Abstract
The transient plane source method was applied to measure the effective thermal conductivity in dimagnesium iron hexahydride (Mg2FeH6) prepared in a high-pressure synthesis of 50 temperature-driven de-/hydrogenation cycles. Temperature- and pressure-dependent measurements of the effective thermal conductivity of the as-synthesized Mg2FeH6 powder have been performed. Measurements for as synthesized Mg2FeH6 were carried out between 2 and 100 bar in a temperature range from 50 °C to 300 °C and at 70 bar in a temperature range from 480 °C to 520 °C during the cycle test. The effective thermal conductivity of the as-synthesized Mg2FeH6 varied between 0.39 W m−1 K−1, recorded at 50 °C and 2 bar of hydrogen gas pressure, and 0.54 W m−1 K−1, measured at 300 °C and 100 bar hydrogen pressure. The effective thermal conductivity increased with elevated hydrogen gas pressure and temperature. An evidence was found that the presence of iron prevents the sintering of the powder, resulting in a constant effective thermal conductivity during all accomplished cycles. The advantage of a non-sintered material resulting in higher hydrogen diffusion, which leads to a faster reaction time. For 50 measured de-/hydrogenation cycles between 480 °C and 520 °C, the thermal conductivity was found to be constant at around ~ 1.0 W m−1 K−1 in the dehydrogenated state (70 bar/520 °C) and between 0.7 W m−1 K−1 and 0.8 W m−1 K−1 in the hydrogenated state (70 bar/480 °C).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
One of the key challenges facing our society is the transition toward an energy system based on renewable energy sources. The renewable energy includes three-energy sources: solar radiation, tidal power and geothermal energy, with solar radiation being the most important. The solar radiation striking the Earth is mainly converted by photovoltaics, concentrating solar power plants and wind turbines into electricity [1]. Provided energy by solar power underlies fluctuations due to the day–night cycle and changing weather situations (clear/cloudy sky and variable wind conditions). To overcome these issues and to provide continuous power to the electrical grid, surplus solar energy must be stored during times of excess energy and released in periods of low supply. There are numerous technological solutions to face the mismatch of energy supply and energy demand, which can be used for different scenarios. An interesting solution to achieve these goals is the conversion of solar energy into heat and its subsequent storage. This conversion can be done directly in concentrating solar power plants or indirectly using the power-to-heat option via photovoltaics or wind energy converters. It is worth mentioning that another possibility might be the heat storage in a heat pump system, being operated for instance as a gas turbine cycle (Brayton or Joule cycle) [2, 3]. With this pumped heat electricity storage (PHES), the electrical energy for driving the compressor could be provided directly from the wind energy converter or photovoltaics. Not only is the storage of heat from the concentrated solar power plants of critical importance, but also the storage of heat used in industry or for transition to flexible fossil fuels supplied power plants, working in combined heat and power mode, is becoming increasingly important nowadays. Heat storage techniques are usually divided into sensible, latent and thermochemical heat storage with all their known advantages and disadvantages [4]. One advantage of the thermochemical heat storage based on reactions of suitable fluids with metals is possibility of coupling them to existing sources of the required fluids as reaction partners. For example, oxygen can be taken from the air for reactions to form metal oxides, water (steam in steam power plants) to form metal hydroxides and hydrogen (refineries, pipelines with natural gas and hydrogen) for metal hydrides. Among others, high gravimetric and volumetric heat storage capacities of the thermochemical heat storage techniques are the most known advantages. Due to the concurrent growth of interest in the hydrogen economy, the application of metal hydrides for heat storage may increase their popularity. The most discussed metal forming metal hydride with promising heat storage properties is magnesium (Mg). Magnesium and its combination with some other metals such as transition metals [(iron (Fe), nickel (Ni) and cobalt (Co)] or alkali earth metals [sodium (Na)] have been studied and investigated for years [5,6,7,8]. A low-cost heat storage material, which can be used for heat storage applications in the temperature range of 450–550 °C, is dimagnesium iron hexahydride (Mg2FeH6.) The usability of this magnesium-based hydride has been mentioned in several publications, highlighting its suitability as heat storage material [9,10,11,12]. To date there is no complete description of the formation process of Mg2FeH6. There is evidence that MgH2 exists as an intermediate during formation [13,14,15]. However, the overall process of storing and releasing heat in dimagnesium iron hexahydride using the reversible de-/hydrogenation can be described by following equation:
The amount of heat that can be stored by dehydrogenation is defined by the enthalpy of reaction \({\mathrm{\Delta H}}_{\mathrm{R}}\) (Eq. 1), which is 77. 4 kJ mol−1 [15] and the amount of hydrogen. To store heat, dimagnesium iron hexahydride is decomposed into magnesium, iron and gaseous hydrogen (dehydrogenation). Subsequently, the recombination of magnesium, iron and hydrogen to form dimagnesium iron hexahydride (hydrogenation) releases the same amount of heat as occurred during heat storage, assuming that the enthalpy of reaction in both cases is the same. Whether the reaction runs to the right or the left side depends on temperature and H2-pressure.
Figure 1 shows the equilibrium pressures of Mg2FeH6 (red dashed curve) and MgH2 (black curve). Above the curve a hydrogenation takes place (Eq. 1 left to right) and below the curve dehydrogenation (Eq. 1 right to left). For the synthesis (Paragraph 2.1.), de-/hydrogenation temperatures of 550 °C/470 °C were chosen. Therefore, the experimental pressures must be lower/higher than the corresponding equilibrium pressures at those temperatures (~ 134 bar/40 bar). During the cycle test (Paragraph 2.4.) the de-/hydrogenation temperatures are 520 °C/480 °C. The experimental pressures must be lower/higher than the corresponding equilibrium pressures during the cycle test (~ 87 bar/47 bar).
Equilibrium pressures of Mg2FeH6 (red dashed) and MgH2 (black). Reprinted with permission of Elsevier from [15]
A promising and cheap method to produce metal hydrides for large-scale heat storage applications is using metal powders or powder mixtures as feedstock without any further processing technology such as ball milling. Simple powder mixing in commercially available powder mixers is considered sufficient. This approach ensures a simple and economical method to produce a large amount of material for a storage tank.
Dimagnesium iron hexahydride has a relatively high enthalpy of reaction, operating temperatures of up to 550 °C and superior cycle stability. A significant drawback of all metal hydride powder systems is their low heat transfer capability [16, 17], which is a critical and limiting factor, especially for materials with a high volumetric energy density (e.g., Mg2FeH6). Crucial to the operation of a metal hydride-based heat storage systems is their effective thermal conductivity [18,19,20], although it has been reported that the thermal conductivity does not influence the thermal behavior of metal hydride reactors [21]. Supposing the heat transfer coefficients at the walls of the heat exchanger, to which the packed metal hydride powder bed is attached, are relatively low and the resultant heat transfer resistance in this particular region is high. In this case the heat transfer coefficient at the wall is the limiting factor for the heat transfer and not the effective thermal conductivity of the powdered metal hydride bed [22]. A fundamental understanding of the heat transfer capability is essential to design and operate a large-scale heat storage systems. Accurate modelling of a powder bed’s thermal conductivity is challenging, because there are many parameters that affect the overall heat conductivity, such as thermal conductivity of the solid (magnesium and iron or dimagnesium iron hexahydride) and the hydrogen gas. Furthermore, the thermal conductivity is a function of the void fraction in the bed, the particle size distribution and the shape of the particles [16, 20]. In a powder bed, four different heat conduction pathways must be considered: conduction through the bulk particle (1), conduction from one particle to the other via the surfaces (2), conduction between particle surfaces and gas particles (3) and conduction through the gas (4). Each conduction pathway has a different thermal conductivity and contributes differently to the overall thermal conductivity, called Effective Thermal Conductivity (ETC). There are some attempts in the literature to describe the thermal conductivity of low temperature metal hydrides successfully, also considering the uptake and release of hydrogen [16, 17, 19, 23,24,25,26]. The thermal conductivity of high-temperature metal hydrides such as MgH2 with different additional metals, some of which serve as active catalytic species, has been studied in the past [27,28,29,30,31]. The ETC of compressed Mg2FeH6-pellets as well as its dehydrogenation products (2 Mg + Fe) under ~ 1 bar Ar at room temperature could be determined to 1.8 Wm−1 K−1 (Mg2FeH6) and 2.2 Wm−1 K−1 (2 Mg + Fe), respectively [32]. Addition of 10 wt-% expanded natural graphite increased the thermal conductivity of Mg2FeH6 to 6.76 Wm−1 K−1. Since gas type and pressure, temperature and particle size will influence the ETC, it is of interest to know the ETC values of a material over a wide range of temperatures and H2-pressures. In addition, the long-term behavior of the ETC should be studied, since the shrinking/expansion of the powder during the de-/hydrogenation cycles have influence on the physical properties of the powder (e.g., particle size, distribution, contact between neighboring particles, etc.). Unfortunately, apart from one dissertation [22], neither simulated nor experimental thermal conductivity values of Mg2FeH6 powder beds describing heat transfer under operating conditions have been published. A predicted effective thermal conductivity of Mg2FeH6 after improvements by the addition of expanded natural graphite achieved 7 W m−1 K−1 [9, 12].
2 Materials and methods
2.1 Synthesis of dimagnesium iron hexahydride
The synthesis of the metal hydride was carried out by hand-mixing 6.52 g (0.268 mol) of magnesium powder from Alfa Aeser (mesh 325) with 7.48 g (0.134 mol) of iron powder from Sigma Aldrich (mesh 325) under an ambient atmosphere, to simulate an up-scaled process. Then, the metal powder mixture was placed in a gas tight autoclave, which was attached to a hydrogen gas test rig (Fig. 2). The magnesium iron powder mixture was exposed to 470 °C at 95 bar hydrogen for 24 h to initiate the first hydrogenation.
The following hydrogenations were carried out according to a fixed temperature program (Table 1). It consists of four steps. At first, the dimagnesium iron hexahydride was dehydrogenated by increasing its temperature from 470 to 550 °C with a heating rate of 5 K min−1. Dehydrogenation pressure was 101 bar in the first half of the cycle and increased to a maximum of 105 bar in the second half. Next, the temperature was kept constant at 550 °C for 3 h to ensure that all hydrogen was released from the material. In third step, the hydrogenation reaction is initiated by reducing the temperature to 470 °C, with a temperature ramp of 5 K min−1. It should be mentioned that no active cooling system is attached to the setup; therefore, the experiment temperature ramp may differ from the programmed ramp. In the last step, the heater’s temperature was kept constant at 470 °C for 3 h to ensure complete hydrogenation of the sample. The resulting gas pressure was 91 bar. These four steps were repeated 20 times, beginning with the dehydrogenation step, to accomplish 21 cycles of dehydrogenation and hydrogenation. Due to minor leakages in the test rig, the overall pressure in the last cycle for the hydrogenation decreased to around 83 bar.
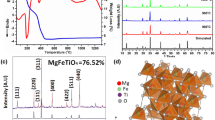
A picture of the dimagnesium iron hexahydride prepared by 21 de-/hydrogenation cycles is shown in Fig. 3a. It is a fine, loosely dispersed green powder as described in the literature [33]. Thermogravimetric analysis with simultaneous differential scanning calorimetry (Fig. 3b) shows a hydrogen mass loss of 3.9 wt-% and an endothermic heat flow in a temperature window between 260 °C and 330 °C. The theoretical hydrogen content in Mg2FeH6 is 5.47 wt-%. The difference between the theoretical value and the obtained hydrogen content can be explained by the results from a Rietveld analysis (Fig. 3c). These show that the sample material contains 64 wt-% of Mg2FeH6, 10 wt-% of MgH2 and the rest is Fe, Mg and MgO. A reason for the relatively low dimagnesium iron hexahydride content might be the low number of de-/hydrogenation cycles (20) and its relatively simple synthesis method, in which coarse particles (mesh 325, < 45 µm) were initially hand-mixed. Several different synthesis routes were investigated in the literature with reported yields of Mg2FeH6 ranging from 25 to 90% [34,35,36]. However, only one synthesis process achieved a yield above 97% [37]. For thermal conductivity experiments, 13.27 g of the dimagnesium iron hexahydride material was transferred to the measurement autoclave under argon atmosphere.
2.2 Transient plane source method
For the determination of the ETC in the dimagnesium iron hexahydride powder bed, the transient plane source method with the TPS 2500S system from HotDisk AB® was applied. The nickel sensor consists of a double spiral head connected by four leads and is encapsulated in a sheet of silicate (mica) layers. The sensor spiral heats up if an electrical current is applied. The temperature rise depends on the thermal conductivity of the surrounding sample material. The electrical resistivity of the nickel sensor increases with increasing temperature and the effective thermal conductivity can be calculated from this temperature change using the TPS 2500S software [38].
The measurements were always carried out under thermally stable conditions at selected gas pressures and temperatures. Each of the measurements was performed three times to calculate the mean value, even though the reproducibility is given by the manufacturer to be in a range of ± 1% [39].
2.3 Measurement setup for high temperature
To enable measurements of the ETC in packed beds of dimagnesium iron hexahydride at hydrogen pressures up to 100 bar and temperatures up to 520 °C, an autoclave made from W.–Nr.: 1.4980 steel (European Norm X6NiCrTiMoVB25-14–2 or Alloy A-286), was constructed, containing the TPS sensor insulated with mica. This autoclave is placed in a furnace with four 250 W infrared heaters covered with silicate plates to reduce heat losses to the environment (cf. Figure 4b).
A hydrogen reservoir of 1.000 cm3 was connected to the measurement autoclave in the test rig (Fig. 4a) to perform a cycle test. The test rig is equipped with a pressure transducer from Jumo GmbH. The pressure reducer and the four needle valves V1, V2, V7 and V8 are located outside a high pressure box, allowing to regulate the gas pressure inside the test rig and the measurement autoclave.
The gas pressure is measured with the pressure transducer pH2 controlled by the pressure reducer and displayed with the Jumo Dicon touch controller. The Jumo Dicon touch controller also regulates the temperature through cascaded heating and records one temperature value per second. A gas-purging program was run after connecting the measurement autoclave to the test rig to ensure an oxygen free atmosphere for the ETC-measurements. Before valve V5 is opened, the residual air or argon in the test rig is removed with a vacuum pump, connected via valve V4.
After the air is removed and the gas pressure is kept below 10–2 mbar, valve V4 is closed for a few minutes. Hydrogen gas is introduced to the test rig via the pressure reducer and V1 and V2 (V7 and V8 are normally closed and V3 is normally opened) until the gas pressure is stable between 5 and 10 bar. This gas is then vented through the valves V7 and V8 to ensure atmospheric pressure in the test rig before valve V4 is opened again for the second gas removal step. These process steps are repeated two more times before V5 can be opened. This whole procedure of gas exchange will be repeated three more times to remove residual argon gas from the measurement autoclave that was introduced into it during the sample preparation in the glovebox. The gas pressure in the test rig can be set, using the valves V1, V2 and the pressure reducer. The hydrogen gas reservoir is connected to the test rig via valve V6 and stores hydrogen gas during dehydrogenation steps, thus enabling cycle tests. The reservoir is equipped with an electrical heater to keep a constant hydrogen gas temperature at 30 °C.
2.4 Cycle test for ETC-measurement of Mg2FeH6 powder bed under operating conditions
To perform ETC-measurements on Mg2FeH6-powder, a temperature-controlled program was started to repeat the de-/hydrogenation cycles. The parameters for the de-/hydrogenation steps were chosen to simulate as realistic as possible conditions (see Table 2). For 50 cycles, ETC-measurements were performed after every 1–3 hydrogenation and dehydrogenation cycles at a pressure of 70 bar and temperatures of 480 °C and 520 °C, in the hydrogenated and dehydrogenated state, respectively. The corresponding equilibrium pressures (Fig. 1) are 47 bar (@ 480 °C) and 87 bar (@ 520 °C).
3 Results
3.1 ETC-Measurement of Mg2FeH6 Powder at Temperatures up to 300 °C
The first measurements were performed on hydrogenated dimagnesium iron hexahydride material at temperatures up to 300 °C and different hydrogen pressure conditions. ETC results are presented at temperatures between 50 and 300 °C as a function of applied hydrogen pressures up to 100 bar H2 in Fig. 5. A strong dependence of the ETC on the applied gas pressure can be observed. This is well-pronounced in the range of up to 10 bar. The lowest ETC value measured was 0.36 W m−1 K−1 recorded at 50 °C and 1 bar hydrogen gas pressure. The ETC increased up to 0.44 W m−1 K−1 at this temperature and 20 bar H2 pressure. Only a small increase in ETC was measured in the range from 20 to 100 bar H2. At 50 °C and 100 bar, the ETC increased to 0.45 W m−1 K−1 due to the higher hydrogen pressure. Similar effects of increasing ETC values could be observed at 100, 150 and 200 °C with increasing pressure. The highest values were observed in the measurements at 300 °C with an ETC value of 0.54 W m−1 K−1 at 100 bar H2 pressure. At this temperature, the ETC increased from 0.45 W m−1 K−1 to 0.54 W m−1 K−1 with increasing hydrogen gas pressure from 2 to 100 bar. The substantial ETC increase at higher hydrogen pressure (up to 5 bar) in Mg2FeH6 is consistent with the Smoluchowski effect, which describes the pressure-dependent behavior of ETC for nonreactive powder beds [39].
In general, three different transport regimes can be described for packed beds. The ETC can be assumed to be constant in the so-called free molecular regime at low pressures. At high pressures (continuum regime), the ETC can also be assumed to be constant but with a higher total value. In the transition region, the thermal conductivity increases with increasing pressure. The measurements clearly show a linear behavior of the ETC values at all measured temperatures and pressures higher than 10 bar.
3.2 ETC-Measurement of Mg2FeH6 material during cycle test
The ETC values of the synthesized dimagnesium iron hexahydride material during the cycle test under operating conditions at temperatures up to 520 °C and a hydrogen gas pressure up to 70 bar are presented in Fig. 6. The red dots represent the ETC values of the dehydrogenated dimagnesium iron hexahydride (magnesium and iron) measured at 520 °C and 70 bar. The black stars show the ETC values of the hydrogenated dimagnesium iron hexahydride at 480 °C and 70 bar hydrogen. The ETC values of the dehydrogenated sample are ~ 1.0 W m−1 K−1. The investigated powder in dehydrogenated state exhibits a higher thermal conductivity than its hydride. Values between 0.7 and 0.8 W m−1 K−1 were observed for the hydrogenated material. This value is higher than the measured ETC between 0.45 W m−1 K−1 and 0.54 W m−1 K−1 in Fig. 5 (Tmax = 300 °C) as a consequence of the higher temperature (T = 480 °C). The total values obtained did not change with an increasing cycle number, as observed for the Ni-activated MgH2 system [31]. According to a rough estimation, the minimum sintering temperature of a pure metal solid solution is 2/3 of its melting temperature and is close to the lower eutectic melting point for powder mixtures [40]. In the case of magnesium, the sintering temperature is ~ 342 °C and for iron, the sintering temperature is ~ 934 °C.
During cycling, the powder experiences temperatures of 480 °C to 520 °C throughout ~ 1000 h without interruption. Therefore, sintering of the magnesium would be expected, which should result in an increased thermal conductivity. The immiscibility of magnesium and iron could explain absence of sintering. They do not form alloys or intermetallic compounds [41].
No change in the ETC of Mg2FeH6/2 Mg + Fe is observed after 50 de-/hydrogenation cycles. However, during one cycle different ETC values can be observed. This could be a sign for an incomplete de-/hydrogenation at the time of the measurement. Figure 7a shows the sample’s temperature (black line) and the measured ETC values (dots/stars) for one de-/hydrogenation cycle during the test. After increasing/decreasing the temperature to the de-/hydrogenation temperature (520 °C/480 °C), it is kept constant for 3 h. During that time, the reaction takes place consuming hydrogen from the reservoir (hydrogenation) and releasing hydrogen into the reservoir (dehydrogenation). If no reaction is taking place, the pressure stays constant. Therefore, the pressure seems to be a good indicator whether a reaction is complete or not. The cycle length was chosen observing the pressure change, respectively.
a ETC-measurement of synthesized dimagnesium iron hexahydride material as a function of time in the 25th de-/hydrogenation cycle. The measurements were performed at a hydrogen pressure of 70 bar and at temperatures of 480 °C (hydrogenated) and 520 °C (dehydrogenated). b Rietveld refined XRD of the material after test ending
After ~ 2 h, 2.5 h and 3 h during the de-/hydrogenation, ETC-measurements were performed. During the time of the measurements, there was no significant pressure change observable.
The dehydrogenated material (metal) shows a higher thermal conductivity then the hydrogenated as can be seen in Fig. 6. Therefore, during the hydrogenation, the measured ETC values should decrease with increasing reaction time, while during the dehydrogenation the ETC values should increase with increasing reaction time until the reaction is complete. The reason is the increasing/decreasing content of metallic powder in the mixture during the de-/hydrogenation. In case of a complete de-/hydrogenation the ETC values should stay constant.
While there was no pressure change observable during the ETC measurements shown in Fig. 7a it can be concluded from the changing ETC values that the reaction is not complete. The experimental setup in that state was not able to record pressure, therefore the described observation cannot be shown in the plot. Minor changes in the material, which are not detectable due to pressure change, probably due to large reservoir volume (1 l), are still pronounced in the change of effective thermal conductivity of the material. The ETC seems to be a reliable measure to check for completeness of de-/hydrogenation reactions. After the test, the material was kept in hydrogenated state. The Rietveld refinement of the XRD (Fig. 7b) shows 84 wt-% Mg2FeH6, 10 wt-% Fe and 6 wt-% MgO.
4 Discussion
This work presented the effective thermal conductivity of the 2 Mg:Fe (2:1)/Mg2FeH6-system for a total of 50 de-/hydrogenation cycles under operating conditions. To synthesize the dimagnesium iron hexahydride, 21 cycles were performed in a high-pressure autoclave before loading the material into a thermal conductivity measurement cell. To measure the effective thermal conductivity under operating conditions (e.g., hydrogen pressure up to 150 bar and temperature up to 550 °C), the transient plane source method was used with a mica-insulated TPS sensor housed in the measuring cell. Measurements were carried out in the range from 480 to 520 °C (70 bar) during the cycle test and 50 °C up to 300 °C (2 bar–100 bar) before the cycle test.
In agreement with other investigated metal hydrides the ETC of the hydrogenated phase shows lower thermal conductivity values (~ 0.7 W m−1 K−1–0.8 W m−1 K−1) compared to the dehydrogenated phase (~ 1 W m−1 K−1). The pressure dependence of the ETC was in good agreement with the expectations for a powder bed described by the Smoluchowski effect. No significant change in ETC was observed over 50 cycles. Minor changes could be induced by orientation and pulverization [22] of the metal/metal hydride powder during cycling and incomplete de-/hydrogenation.
Overall, the investigated powder showed smaller ETC values then the compressed pellets measured by Bird et al. 2020 [32], because compression of a powder increases the contact area between particles and, respectively, the effective heat conductivity as well.
In a previous publication, we presented the formation of a percolated network in nickel-activated magnesium [31]. The formation of such a network is associated with a dramatic increase in ETC from 2 to more than 7 W m−1 K−1 after about 70 hydrogenation and dehydrogenation cycles. In the case of the Mg2FeH6/2 Mg + Fe system, sintering of Mg-particles could be expected under the given temperatures (> 342 °C), which should lead to higher effective thermal conductivity, but this could not be observed in the experiments which were carried out. This may be caused that Mg and Fe do not form any alloy or intermetallic compound.
The feature of stable thermal conductivity over the first 50 cycles in the powder bed of dimagnesium iron hexahydride confirms the hypothesis that the iron could prevent the formation of coarse particle structures, as shown in the case of magnesium hydride combined with nickel. It would be advantageous to verify the proposed hypothesis with SEM/EDX measurements, but due to the remains of small, air-sensitive Fe powder particles, these investigations cannot be provided.
Within one cycle, ETC-measurements have shown that under the selected parameters, the de-/hydrogenation takes at least 3 h. The deviation between some measured ETC values in a single cycle can be explained by an incomplete conversion of the two metals to metal hydride and vice versa.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
H.P. Garg, S.C. Mullick, V.K. Bhargava, Solar thermal energy storage (D. Reidel Publishing Company, Dordrecht, 1985), pp.292–427
A. Thess, Phys. Rev. Lett. 111, 110602 (2013). https://doi.org/10.1103/physrevlett.111.110602
R.B. Laughlin, J. Renew. Sustain. Energy 9, 44103 (2017). https://doi.org/10.1063/1.4994054
M. Felderhoff, R. Urbanczyk, S. Peil, Green 3, 113 (2013). https://doi.org/10.1515/green-2013-0011
A. Reiser, B. Bogdanović, K. Schlichte, Int. J. Hydrogen Energy 25, 425 (2000). https://doi.org/10.1016/s0360-3199(99)00057-9
J.-C. Crivello, R.V. Denys, M. Dornheim, M. Felderhoff, D.M. Grant, J. Huot, T.R. Jensen, P. de Jongh, M. Latroche, G.S. Walker, C.J. Webb, V.A. Yartys, Appl. Phys. A 122, 85 (2016). https://doi.org/10.1007/s00339-016-9601-1
V.A. Yartys, M.V. Lototskyy, E. Akiba, R. Albert, V.E. Antonov, J.R. Ares, M. Baricco, N. Bourgeois, C.E. Buckley, J.M. Bellosta von Colbe, J.-C. Crivello, F. Cuevas, R.V. Denys, M. Dornheim, M. Felderhoff, D.M. Grant, B.C. Hauback, T.D. Humphries, I. Jacob, T.R. Jensen, P.E. de Jongh, J.-M. Joubert, M.A. Kuzovnikov, M. Latroche, M. Paskevicius, L. Pasquini, L. Popilevsky, V.M. Skripnyuk, E. Rabkin, M.V. Sofianos, A. Stuart, G. Walker, C.J. Hui Wang, M.Z. Webb, Int. J. Hydrogen Energy 44, 7809 (2019). https://doi.org/10.1016/j.ijhydene.2018.12.212
A. Baran, M. Polański, Materials 13, 3993 (2020). https://doi.org/10.3390/ma13183993
D.A. Sheppard, C. Corgnale, B. Hardy, T. Motyka, R. Zidan, M. Paskevicius, C.E. Buckley, RSC Adv. 4, 26552 (2014). https://doi.org/10.1039/C4RA01682C
C. Corgnale, B. Hardy, T. Motyka, R. Zidan, J. Teprovich, B. Peters, Renew. Sustain. Energy Rev. 38, 821 (2014). https://doi.org/10.1016/j.rser.2014.07.049
P.A. Ward, C. Corgnale, J.A. Teprovich, T. Motyka, B. Hardy, D. Sheppard, C. Buckley, R. Zidan, Appl. Phys. A 122, 1114 (2016). https://doi.org/10.1007/s00339-016-9909-x
D.A. Sheppard, C.E. Buckley, Int. J. Hydrogen Energy 44, 9143 (2019). https://doi.org/10.1016/j.ijhydene.2019.01.271
M. Retuerto, J. Sánchez-Benítez, E. Rodríguez-Cañas, D. Serafini, J.A. Alonso, Int. J. Hydrogen Energy 35, 7835 (2010). https://doi.org/10.1016/j.ijhydene.2010.05.062
F.C. Gennari, F.J. Castro, J.A. Gamboa, J. Alloys Compd. 339, 261 (2002). https://doi.org/10.1016/S0925-8388(01)02009-6
B. Bogdanović, A. Reiser, K. Schlichte, B. Spliethoff, B. Tesche, J. Alloys Compd. 345, 77 (2002). https://doi.org/10.1016/S0925-8388(02)00308-0
D.E. Dedrick, Solid-State Hydrogen Storage, 1st edn. (Elsevier, Cambridge, 2008), pp.82–103
E. Hahne, Int. J. Hydrogen Energy 23, 107 (1998). https://doi.org/10.1016/S0360-3199(97)00020-7
M. Groll, Heat Recovery Syst. CHP 13, 341 (1993). https://doi.org/10.1016/0890-4332(93)90059-5
S. Suda, N. Kobayashi, K. Yoshida, Y. Ishido, S. Ono, J. Less Common Met. 74, 127 (1980). https://doi.org/10.1016/0022-5088(80)90082-X
A. Rodŕiguez Sánchez, H.-P. Klein, M. Groll, Int. J. Hydrogen Energy 28, 515 (2003). https://doi.org/10.1016/S0360-3199(02)00057-5
A. Mathew, N. Nadim, T.T. Chandratilleke, T.D. Humphries, M. Paskevicius, C.E. Buckley, Int. J. Hydrogen Energy 46, 1038 (2021). https://doi.org/10.1016/j.ijhydene.2020.09.191
R. Albert, Thermal conductivity measurements of metal hydrides as high temperature heat storage materials under operating conditions, dissertation (University Duisburg-Essen, Duisburg, 2019)
J. Kallweit, (1994) Effektive Wärmeleitfähigkeit von Metallhydrid-Materialien zur Speicherung von Wasserstoff, Dissertation, University Stuttgart, Stuttgart
M.D. Christopher, Application of the transient hot-wire technique for measurement of effective thermal conductivity of catalyzed sodium alanate for hydrogen storage (Virginia Polytechnic Institute and State University, 2006)
S.M. Flueckiger, Thermal property measurements of high pressure metal hydrides (Purdue University, 2009)
W. Zhao, Y. Yang, Z. Bao, D. Yan, Z. Zhu, Int. J. Hydrogen Energy 45, 6680 (2020). https://doi.org/10.1016/j.ijhydene.2019.12.185
J. Kapischke, J. Hapke, Exp. Therm. Fluid Sci. 17, 347 (1998). https://doi.org/10.1016/S0894-1777(98)00007-7
H. Shao, W. Ma, M. Kohno, Y. Takata, G. Xin, S. Fujikawa, S. Fujino, S. Bishop, X. Li, Int. J. Hydrogen Energy 39, 9893 (2014). https://doi.org/10.1016/j.ijhydene.2014.02.063
L. Popilevsky, V.M. Skripnyuk, Y. Amouyal, E. Rabkin, Int. J. Hydrogen Energy 42, 22395 (2017). https://doi.org/10.1016/j.ijhydene.2017.04.088
R. Albert, R. Urbanczyk, M. Felderhoff, Int. J. Hydrogen Energy 44, 29273 (2019). https://doi.org/10.1016/j.ijhydene.2019.01.218
R. Albert, C. Wagner, R. Urbanczyk, M. Felderhoff, Energy Technol. 8, 2000356 (2020). https://doi.org/10.1002/ente.202000356
J.E. Bird, T.D. Humphries, M. Paskevicius, L. Poupin, C.E. Buckley, Phys. Chem. Chem. Phys. 22, 4617 (2020). https://doi.org/10.1039/c9cp05940g
R. Urbanczyk, M. Meggouh, R. Moury, K. Peinecke, S. Peil, M. Felderhoff, Appl. Phys. A 122, 591 (2016). https://doi.org/10.1007/s00339-016-9811-6
A. Asselli, J. Huot, Metals 4, 388 (2014). https://doi.org/10.3390/met4030388
M. Rzeszotarska, T. Czujko, M. Polański, Int. J. Hydrogen Energy 45, 19440 (2020). https://doi.org/10.1016/j.ijhydene.2020.04.247
J. Puszkiel, M. Castro Riglos, J. Ramallo-López, M. Mizrahi, T. Gemming, C. Pistidda, P. Arneodo Larochette, J. Bellosta von Colbe, T. Klassen, M. Dornheim, F. Gennari, Metals 8, 967 (2018). https://doi.org/10.3390/met8110967
S. Brutti, L. Farina, F. Trequattrini, O. Palumbo, P. Reale, L. Silvestri, S. Panero, A. Paolone, Energies 11, 1952 (2018). https://doi.org/10.3390/en11081952
S.E. Gustafsson, Rev. Sci. Instrum. 62, 797 (1991). https://doi.org/10.1063/1.1142087
www.hotdiskinstruments.com/content/uploads/2022/01/TPS2500S.pdf. Accessed 03 August 2022
P.S. Liu, G.F. Chen, Porous Materials, 1st edn. (Elsevier, Amsterdam, 2014), p.p43
R. Ninomiya, H. Yukawa, M. Morinaga, K. Kubota, J. Alloys Compd. 215, 315 (1994). https://doi.org/10.1016/0925-8388(94)90860-5
Acknowledgements
This project (19695N) has been supported by AiF fund as part of the industrial cooperative research and development (IGF) program of the German Federal Ministry of Economic Affairs and Energy (BMWi). The additional financial support of the Max–Planck Society is greatly appreciated. The author R.A. is grateful for the financial support from the IMPRS SurMat (International Max–Planck Research School for Interface Controlled Materials for Energy Conversion) in Düsseldorf, Germany, for granting a scholarship. The authors thank members of the powder diffraction and surface spectroscopy department of the Max–Planck-Institut für Kohlenforschung for their valuable support.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualization, RA; methodology, RA, M.F. and RU, software, RA and CW; validation RA and CW; formal analysis; investigation, RA and CW; resources, RA, MF and RU; data curation, RA and CW; writing—original draft preparation, R.A.; writing—review and editing, MF and RU.; visualization, RA; supervision, MF and RU; project administration, MF; funding acquisition, MF and RU All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Albert, R., Wagner, C., Urbanczyk, R. et al. Effective thermal conductivity of dimagnesium iron hexahydride (Mg2FeH6) for heat storage applications. Appl. Phys. A 129, 62 (2023). https://doi.org/10.1007/s00339-022-06336-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-022-06336-9