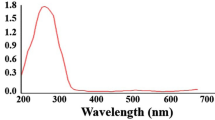
In this investigation, zinc oxide nanoparticles (ZnO NPs) were produced by solution combustion-assisted technique utilising aqueous leaf extract of Piper betle (betel leaf) (PB). Phase formation and the particle size of ZnO-PB-NPs were ascertained by using X-ray diffraction. It was observed that the ZnO-PB-NPs crystallize in the hexagonal phase with an average crystallite size of 24 nm. The morphology, shape, and size of the NPs were studied by Scanning Electron Microscope and Transmission Electron Microscope (TEM). The elemental composition was analysed using energy-dispersive advanced X-ray spectroscopy. Further, Fourier-Transform Infrared (FTIR) spectroscopy confirmed the formation of ZnO bonding. Anticancer activity of ZnO-PB-NPs was evaluated in the MDA-MB-231, human breast cancer cells by MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide] assay. The study findings demonstrated that the ZnO-PB-NPs were able to induce significant cytotoxicity in human breast cancer cells in a dose-dependent manner. ZnO-PB-NPs treatment impaired the Clonogenic potential cells of breast cancer. Additionally, the biocompatibility with blood components of ZnO-PB-NPs was evaluated by blood hemolysis assay. It was observed that, ZnO NPs inhibited breast cancer cell growth and increased the induction of early apoptosis cell population.

















Similar content being viewed by others
Data availability
Not applicable.
References
S. Wang, K. Cheng, K. Chen, C. Xu, P. Ma, G. Dang et al., Nanoparticle-based medicines in clinical cancer therapy. Nano Today 45, 101512 (2022). https://doi.org/10.1016/j.nantod.2022.101512
H. Mohd Yusof, R. Mohamad, U.H. Zaidan et al., Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: a review. J Anim Sci Biotechnol 10, 57 (2019). https://doi.org/10.1186/s40104-019-0368-z
M.C. Sportelli, C. Gaudiuso, A. Volpe, M. Izzi, R.A. Picca, A. Ancona, N. Cioffi, Biogenic synthesis of ZnO nanoparticles and their application as bioactive agents: a critical overview. Reactions 3, 423–441 (2022). https://doi.org/10.3390/reactions3030030
P.G. Krishna, P.P. Ananthaswamy, T. Yadavalli, N.B. Mutta, A. Sannaiah, Y. Shivanna, ZnO nanopellets have selective anticancer activity. Mater. Sci. Eng. C 62, 919–926 (2016). https://doi.org/10.1016/j.msec.2016.02.039
I. Shahine, N. Beydoun, J.J. Gaumet, E.-E. Bendeif, H. Rinnert, P. Magri, A. EnNaciri, P. Miska, S. Jradi, S. Akil, Pure, size tunable ZnO nanocrystals assembled into large area PMMA layer as efficient catalyst. Catalysts 9, 162 (2019). https://doi.org/10.3390/catal9020162
R.A. Salinas, A. Orduña-Díaz, O. Obregon-Hinostroza, M.A. Dominguez, Biosensors based on zinc oxide thin-film transistors using recyclable plastic substrates as an alternative for real-time pathogen detection. Talanta 237, 122970 (2022). https://doi.org/10.1016/j.talanta.2021.122970
H.-Q. Liu, C.-B. Yao, Y. Cai, H.-T. Yin, Synthesis, photoluminescence and photocatalytic characteristics of Ag–ZnO sandwich structures. J.Phys. Chem. Solids 165, 110697 (2022). https://doi.org/10.1016/j.jpcs.2022.110697
P.G. Krishna, P. Chandra Mishra, M.M. Naika, M. Gadewar, P.P. Ananthaswamy, S. Rao, S.R. Boselin Prabhu, K.V. Yatish, H.G. Nagendra, M. Moustafa et al., Photocatalytic activity induced by metal nanoparticles synthesized by sustainable approaches: a comprehensive review. Front. Chem. 10, 917831 (2022). https://doi.org/10.3389/fchem.2022.917831
J. Xu, Y. Huang, S. Zhu, N. Abbes, X. Jing, L. Zhang, A review of the green synthesis of ZnO nanoparticles using plant extracts and their prospects for application in antibacterial textiles. J. Eng. Fibers Fabr. 16, 15589250211046242 (2021). https://doi.org/10.1177/15589250211046242
J. Liu, J. Ma, Y. Bao, J. Wang, H. Tang, L. Zhang, Polyacrylate/surface-modified ZnO nanocomposite as film-forming agent for leather finishing. Int. J. Polym. Mater. Polym. Biomater. 63, 809–814 (2014). https://doi.org/10.1080/00914037.2014.886217
M. Manabeng, B.S. Mwankemwa, R.O. Ocaya, T.E. Motaung, T.D. Malevu, A review of the impact of zinc oxide nanostructure morphology on perovskite solar cell performance. Processes 2022, 10 (1803). https://doi.org/10.3390/pr10091803
P.G. Krishna, P.P. Ananthaswamy, M. Gadewar, U. Bora, N.B. Mutta, In vitro antibacterial and anticancer studies of ZnO nanoparticles prepared by sugar fueled combustion. Synthesis 8, 24–29 (2017). https://doi.org/10.5185/amLett.2017.6424
P.G. Krishna, P.P. Ananthaswamy, N.B. Mutta, K.G. Mariyappa, R. Singh, Comparison of antimicrobial and anticancer activity of ZnO nanoparticles prepared using different precursors by hydrothermal synthesis. J. Chem. Pharm. Sci. 10, 192–197 (2017)
G.K. Prashanth, P.A. Prashanth, P. Singh, B.M. Nagabhushana, C. Shivakumara, G.M. Krishnaiah et al., Effect of doping (with cobalt or nickel) and UV exposure on the antibacterial, anticancer, and ROS generation activities of zinc oxide nanoparticles. J. Asian Ceram. Soc. 8(4), 1175–1187 (2020). https://doi.org/10.1080/21870764.2020.1824328
G.K. Prashanth, P.A. Prashanth, B.M. Nagabhushana, S. Ananda, H.G. Nagendra, R.C. Singh, In vitro antimicrobial, antioxidant and anticancer studies of ZnO nanoparticles synthesized by precipitation method. Adv. Sci. Eng. Med. 8, 306–313 (2016). https://doi.org/10.1166/asem.2016.1854
G.K. Prashanth, P.A. Prashanth, M. Ramani, S. Ananda, B.M. Nagabhushana, G.M. Krishnaiah et al., Comparison of antimicrobial, antioxidant and anticancer activities of ZnO nanoparticles prepared by lemon juice and citric acid fueled solution combustion synthesis. BioNanoScience 9(4), 799–812 (2019). https://doi.org/10.1007/s12668-019-00670-8
L. Palanikumar, S. Ramasamy, C. Balachandran, Antibacterial and cytotoxic response of nano zinc oxide in gram negative bacteria and colo 320 human adenocarcinoma cancer cells. Curr. Nanosci. 9, 469–478 (2013). https://doi.org/10.2174/1573413711309040009
F. Namvar, H.S. Rahman, R. Mohamad, S. Azizi, P.M. Tahir, M.S. Chartrand, S.K. Yeap, Cytotoxic effects of biosynthesized zinc oxide nanoparticles on murine cell lines, Evid. Based Complement. Alternat. Med. 2015, 593014 (2015). https://doi.org/10.1155/2015/593014
R. Wahab, M.A. Siddiqui, Q. Saquib, S. Dwivedi, J. Ahmad, J. Musarrat, A.A. AlKhedhairy, H.-S. Shin, ZnO nanoparticles induced oxidative stress and apoptosis in HepG2 and MCF-7 cancer cells and their antibacterial activity. Colloids Surf. B: Biointerfaces 117, 267–276 (2014). https://doi.org/10.1016/j.colsurfb.2014.02.038
I. Pujalte, I. Passagne, B. Brouillaud, M. Treguer, E. Durand, C. Ohayon-Courtes, B. L'Azou, Cytotoxicity and oxidative stress induced by different metallic nanoparticles on human kidney cells. Part. Fibre Toxicol. 8, 10 (2011). https://doi.org/10.1186/1743-8977-8-10
R. Guan, T. Kang, F. Lu, Z. Zhang, H. Shen, M. Liu, Cytotoxicity, oxidative stress, and genotoxicity in human hepatocyte and embryonic kidney cells exposed to ZnO nanoparticles. Nanoscale Res. Lett. 7, 602 (2012). https://doi.org/10.1186/1556-276X-7-602
T. Kang, R. Guan, X. Chen, Y. Song, H. Jiang, J. Zhao, In vitro toxicity of different-sized ZnO nanoparticles in Caco-2 cells. Nanoscale Res. Lett. 8, 496 (2013). https://doi.org/10.1186/1556-276X-8-496
E. Taylor, T.J. Webster, Reducing infections through nanotechnology and nanoparticles. Int. J. Nanomed. 6, 1463–1473 (2011). https://doi.org/10.2147/IJN.S22021
H. Mirzaei, M. Darroudi, Zinc oxide nanoparticles: biological synthesis and biomedical applications. Ceram. Int. 43(1, Part B), 907–914 (2017). https://doi.org/10.1016/j.ceramint.2016.10.051
Y. Hu, L. Sun, Z. Liu, T. Liu, Controlled solvothermal synthesis of ZnO nanoparticles using non-destructive Mg-based channel templates for enhanced photocatalytic performance. Mater. Chem. Phys. 299 (2023). https://doi.org/10.1016/j.matchemphys.2023.127525
M. Lal, P. Sharma, L. Singh, C. Ram, Photocatalytic degradation of hazardous Rhodamine B dye using sol-gel mediated ultrasonic hydrothermal synthesized of ZnO nanoparticles. Results Eng. 17, 100890 (2023). https://doi.org/10.1016/j.rineng.2023.100890
K.C. Patil, S.C. Aruna, T. Mimani, Combustion synthesis: an update. Curr. Opin. Solid State Mater. Sci. 6(6), 507–512 (2002). https://doi.org/10.1016/S1359-0286(02)00123-7
K.C. Patil, M.S. Hegde, R. Tanu et al., Chemistry of Nanocrystalline Oxide Materials (World Scientific, Singapore, 2008). https://doi.org/10.1016/S1359-0286(02)00123-7
N. Shobha, N. Nanda, A.S. Giresha, P. Manjappa, S. P, K.K. Dharmappa et al., Synthesis and characterization of Zinc oxide nanoparticles utilizing seed source of Ricinus communis and study of its antioxidant, antifungal and anticancer activity. Mater. Sci. Eng.: C 97, 842–850 (2019). https://doi.org/10.1016/j.msec.2018.12.023
T. Nalina, Z.H.A. Rahim, The crude aqueous extract of Piper betle L. and its antibacterial effect towards Streptococcus mutans. Am. J. Biotechnol. Biochem. 13, 10–15 (2007). https://doi.org/10.3844/ajbbsp.2007.10.15
L.S.R. Arambewela, L.D.A.M. Arawwawala, W.D. Ratnasooriya, Antidiabetic activities of aqueous and ethanolic extracts of Piper betle leaves in rats. J. Ethnopharmacol. 13, 239–245 (2005). https://doi.org/10.1016/j.jep.2005.06.016
R. Hajare, V.M. Darvhekar, A. Shewale, V. Patil, Evaluation of antihistaminic activity of piper betel leaf in guinea pig. Afr. J. Pharm. Pharmacol. 13, 113–117 (2011)
N. Nur-Sazwi, T. Nalina, Z.H.A. Rahim, Antioxidant and cytoprotective activities of Piper betle, Areca catechu, Uncaria gambir and betel quid with and without calcium hydroxide. BMC Complement. Altern. Med. 13, 351 (2013). https://doi.org/10.1186/1472-6882-13-351
C.K. Kokate, Practical Pharmacognosy (Vallabh Prakashan, New Delhi, 2000)
J.B. Harbone, Phytochemical Methods (Chapman and Hall, London, 1999)
P. Tiwari, B. Kumar, M. Kaur et al., Phytochemical screening and extraction: a review. Internationale Pharmaceutica Sciencia. 1, 98–106 (2011)
G.K. Prashanth, G.M. Krishnaiah, Chemical composition of the leaves of Azadiracta indica Linn (Neem). Int. J. Adv. Eng. Technol. Manag. Appl. Sci. 1(5), 21–31 (2014)
G.K. Prashanth, G.M. Krishnaiah, H.M. Sathyananda, Qualitative phytochemical screening and GCMS analysis of the leaves of Indigofera tinctoria. Int. J. Innov. Res. Sci. Eng. Technol. 4, 7544–7547 (2015). https://doi.org/10.15680/IJIRSET.2015.0408080
G.K. Prashanth, G.M. Krishnaiah, Phytochemical screening and GC-MS analysis of the leaves of Pongamia Pinnata linn. Int. J. Innovative Res. Sci. Eng. Technol. 3(11), 17329–17334 (2014). https://doi.org/10.15680/IJIRSET.2014.0311034
T. Mosmann, Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65, 55–63 (1983). https://doi.org/10.1016/0022-1759(83)90303-4
G.K. Prashanth, H.M. Sathyananda, P.A. Prashanth, M. Gadewar, M. Mutthuraju, S.R. Boselin Prabhu et al., Controlled synthesis of Ag/CuO nanocomposites: evaluation of their antimycobacterial, antioxidant, and anticancer activities. Appl. Phys. A 128, 614 (2022). https://doi.org/10.1007/s00339-022-05748-x
H.M. Sathyananda, P.A. Prashanth, G.K. Prashanth, B.M. Nagabhushana, C. Shivakumara, S.R. Boselin Prabhu et al., Evaluation of antimycobacterial, antioxidant, and anticancer activities of CuO nanoparticles through cobalt. Doping. Appl. Nanosci. (2021). https://doi.org/10.1007/s13204-021-02156-0
H.M. Sathyananda, P.A. Prashanth, G.K. Prashanth, B.M. Nagabhushana, G.M. Krishnaiah, H.G. Nagendra, Antimicrobial, antioxidant, and cytotoxicity activities of CuO nanopellets synthesized by surfactant-free hydrothermal method. J. Test. Evaluat. (2021). https://doi.org/10.1520/JTE20200538
P. Gopala Krishna, P. PaduvarahalliAnanthaswamy, P. Trivedi, V. Chaturvedi, N. Bhangi Mutta, A. Sannaiah et al., Antitubercular activity of ZnO nanoparticles prepared by solution combustion synthesis using lemon juice as bio-fuel. Mater. Sci. Eng. C 75, 1026–1033 (2017). https://doi.org/10.1016/j.msec.2017.02.09
N.A.P. Franken, H.M. Rodermond, J. Stap, J. Haveman, C. van Bree, Clonogenic assay of cells in vitro. Nat. Protoc. 1(5), 2315–2319 (2006). https://doi.org/10.1038/nprot.2006.339
J.S. Köerich, D.J. Nogueira, V.P. Vaz, C. Simioni, M.L.N.D. Silva, L.C. Ouriques, D.S. Vicentini, W.G. Matias, Toxicity of binary mixtures of Al2O3 and ZnO nanoparticles toward fibroblast and bronchial epithelium cells. J. Toxicol. Environ. Health A 83(9), 363–377 (2020). https://doi.org/10.1080/15287394.2020.1761496
K. Rjiba-Touati, I. Ayed-Boussema, H. Hamdi, S. Abid, Genotoxic damage and apoptosis in rat glioma (F98) cell line following exposure to bromuconazole. Neurotoxicology 94, 108–116 (2023). https://doi.org/10.1016/j.neuro.2022.11.006
M.C. Pico, A. Basulto, A. del Monte, A. Hidalgo, M.E. Lanio, C. Alvarez et al., Cross-reactivity and inhibition of haemolysis by polyclonal antibodies raised against St II, a cytolysin from the sea anemone Stichodactyla helianthus. Toxicon 43, 167–171 (2004). https://doi.org/10.1016/j.toxicon.2003.11.020
D. Das, B.C. Nath, P. Phukon, S.K. Dolui, Colloid. Surf. B: Bio Interfaces. 101, 430–433 (2013)
S. Biswal, Phytochemical analysis and a study on the antiestrogenic antifertility effect of leaves of Piper betel in female albino rat. Anc. Sci. Life. 34(1), 16–22 (2014). https://doi.org/10.4103/0257-7941.150770
R. Rajamani, K. Selvam, S. Muthusamy, R. Devadass, Preliminary phytochemical screening of aqueous extract of betel nut and betel leaves. Int. J. Biosci. Nanosci. 3(1), 14–18 (2026)
N.M. Patel, D.D. Jain, H.P. Suryawanshi, S.P. Pawar, Phytopharmacological study of Piper Betle leaf, Saudi. J. Med. Pharm. Sci. (2019). https://doi.org/10.36348/sjmps.2019.v05i11.008
A.K. Jha, K. Prasad, V. Kumar, K. Prasad, Biosynthesis of silver nanoparticles using eclipta leaf. Biotechnol. Prog. 25, 1476–1479 (2009). https://doi.org/10.1002/btpr.233
S. Wongrerkdee, S. Wongrerkdee, C. Boonruang, S. Sujinnapram, Enhanced photocatalytic degradation of methylene blue using Ti-doped ZnO nanoparticles synthesized by rapid combustion. Toxics 11, 33 (2023). https://doi.org/10.3390/toxics11010033
K. Handore, S. Bhavsar, A. Horne, P. Chhattise, K. Mohite, J. Ambekar, N. Pande, V. Chabukswar, Novel green route of synthesis of ZnO nanoparticles by using natural biodegradable polymer and its application as a catalyst for oxidation of aldehydes. J. Macromol. Sci. Part A 51(12), 941–947 (2014). https://doi.org/10.1080/10601325.2014.967078
R.J. Gonzalez, J.B. Tarloff, Evaluation of hepatic subcellular fractions for Alamar blue and MTT reductase activity. Toxicol. In Vitro 15, 257–259 (2001). https://doi.org/10.1016/S0887-2333(01)00014-5
L. Kangas, M. Grönroos, A.L. Nieminen, Bioluminescence of cellular ATP: a new method for evaluating cytotoxic agents in vitro. Med. Biol. 62, 338–343 (1984)
F. Blankenberg, J. Narula, H.W. Strauss, In vivo detection of apoptotic cell death: a necessary measurement for evaluating therapy for myocarditis, ischemia, and heart failure. J. Nucl. Cardiol. 6, 531–539 (1999). https://doi.org/10.1016/S1071-3581(99)90026-0
M.A. Swairjo, N.O. Concha, M.A. Kaetzel, J.R. Dedman, B.A. Seaton, Ca2+-bridging mechanism and phospholipid head group recognition in the membrane-binding protein annexin V. Nat. Struct. Biol. 2, 968–974 (1995). https://doi.org/10.1038/nsb1195-968
J. Zhang, L. Tan, C. Wu, Y. Li, H. Chen, Y. Liu et al., Discovery and biological evaluation of 4,6-pyrimidine analogues with potential anticancer agents as novel colchicine binding site inhibitors. Eur. J. Med. Chem. 248, 115085 (2023). https://doi.org/10.1016/j.ejmech.2022.115085
I.K. Lindamulage, H.-Y. Vu, C. Karthikeyan, J. Knockleby, Y.-F. Lee, P. Trivedi et al., Novel quinolone chalcones targeting colchicine-binding pocket kill multidrug-resistant cancer cells by inhibiting tubulin activity and MRP1 function. Sci. Rep. 7, 10298 (2017). https://doi.org/10.1038/s41598-017-10972-0
Acknowledgments
The author Dr. Shobha Nagarajaiah is thankful to Dr. Shivashankarappa L.H., Principal, Maharani’s Science College for Women, Bengaluru for the support. Dr. Prashanth GK would like to express his gratitude to the management of Sri KET for the constant encouragement provided towards research activities.
Author information
Authors and Affiliations
Contributions
Conceptualization, generation of the hypothesis, experimentation, and manuscript writing SN, PGK and NN; software analysis, SN, PGK, NN, PM, MGG, and SR; review and editing PGK, SN, and NN: revision plagiarism errors and proofread, PGK, SN, NN, PM, BMN, MG, and SR. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nagarajaiah, S., Nanda, N., Manjappa, P. et al. Evaluation of apoptosis in human breast cancer cell (MDA-MB-231) induced by ZnO nanoparticles synthesized using Piper betle leaf extract as bio-fuel. Appl. Phys. A 129, 461 (2023). https://doi.org/10.1007/s00339-023-06731-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06731-w