Abstract
Aluminum-substituted M-type strontium hexaferrite (SrM-Al, SrFe12−xAlxO19, 0 ≤ x ≤ 4) with uniform microstructure and excellent magnetic properties was synthesized by conventional ceramic processing based on solid state reaction. Coercive field was highly enhanced by heavy doping with Al3+ although magnetization was significantly reduced. However, formation of coarse microstructure and poor development of Hc with low level doping of Al3+ disturbed practical application. To promote phase formation and to suppress growth of coarse grains which were induced by incorporation of Al3+ ions, SrM-Al ceramics was processed with NaOH. Such Na-assisted processing of SrM-Al reduced formation of non-magnetic phases and assisted development of uniform fine grains. In addition, the large residual strain by Al3+ doping was partially relieved by the same approach. As a result, saturation magnetization of SrFe12−xAlxO19 was remarkably enhanced and the BHmax of randomly oriented hard magnetic ceramics was improved to 1.23MGOe by concomitant addition of Na and Al ions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Permanent magnetic materials with high coercivity and high remanent magnetization have wide application fields including cost-effective electric motors, long-lasting data recording media, high frequency communication devices, electroacoustic devices, etc. [1,2,3,4,5]. Among these, strontium hexaferrite (SrM; SrFe12O19) with magnetoplumbite structure comprises one of the most important class of high-performance magnetic materials. SrM exhibits a large magnetocrystalline anisotropy constant (K1 ~ 3.6 × 106 erg/cm3) and a high saturation magnetization (Ms ~ 74 emu/g) suitable for permanent magnet [6].
Since their first development in twentieth century, much interest on SrM ceramics has been attracted owing to their excellent ferromagnetic properties, low production cost and outstanding chemical stability [7, 8]. Then, various efforts including doping foreign atoms to the lattice of SrM or controlling particle size have been devoted to improve magnetic properties. Recently, cation doping with La, Co improved both remanent magnetization (Mr) and coercive field (Hc) as reported by many groups [9,10,11]. Hc was increased up to a certain low doping level without noticeable influence on Mr, but excessive doping reduced Hc significantly [12]. As a result, the available maximum energy product (BHmax) exceeded that of undoped SrM [13] and industrial production of ferrite permanent magnets mainly relies on this approach. However, recent explosively increasing demand on cobalt resources induced surge of their price and ferrites free of rare elements are highly required for sustainable industrial production.
Interestingly, addition of non-magnetic aluminum ions has turned out to be useful for multiplication of Hc via substitution of Al3+ for Fe3+ in M-type hexaferrites [14]. The large Hc of Al3+ doped hexaferrite throws light on possible improvement of the energy density of permanent magnets. Recently, Kazin et al. reported extremely large Hc approaching 40 kOe for SrFe12−xAlxO19 (SrM-Al) with x = 5.5 via solution auto-combustion route although the Mr is highly limited and non-ferromagnetic phases are still present [15]. However, most reports on the synthesis of SrM-Al have been based on non-conventional routes such as sol–gel autocombustion [14,15,16], co-precipitation [17], and salt-mediated synthesis [18]. Synthesis of SrM-Al has rarely been reported via industrially important ceramic processing based on solid state reaction [19] owing to large grain size distribution and unreacted impurities [14] although small amount of Al2O3 were occasionally adopted to support consolidation of particles during sintering of hexaferrites [2]. Besides, systematic analyses of structural and magnetic properties of SrM-Al with low Al content are quite rare.
Here, we synthesized SrM-Al having low Al content (0 ≤ x ≤ 4) via conventional ceramic processing based on solid state reaction. A significant degradation of magnetic property was observed with increasing amount of Al3+ addition, which was attributed to large grain size and formation of a variety of non-magnetic phases. Here, sodium ions were introduced as a form of NaOH to promote phase formation and to suppress rapid crystal growth by incorporation of Al3+. Homogeneous microstructures with trace of non-magnetic phases were obtained for SrM-Al by processing with NaOH. As a result, BHmax of randomly oriented crystallites were highly improved.
2 Materials and methods
2.1 Preparation of SrFe12−xAlxO19
SrFe12−XAlXO19 was synthesized with conventional ceramic processing route. In detail, SrCO3 (99%, Sigma-Aldrich), Fe2O3 (96%, Sigma-Aldrich) and Al2O3 (99%, Alfa Aesar) were weighed according to nominal composition of SrFe12−xAlxO19 (0 ≤ x ≤ 4) and subsequently ball milled with stabilized zirconia ball using de-ionized water as media for dispersion. Series of samples with composition of SrFe12−xAlxO19 (0 ≤ x ≤ 4) were also prepared with SrCO3, Fe2O3, Al2O3 and NaOH (95%, Samchun Chemicals). Here, the molar ratio of NaOH to SrCO3 (hereafter denoted as xNa) was set to be 0.05.
The milled powders were dried and calcined at 1100 °C for 4 h in ambient atmosphere to synthesize hexagonal ferrite phase. Again, calcined ferrite powders were ball milled in de-ionized water. Bulk samples were shaped as disks by mechanical forming and were subsequently sintered at 1200 °C for 2 h in air.
2.2 Characterization
X-ray diffraction (XRD) of crystalline phases was carried out using a horizontal diffractometer (D/Max 2500, Rigaku) using Cu Kα X-ray lines. The lattice parameters were calculated from XRD peaks with software Unit Cell after extracting diffraction angles by numerical fitting of XRD profiles with pseudo-Voigt function using software XFIT [20, 21]. The microstructures were detected with scanning electron microscopy (SEM) using a field-emission electron microscope (JEM6700F, JEOL). Average grain sizes were estimated from line intercept method. Microscopic chemical composition was characterized with energy dispersive X-ray spectroscopy (EDS) system equipped with SEM. Magnetic properties were characterized with vibrating sample magnetometer (VSM8600, Lakeshore) with the maximum field of 25 kOe at ambient temperature. Also, physical properties measurement system (PPMS, Quantum design) with VSM option was utilized to measure high field magnetization curves with maximum field of 70 kOe.
3 Results and discussions
3.1 Phase formation and lattice distortion of SrFe12−xAlxO19
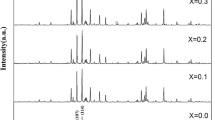
Series of SrFe12−xAlxO19 (SrM-Al) with different content of Al3+ were prepared by solid state reaction with calcination at 1100 °C for 4 h and subsequent annealing at 1200 °C for 2 h in ambient atmosphere. X-ray diffraction (XRD) profiles shown in Fig. 1a indicated formation of M-type hexaferrites of SrM-Al and non-magnetic secondary phases such as Fe2O3, SrFe2O4, etc. were also identified from XRD. Profile fitting based on hexagonal symmetry showed lattice contraction of the unit cell by incorporating more Al3+ ions. As shown in Fig. 1b, volume change of hexaferrite lattice (VR) denoted by VR = (Vo − V)/Vo was estimated by profile fitting based on hexagonal symmetry. Here, Vo and V denote cell volume of undoped and doped SrM, respectively. Origin of lattice contraction can be attributed to substitution of Al3+ ions for Fe3+ ions in the lattice of M-type hexaferrites, since the ionic radius of Al3+ is much smaller than that of Fe3+ (r[Fe3+] ~ 0.645 Å, r[Al3+] ~ 0.535 Å). Clear linearity between lattice contraction and amount of Al3+ doping was confirmed with near unity correlation coefficient (rcorr) from linear fitting. Linearity in lattice contraction and dopant concentration is popularly found from other materials doped with foreign atoms [22].
One interesting feature of SrM-Al was identified from variation of linewidths in diffraction profiles of SrM-Al. As shown in Fig. 1c, the line width of XRD profile was increased with enlarged Al content. It is known that both size of crystallite and residual strain can contribute to broadening in the XRD profile [23]. To check the characteristic origin of broad feature in the diffraction peak, numerical analysis based on Williamson–Hall method was carried out with the observed broad diffraction profile from sample with x = 4.0 (see Fig. S1 in the Supporting information) [23]. As a result, the residual strain was identified to be one of the dominantly contributing factors to peak broadening in XRD profiles for SrM-Al and the residual lattice strain of the same specimen was estimated to be 0.18%. Here, the large residual strain in samples can be attributed to local inhomogeneous distortion of lattice by substitution of smaller Al3+ for larger Fe3+. In addition, residual strain by cation doping can partially contribute enhanced coercivity of magnetic materials by affecting domain motion [24, 25].
3.2 The magnetic properties and microstructures of SrFe12−xAlxO19
The magnetic properties were measured with a vibrational sample magnetometer (VSM). Magnetization was almost saturated by applying highest field of 25 kOe and magnetization at 25 kOe was regarded as a saturation magnetization (Ms). Figure 2a shows magnetization-applied field (MH) curves of various SrM-Al samples. Ms of SrM-Al decreased with increasing Al3+ content although Ms was underestimated owing to presence of non-magnetic phases. Clear linear correlation between the Al3+ content and the Ms was shown from linear regression in Fig. 2c. Then, variation of Ms with Al content in series of SrM-Al specimen can be attributed to substitution of magnetic Fe3+ ions for non-magnetic Al3+ ions. Occupation of octahedral sites (2a, 12k, 4f2) with upward spin alignment by non-magnetic Al3+ ions can reduce net magnetic moments of hexaferrites since total magnetic moments of hexaferrite were originated from uncompensated upward spins [16].
a Magnetization (M) versus field (H) curves and b magnetic parameters curves of SrFe12−xAlxO19 after annealing at 1200 °C for 2 h. c Variation of Ms with respect to measured Al content (xm) d Magnetization curves scanning from zero to 70 kOe and numerical fitting based on law of approach to saturation (LAS) denoted with solid lines
In contrast to previous report based on hexaferrites with high Al content [14,15,16], coercive field (Hc) was slightly dropped by minimal addition of Al3+ ions (x ≤ 0.5). However, further addition of Al3+ ions (x ≥ 1) enhanced the Hc up to ~ 14 kOe without further depression. The derivative of magnetization (dM/dH) as a function of applied field shows distribution of switching field during sweeping of magnetic field (see Figure S2 in the supporting information) [15]. Nearly symmetric single peak was observed for each specimen and the peaked field for specimen with low Al content (x ≤ 1) remained at the same level despite of increasing Hc with more Al3+ addition. Interestingly, a variety of kinks were found from MH curves of some specimen. Careful investigation of MH curves and their derivatives disclosed that significant inconsistency between peaked switching field and Hc which could be related to the presence of kinks in the hysteresis curves for SrM-Al (0.1 ≤ x ≤ 2). Kinks separated the MH curves of samples from boundary of constant BHmax (see Figure S3 in the supporting information) and should be removed to synthesize hard magnetic materials with excellent magnetic properties.
Magnetic properties of the hard magnetic materials are empirically described by Kronmüller equation as:
using experimental parameters, αK and Neff. The first term in (1), 2K1/μoMs is the magnetic anisotropy field (HA) which is the upper limit of Hc corresponding to magnetic field required to coherent nucleation of domain walls. The discrepancy in HA and Hc is originated from non-ideal motion of magnetic domains by non-uniform magnetization reversal, heterogeneities in microstructures, etc. [26]. Analysis of HA may offer information on the nature of coercivities without perturbation by the effect of microstructures. We estimated the magnetic anisotropy field (HA) via law of approach to saturation magnetization (LAS) fitting [27, 28] to investigate the effect of Al addition:
where χh denotes high field susceptibility including field-induced band splitting [1]. The fitting constant A was set to 0 and value of B was used for calculation of HA with following relation:
Numerical fitting of the magnetization curve was limited to high field region with magnetization exceeding 90% of Ms to remove domain structure effect [1]. Table 1 lists extracted magnetic parameters from magnetization curves at high field of some samples with LAS fitting displayed in Fig. 1d. HA was greatly enhanced by substitutional incorporation of Al3+ to SrM without depression at low Al3+ content (x ≤ 0.5). In particular, HA was enhanced to 36 kOe by addition of large amount of Al3+ (x = 2) although significant reduction in saturation magnetization was observed. In contrast to variation of Hc for SrM-Al, almost linear correlation of HA with Al3+ content was found. Since HA corresponds to intrinsic limit of coercive field of ferromagnetic materials, enhanced HA manifests effect of Al-doping on magnetic properties of SrM which was not perturbed by development of microstructure. In addition, discrepancies in variation of Hc and HA can be attributed to development of non-ideal microstructures or defects by Al3+ addition.
Figure 3 shows microstructures of SrM-Al with various Al3+ content. One can see development of significantly large facetted grains (diameter ~ 2 μm) even with lowest level of Al3+ addition in this study. That is, low level addition of Al2O3 (~ 0.3 wt.%) is enough to promote grain growth of hexaferrites. The typical size of crystalline grains in SrM-Al is much beyond the critical size of single domain for strontium hexaferrite (Dc < 1 μm) [29]. Then, initial depression of Hc may be attributed to formation of multi-domains and easy movement of magnetic domain walls as expected from the coarsened microstructure of SrM-Al. However, further addition of Al3+ for SrM-Al (x > 2) resulted in fine microstructure with grain size comparable to Dc. As depicted in Fig. 1d, both coarse grains (> 2 μm) and fine grains (< 1 μm) were identified from microstructure of SrM-Al with low Al3+ content. It is known that kinks in the MH hysteresis curve are usually caused by inhomogeneity in morphology and chemical composition in various ferromagnetic materials [30, 31]. The morphological inhomogeneity depicted from the microscopic images can be attributed to one possible origin of kinks in hysteresis curves for SrM-Al. In addition, possible existence of chemical inhomogeneity including variety of lattice defects and non-magnetic secondary phases could also contribute such distorted hysteresis curves.
3.3 Addition of NaOH during the synthesis of SrFe12−xAlxO19
AS mentioned in the previous section, introduction of Al3+ promoted grain growth of SrM even with very low level of addition and the significant grain growth would be related to reduced coercivity of hexaferrites through formation of multi-domain magnetic structures. Hence, rapid grain growth should be suppressed to enhance coercivity of SrM-Al at low level doping of Al3+. To control the grain growth of ceramic materials, various additives have been attempted [27, 32,33,34,35,36,37]. For example, it was shown that application of only 1000–2000 ppm of sodium oxide successfully suppressed grain growth of various oxide ceramics by controlling formation of liquid phases and dragging the grain boundaries [35,36,37]. Here, we pursued enhancement of magnetic properties through microstructural control of SrM-Al through minor addition of NaOH during solid state synthesis of the series of SrM-Al. For convenience of description, SrM-Al processed with NaOH is named as SrM-Al-Na.
Figure 4a, b shows magnetic hysteresis curves of SrM-Al-Na prepared with small amount of NaOH. All of measured curves showed monotonous change with variety of applied field and reduction of magnetization was also observed with increasing Al3+ content. The value of Hc increased without local dropping with increasing amount of Al3+. All of measured MH curves followed traces of typical magnetic hysteresis curves from ferrite particles and no shape modification such as kinks have been found, which denotes formation of homogeneous microstructure and reduction of chemical inhomogeneity. One of the most important figure of merits from performance of hard magnetic materials is the maximum energy product (BHmax). Figure 4c shows BHmax of the powdered samples with random orientation and the values of BHmax have been extracted from MH curves for the same samples [27, 28] BHmax of undoped SrM was about 1 MGOe and processing with NaOH enhanced BHmax to 1.15 MGOe in the absence of Al3+ doping. However, mere addition of Al3+ ions resulted in a significant reduction of the same values. Such depression in BHmax can be attributed to much fast reduction in magnetization than slow increase in coercivity by Al3+ doping. In contrast, processing with Na ions showed a remarkable enhancement in BHmax of the Al3+-doped strontium hexaferrites throughout all composition range. When the Al content is limited to be below x = 1, SrM-Al-Na showed much higher BHmax compared with those from SrM-Al processed without NaOH and best result of BHmax = 1.23 MGOe was obtained with x ≤ 0.5. Thus, we can conclude that concurrent application of optimal Al3+ doping and processing with NaOH can enhance the ferromagnetic performance of strontium hexaferrites.
a Magnetization (M) versus applied magnetic field (H) curves and representation of 2nd quadrant with range of high energy product (BHmax > 1MGOe) denoted as a light shade. b magnetic parameters (Hc and Ms) of SrFe11−xAlxO19 processed with Na ions; c BHmax of SrFe11−xAlxO19 processed with Na ions (denoted as circles) and without Na ions (denoted as squares). d, a Images from microscopic investigation of SrFe11−xAlxO19 after annealing at 1200 °C for 2 h
Figure 5a shows microstructure of SrM-Al-Na. In contrast to the same series of samples processed without Na ions, all SrM-Al-Na samples exhibited fine and uniform microstructure with average diameter less than 1 μm. In addition, facetted hexagonal grains with sharp edges were rounded and uniform microstructure free of rapid grain growth was obtained. Then, it can be concluded that application of NaOH during synthesis of SrM-Al successfully inhibited significant grain growth of hexaferrites. The measured grain size of SrM-Al-Na was comparable to the critical radius for single domain motion and formation of single domain is more probable. Formation of fine and uniform microstructure in SrM-Al-Na explains the monotonic increase in Hc by increasing Al3+ content. In addition, uniform microstructures of SrM-Al-Na explains absence of kinks in MH curves and enhanced switching field for spin alignment.
a Images from microscopic investigation of SrFe12−xAlxO19 synthesized with NaOH after annealing at 1200 °C for 2 h. Grain sizes of SrFe12−xAlxO19 processed without NaOH were also displayed for comparison. b XRD profiles of SrFe11.5Al0.5O19 processed with and without NaOH. Plane index of main phase were indexed based on M-type hexaferrites. Asterisks (*) denote peaks from unidentified impurity phases. c Centered and normalized XRD profiles from (114) reflections of SrFe12−xAlxO19 processed with and without NaOH. Open symbols (open square, open circle) denote samples processed with NaOH
XRD profiles of SrM-Al-Na were also denoted in Fig. S2 and small amount of α-Fe2O3 were identified from X-ray diffractograms (see the supporting information). Comparison of detailed XRD profiles from SrFe11.5Al0.5O19 processed with and without NaOH showed a clear difference in phase formation. As shown in Fig. 5b, the XRD profile from SrFe11.5Al0.5O19 processed without NaOH contains several unidentified peaks denoted with asterisks (*). Although both of two samples indicate presence of SrFe2O4 and Fe2O3 phases in common, unidentified phases deviated from ferromagnetic M-type hexaferrites were found for SrFe11.5Al0.5O19 processed without NaOH. So, processing with NaOH assisted phase formation of SrFe11.5Al0.5O19 and total amount of secondary phases was successfully reduced by processing with NaOH. Higher level of Ms of SrM-Al-Na throughout all composition range can be explained with absence of some non-magnetic secondary phases by processing with NaOH.
Interestingly, the width of diffraction profiles was remarkably reduced by application of NaOH during synthesis as shown in Fig. 5c. As mentioned in the previous section, incorporation of Al3+ ions in the lattice of SrM induced residual lattice strain which was revealed as peak broadening in the XRD profile of SrM-Al. Hence, the highly reduced width of the diffraction profile implies reduction of residual strain in SrM-Al-Na. It is known that the lattice strain can reduce saturation magnetization of hexaferrites by disturbing magnetic networks consisting of Fe–O–Fe bondings [38]. Also, residual strain can affect spin alignment in magnetic materials and may enhance the coercive field [27, 28, 39].
Thus, microstructure and phase formation of SrM-Al were highly improved by processing with NaOH. In addition, residual strain formed by doping with Al3+ ion was also reduced. As a result, variation of magnetic properties, Hc, Ms, BHmax were remarkably improved by incorporation of Al3+ ions.
4 Conclusions
SrFe12−XAlXO19 having low Al content (0 ≤ x ≤ 4) was synthesized via conventional ceramic processing based on solid state reaction after calcination at 1100 °C for 4 h and subsequent annealing at 1200 °C for 2 h in ambient atmosphere. Significant inhomogeneity in microstructure and large residual strain were identified. As a result, poor magnetic performance was obtained. Sodium ions were introduced with a form of NaOH to promote phase formation and to suppress rapid grain growth induced by incorporation of Al3+ ions. Non-magnetic secondary phases were partially relieved and grain size of constituting crystal grains was reduced to be smaller than the critical size of single domain formation via solid state processing with NaOH. As a result, Ms and Hc of SrFe12-−xAlxO19 were remarkably enhanced and the maximum energy product of randomly oriented hard magnetic ceramics was improved to be 1.23MGOe by concomitant addition of Na and Al ions during synthesis. We expect that permanent magnet with high energy density can be synthesized by solid state reaction with help of Al and Na ions if supported by suitable magnetic texturing and densification process.
Data availability statement
The data that support the findings of this study are available from the corresponding author, Y. K. Kim, upon reasonable request.
References
J.M.D. Coey, Magnetism and Magnetic Materials (Cambridge University Press, Cambridge, 2010)
R.C. Pullar, Hexagonal ferrites: a review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater. Sci. 57, 1191–1334 (2012)
K. Katayama, Y. Chinda, O. Shimizu, Y. Goto, M. Suzuki, H. Noguchi, Long term stabilities of magnetic tape for data storage in office environment. J. Appl. Phys. 117, Article 17E305 (2015)
S.P. Gairola, V. Verma, A. Singh, L.P. Purohit, R.K. Kotnala, Modified composition of barium ferrite to act as a microwave absorber in X-band frequencies. Solid State Commun. 150, 147–151 (2010)
K. Kimiabeigi, J.D. Widmer, R. Long, Y. Gao, J. Ross, R. Martin, T. Lisle, J.M. Soler Vizan, A. Michaelides, B. Mecrow, High performance low cost electric motor for electric vehicles using ferrite magnets. IEEE Trans. Ind. Electron. 63, 113–122 (2016)
A. Goldman, Modern Ferrite Technology (Springer Science+Business Media, Berlin, 2006)
H.P.J. Wijn, New method of melting ferromagnetic semiconductors: BaFe18O27, a new kind of ferromagnetic crystal with high crystal anisotropy. Nature 170, 707–708 (1952)
L.H. Brixner, Preparation of the ferrites BaFe12O19 and SrFe12O19 in transparent form. J. Am. Chem. Soc. 81, 3841–3843 (1959)
Y. Yang, X. Liu, D. Jin, Y. Ma, Structural and magnetic properties of La–Co substituted Sr–Ca hexaferrites synthesized by the solid state reaction method. Mater. Res. Bull. 59, 37–41 (2014)
H. Taguchi, Y. Minachi, K. Masuzawa, High performance SrLaCo ferrite magnets with M structure, in Proceedings of the 8th International Conference Ferrites, Kyoto, Japan, September 18–21, (2000), pp. 405–409
F. Kools, A. Morel, P. Tenaud, M. Rossignol, O. Isnard, R. Grössinger, J. M. Le Breton, P. Tenaud, La–Co substituted Sr and Ba M-ferrites magnet properties versus intrinsic and microstructural factors, in Proceedings of the 8th International Conference Ferrites, Kyoto, Japan, Sept. 18–21, (2000), pp. 437–439
T. Takami, Y. Ogata, Y. Kubota, Development of La–Co substituted ferrite magnets. J. Jpn. Soc. Powder Metall. 56, 636–641 (2000)
Y. Yang, F. Wang, J. Shao, D. Huang, Z. Gao, Effects of La doping on the structural and magnetic properties of Sr1−xLaxFe11.75Co0.10Zn0.15O19 hexagonal ferrites. J. Mater. Res. 30, 1844–1851 (2015)
L.A. Trusov, E.A. Gorbachev, V.A. Lebedev, A.E. Sleptsova, I.V. Roslyakov, E.S. Kozlyakova, A.V. Vasiliev, R.E. Dinnebier, M. Jansen, P.E. Kazin, Ca-Al double-substituted strontium hexaferrites with giant coercivity. Chem. Comm. 54, 479–482 (2018)
E.A. Gorbachev, L.A. Trusov, A.E. Sleptsova, S. Kozlyakova, L.N. Alyabyeva, S.R. Yegiyan, A.S. Prokhorov, V.A. Lebedev, I.V. Roslyakov, A.V. Vasiliev, P.E. Kazin, Hexaferrite materials displaying ultra-high coercivity and sub-terahertz ferromagnetic resonance frequencies. Mater. Today 32, 13–18 (2020)
H. Luo, B.K. Rai, S.R. Mishra, V.V. Nguyen, J.P. Liu, Physical and magnetic properties of highly aluminum doped strontium ferrite nanoparticles prepared by auto-combustion route. J. Magn. Magn. Mater. 324, 2602–2608 (2012)
J. Qiu, Q. Zhang, M. Gu, H. Shen, Effect of aluminum substitution on microwave absorption properties of barium hexaferrite. J. Appl. Phys. 98, article 103905 (2005)
F. Rhein, R. Karmazin, M. Krispin, T. Reimann, O. Gutfleisch, Enhancement of coercivity and saturation magnetization of Al3+ substituted M-type Sr-hexaferrites. J. Alloys Compd. 690, 979–985 (2017)
F. Sandiumenge, S. Gali, J. Rodrigues, X-ray profile analysis of cation distribution in SrAlxFe12−xO19. Mater. Res. Bull. 23, 685–692 (1988)
T.J.B. Holland, S.A.T. Redfern, Unit cell refinement from powder diffraction data: the use of regression diagnostics. Miner. Mag. 61, 65–77 (1997)
R.W. Cheary, A.A. Coelho, Programs XFIT and FOURYA, deposited in CCP14 Powder Diffraction Library, Engineering and Physical Sciences Research Council, Daresbury Laboratory, Warrington, England (1996). http://www.ccp14.ac.uk/tutorial/xfit-95/
S. Kobayashi, Y. Ikuhara, T. Mizoguchi, Lattice expansion and local lattice distortion in Nb- and La-doped SrTiO3 single crystals investigated by x-ray diffraction and first-principles calculations. Phys. Rev. B 98, 134114 (2018)
D. Nath, F. Singh, R. Das, X-ray diffraction analysis by Williamson-Hall, Halder–Wagner and size-strain plot methods of CdSe nanoparticles-a comparative study. Mater. Chem. Phys. 239, 122021 (2020)
J.J. Deng, M.Y. Zhao, Y. Wang, X. Wu, X.T. Niu, L. Ma, D.W. Zhao, C.M. Zhen, D.L. Hou, Effect of residual strain on magnetic properties and Hall effect in chiral antiferromagnet Mn3Sn. J. Phys. D: Appl. Phys. 55, 275001 (2022)
O. Perevertov, Influence of the residual stress on the magnetization process in mild steel. J. Phys. D: Appl. Phys. 40, 949–954 (2007)
D. Givord, M. Rossignol, D. Taylor, Coercivity mechanisms in hard magnetic materials. J. Phys. IV 2, 95–104 (1992)
Y. Du, Y. Liu, L. Lian, J. Du, Structural and magnetic properties of Sr0.8La0.2Co0.2Fe11.8−xAlxO19 hexaferrite particles prepared via sol-gel auto-combustion method. J. Magn. Magn. Mater. 469, 189–195 (2019)
R. Skomski, J.M.D. Coey, Giant energy product in nanostructured two-phase magnets. Phys. Rev. B 48, 15812 (1993)
C.J. Fernández, C. Sangregorio, J. Figuera, B. Belec, D. Makovec, A. Quesada, Progress and prospects of hard hexaferrites for permanent magnet applications. J. Phys. D 54, 153001 (2021)
A.P. Roberts, Y. Cui, K.L. Verosub, Wasp-waisted hysteresis loops: mineral magnetic characteristics and discrimination of components in mixed magnetic systems. J. Geophys. Res. 100, 17909 (1995)
U. Khan, N. Adeela, K. Javed, S. Riaz, H. Ali, M. Iqbal, X.F. Han, S. Naseem, Influence of cobalt doping on structural and magnetic properties of BiFeO3 nanoparticles. J. Nanopart. Res. 17, 429 (2015)
F. Guo, X. Zhang, H. Cai, X. Fan, L. Hu, W. Sun, X. Zhao, Effect of doping location induced anisotropy on thermophysical properties of dilute Fe2O3–Y2O3–ZrO2 solid solutions. J. Am. Ceram. Soc. 104, 4742–4758 (2021)
B. Palanki, Some factors affecting densification and grain growth in the sintering of uranium dioxide—a brief review. J. Nucl. Mater. 550, 152918 (2021)
H. Björklund, L.K.L. Falk, K. Rundgren, J. Wasén, β-Si3N4 grain growth, part I: effect of metal oxide sintering additives. J. Eur. Ceram. Soc. 17, 1285–1299 (1997)
N. Louet, M. Gonon, G. Fantozzi, Influence of the amount of Na2O and SiO2 on the sintering behavior and on the microstructural evolution of a Bayer alumina powder. Ceram. Int. 31, 981–987 (2005)
M.H. Lin, J.F. Chou, H.Y. Lu, Grain-growth inhibition in Na2O-doped TiO2-excess barium titanate ceramics. J. Am. Ceram. Soc. 83, 2155–2162 (2000)
T. Watari, R.C. Bradt, Grain growth of sintered ZnO with alkali oxide additions. J. Ceram. Soc. Jpn. 101, 1085–1089 (1993)
S. Kumar, S. Supriya, R. Pandey, L.K. Pradhan, R.K. Singh, M. Kar, Effect of lattice strain on structural and magnetic properties of Ca substituted barium hexaferrite. J. Magn. Magn. Mater. 458, 30–38 (2018)
H. Kronmüller, M. Fähnle, Micromagnetism and the Microstructure of Ferromagnetic Solids (Cambridge University Press, Cambridge, 2009)
Acknowledgements
Authors would like to thank financial support by the National Research Foundation (#NRF-2020M3H4A2084780) of Korea was also greatly acknowledged.
Funding
This work was supported by the Nano and Materials Technology Development Project (NRF-2020M3H4A2084780) of the National Research Foundation of Korea.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
339_2022_6238_MOESM1_ESM.docx
Estimation of residual strain of SrM-Al from broadening of XRD peaks and first derivative of magnetization curves. X-ray diffraction profiles of SrM-Al-Na. Summary of composition analysis for SrM-Al processed with Na ions for low level of Al addition were also included (DOCX 2956 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Choi, JY., Baek, YK., Lee, JG. et al. Effect of sodium addition on structural and magnetic properties of solid state processed SrFe12−xAlxO19 (x ≤ 4). Appl. Phys. A 128, 1127 (2022). https://doi.org/10.1007/s00339-022-06238-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-022-06238-w