Abstract
In this work, ex situ MgB2 bulks were added with (1.5 Mg + 2B) and sintered in an attempt to enhance its intergrain connectivity. The addition was varied within the range of 0–50 wt.%, and the sintering was undertaken at 700 °C, 800 °C, and 1000 °C, respectively, for 1 h. Superconducting critical temperature, Tc of the samples was determined to be around 38 K as shown by the temperature dependence of susceptibility measurement. It was found that critical current density, Jc increased with the increased amount of the addition. Jc was further enhanced to 2 × 104A cm2 (0 T, 20 K) as the sintering temperature was raised. The increase of Jc is due to improved grain coupling as a result of in situ formation of MgB2, which fills the voids and connects the ex situ MgB2 grains. Additionally, the grain coupling was further strengthened by solid-state self-sintering at higher temperatures. The increment of Jc was accompanied by a narrower width of double-step transition of Tc attributable to a more complete MgB2 phase formation of the samples as the sintering temperature was increased.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Superconducting critical temperature, Tc ≈ 39 K of magnesium diboride, MgB2 is one of the highest among the non-cuprate-based superconductors [1]. Like other superconducting compounds such as high-temperature superconductors (HTS) [2,3,4,5] and the iron-based one [6], MgB2 has a great potential for practical applications, especially in liquid helium-free temperatures (15–20 K). Besides, MgB2 consists of two light elements of magnesium (Mg) and boron (B) which are relatively inexpensive as compared to those used to synthesize iron-based and HTS. Other features such as weak-link free behavior across grain boundaries, larger coherence length, and lower electronic anisotropy [7,8,9,10] are the additional advantages of MgB2.
Two common approaches for preparing MgB2 bulk samples are in situ [11,12,13,14], and ex situ [15,16,17] methods. For in situ reaction process, Mg grains melt and diffuse into B grains to form MgB2 through liquid–solid reaction during heat treatment. The sample prepared by this method yields a strong intergrain coupling and can easily attain high critical current density, Jc. Similar to the cuprate HTS [18,19,20], the addition of impurities into MgB2 during the in situ reaction enhances Jc enormously [7, 21]. Due to the liquid–solid reaction, Mg sites turn into voids resulting in a low bulk density (P ~ 50%) [9]. On the other hand, a much higher packing factor of about 75% can be achieved in ex situ MgB2, which is a pre-reacted MgB2 prepared via the in situ reaction. Nevertheless, intergrain coupling of ex situ MgB2 samples is much weaker than that of the in situ ones [9, 22].
Hence, we are motivated to improve the intergrain connectivity of ex-situ MgB2 in an attempt to enhance its Jc. To do so, ex situ MgB2 was mixed with a mixture of Mg and B according to the proportion of (1.5Mg + 2B). This means of powder technology is cost-effective as no additional chemical dopants are required. The addition of mixed powder (1.5Mg + 2B) during in situ reaction is expected to strengthen the grain connectivity of ex situ MgB2 due to the higher bulk density of ex situ MgB2 [23]. To study the effect of (1.5Mg + 2B) addition on Tc and Jc, the amount of the addition was varied according to x (wt %) = 0, 10, 30, and 50 for sintering at 700 °C for 1 h. Another batch of samples was prepared by adding 30 wt.% of (1.5 Mg + 2 B) into the ex situ MgB2 powder and sintered at 700 °C, 800 °C, and 1000 °C, respectively, for 1 h. The purpose of the heat treatment is twofold: (i) for ex situ MgB2, it is expected to increase the cross-section of the grain-to-grain current path through solid-state self-sintering [15] (ii) for in situ MgB2, it optimizes formation of MgB2 phase, which in turn assists grain connectivity [24] in (i). Excess Mg was used in the mixed (1.5Mg + 2B) to compensate for Mg loss due to the high volatility of Mg at elevated temperatures.
2 Experimental details
Commercially available MgB2 powder (Alfa Aesar, 99.0% purity) was used to serve as ex situ MgB2. Varying amounts (x weight percentage, wt.%, for x = 0, 10, 30, and 50) of mixed magnesium, Mg (Tangshan Wei Hao, 99.0% purity) and boron, B (Tangshan Wei Hao, 97.0% purity) powders with a molar ratio of 1.5: 2 (Mg: B) were added to the ex situ MgB2 powder and ground for 1 h in open air. The mixture was pressed into circular pellets with dimensions of ~ 13 mm diameter and ~ 1 mm thickness using a hydraulic press under a pressure of five tons. Then, the pellets were loaded into stainless-steel tube, and both ends of the tube were sealed. Finally, the tube was loaded into a tube furnace for heat treatment at 700 °C, 800 °C, and 1000 °C, respectively, for 1 h under a constant argon gas flow.
The samples were checked by X-ray diffraction (XRD) method using the PW 3040/ 60 MPD X’pert Pro Panalytical Philips DY 1861 X-ray diffractometer with Cu-Kα radiation source (λ = 1.5406 Å). The scanning was carried out in the 2θ range of 20°–80° with the increment step size of 0.03°. Microstructure imaging was performed using a scanning electron microscope (SEM-LEO 1455 VPSEM). Superconducting critical temperature, Tc, and critical current density, Jc was measured using a SQUID magnetometer (Quantum Design: MPMS5). Tc was measured using zero-field cooled conditions from 10 to 50 K under an applied field of 10 Oe. Magnetization hysteresis (M-H) loops were acquired in applied magnetic fields from 0 to + 3 T at 20 K. Jc of the bulk samples with dimensions of approximately 1.0 × 1.0 × 0.5 mm3 was calculated from the M-H loops based on the extended Bean critical state model equation [25]:
where d is the sample thickness, a, b (a < b) are cross-sectional dimensions of a rectangular sample and Δm (in emu units, 1 emu = 10–3 A m2) is the hysteresis loop width.
3 Results and discussion
3.1 X-ray diffraction (XRD) analysis
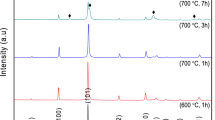
Figure 1 shows XRD patterns of the ex situ MgB2 samples and that added with x wt.% of (1.5Mg + 2B) (x = 0, 10, 30 and 50). XRD patterns of the ex situ MgB2 samples added with 30 wt.% of (1.5 Mg + 2 B) and sintered at different temperatures for 1 h are shown in Fig. 2. Majority of the diffraction peaks (Fig. 1 and Fig. 2) were indexed to MgB2 phase (ICSD: 98-010-6149), having hexagonal crystal structure with P6/mmm space group. MgB2 peak with the highest intensity was observed at 2θ ≈ 42.4° which corresponds to the (1 0 1) plane.
Some minor peaks observed at 2θ ≈ 62.2° and 78.6° were indexed to MgO (ICSD: 98-009-4096) in all the samples (Figs. 1 and 2). Intensity of these peaks increased with the increasing addition amount of (1.5 Mg + 2B). For the sample added with 30 wt.% of (1.5Mg + 2B) and sintered at 1000 °C, MgO peak was clearly observed at 2θ ≈ 42.9° which is near the (1 0 1) peak of MgB2 at 2θ ≈ 42.4° (Fig. 2) indicating the presence of significant fraction of MgO in the sample. In addition to the MgB2 and MgO phases, a peak with a weak intensity corresponding to MgB4 (ICSD: 98–009-1660) at 2θ ≈ 45.9° was noticeable for the ex-situ sample sintered at 700 °C for 1 h (Fig. 1) and the samples added with 30 wt.% of (1.5Mg + 2B) and sintered at 800 °C for 1 h (Fig. 2). However, multiple peaks of MgB4 (ICSD: 98-009-1660) appeared in the XRD pattern of the sample added with 30 wt.% of (1.5Mg + 2B) and sintered at a higher temperature of 1000 °C (Fig. 2). Without an excessive amount of MgO and MgB4, the presence of these phases may assist in pinning the movement of flux lines for enabling the samples to carry high Jc [26, 27].
Tables 1, 2 show intensity fractions of the phases formed and lattice parameters of a and c axis for all the samples. The intensity fractions were estimated using the following formula [28,29,30]:
where I is the peak intensity of the respective phases. As shown in Table 1, the intensity fraction of MgB2 phase changes very little between 97 and 98% with addition of up to 50 wt.% of (1.5Mg + 2B). The slight decrease was probably due to the availability of more Mg [from the mixture of (1.5Mg + 2B)], thus increasing the formation of MgO phase (Table 1). With the addition of (1.5Mg + 2B), the formation of MgB4 did not occur (Table 1). This suggests that the (1.5Mg + 2B) addition increased partial pressure of Mg (due to the Mg there) and thus suppressed decomposition of MgB2 into MgB4 and Mg [31,32,33]. Unit cell lattice parameter of a axis is larger for the sample with addition of 50 wt.% of (1.5Mg + 2B). While considering the c axis lattice parameter, its value increased starting from the addition level of 30 wt.% of (1.5Mg + 2B). Consequently, the ratio of the c axis to a axis (c/a) is larger. The increase in the lattice parameters may be associated with in situ formation of MgB2 as a result of the addition of (1.5Mg + 2B) into the ex situ powder leading to buckling in the unit cell of MgB2.
For the ex situ MgB2 added with 30 wt.% (1.5Mg + 2B), increasing the sintering temperature from 700 °C to 1000 °C abruptly decreased MgB2 phase from 97.6 to 68.0% (Table 2). The decrease in MgB2 phase is due to the increase of both MgO and MgB4 phases. At higher sintering temperatures, MgB2 decomposed into MgB4 and Mg [15, 32, 34] giving rise to the availability of more Mg to react with oxygen to form MgO. These processes were accelerated as the sintering temperature was increased. As shown in Table 2, the intensity fraction of MgB4 phase increased from 0.0 to 15.3% as the sintering temperature was raised from 700 °C to 1000 °C. Concurrently, the intensity fraction of MgO increased from 2.4 to 16.7%. The chemical reaction equation below shows the decomposition of MgB2 to MgB4 and Mg [35]:
While the c axis lattice parameter remained relatively unchanged as the sintering temperature was raised from 700 to 1000 °C, the a axis decreased more significantly for the samples sintered at 1000 °C (Table 2). Accordingly, the ratio c/a increased with the sintering temperature. This behavior may be related to strain resulting from the presence of considerable fraction of impurity phases [36].
3.2 Microstructure analysis
Figure 3 shows SEM images taken on the fractured surfaces of the samples at a magnification of 5000 Χ. As can be observed from the images, the samples show randomly oriented grains with irregular shapes. Voids (dark regions) between the grains can be clearly seen for 0 wt.% added sample (Fig. 3a). However, the gap between the voids appeared to be reduced as the addition level and the sintering temperature increased (Fig. 3b, c, d, e, f and g), suggesting an improvement of grain coupling for the samples. It is known that the presence of voids will reduce the bulk density and disrupt the grain connectivity of the samples leading to a reduction of Jc [37, 38]. As the amount of the addition was increased, the samples appeared to be less porous most probably due to the in situ formation of MgB2 [from the addition of (1.5Mg + 2B)] that filled some of the voids [39].
SEM images showing fractured surfaces of ex situ MgB2 samples added with a 0 wt.%, b 10 wt.%, c 30 wt.%, d 50 wt.% of (1.5Mg + 2B), respectively, and sintered at 700 °C for 1 h; ex situ MgB2 added with 30 wt % (1.5Mg + 2B) and sintered at e 700 °C, f 800 °C, g 1000 °C, respectively. Distribution of grain size is shown on the right-hand side of the images
Figure 3 right shows the grain size distribution of the samples. The grain size distribution was calculated from the randomly selected 100 grains according to the SEM images using the Image-J software. Overall, the average grain size of (1.5Mg + 2B) added sample varied slightly only within the range of 0.52 µm–0.56 µm (Fig. 4b–d) as compared with 0.50 µm of the 0 wt.% added sample (Fig. 4a). This result agrees with the previous finding that no significant change in the grain size was observed for the ex situ MgB2 sintered at 900 °C for even up to 240 h [15]. The slight increase in the average grain size may be due to Mg melting at around 650 °C resulting in neck formation and aggregation between the grains [16] leading to grain growth. Besides, Mg melting plays an important role in cleaning grain boundaries and healing microcracks which enhance grain connectivity of the samples [40,41,42]. For the samples added with 30 wt.% of (1.5Mg + 2B), the average grain size decreased slightly from 0.52 to 0.48 µm as the sintering temperature was increased from 700 to 1000 °C. Such a small decrease may be linked to Mg deficiency caused by Mg loss at the higher sintering temperature [43]. With the increase in the addition levels and the sintering temperatures, the reduction in the average grain size (Fig. 4) may contribute to the enhancement of Jc as a result of the increased number of grain boundaries for flux pinning [42].
3.3 Superconducting analysis
3.3.1 Critical temperature, T c
Figure 5a, b shows the temperature dependence of magnetic susceptibility between 10 and 50 K for all the samples. In order to determine onset (Tc-onset), onset of the second transition, Tc-onset2, and offset (Tc-offset) of superconducting critical temperature, derivatives of the magnetic susceptibility with respect to the temperature ([δχ/δT]) versus temperature were plotted and shown in Fig. 5c, d. As shown in Table 3, all the samples show a Tc-onset ≈ 38 K. Such a minor variation in Tc-onset among the samples indicates that the addition of (1.5Mg + 2B) powder did not give rise to significant perturbation on the electronic structure of MgB2 [44]. Tc-onset2 was determined from the peak at the lower temperature as shown in Fig. 5c, d. Tc-onset2 is 30 K and 33 K for the 30 wt.% and 50 wt.% added sample, respectively (Table 3). For the samples sintered at 700 °C for 1 h (Fig. 5a), the addition of a higher amount of (1.5Mg + 2B) (30 wt.% and 50 wt.%) resulted in a double-step transition. The double-step transition indicates inhomogeneity within the samples [22], which may be caused by the incomplete reaction between Mg and B during the heat treatment after the addition of (1.5Mg + 2B). The presence of ex situ MgB2 particles may slow down MgB2 phase formation as they hinder the diffusion of Mg and B in the mixture. Moreover, the width transition, ΔTc (Tc-onset – Tc-offset) of the 30 wt.% sample is larger than that of the 50 wt.% one. The larger ΔTc of the former suggests the presence of a higher density of defects in the sample. Similar double-step transition was also observed in the 30 wt.% of (1.5Mg + 2B) added samples sintered at 800 °C and 1000 °C. Nevertheless, the transition width was narrower, and the double-step transition became less obvious as the sintering temperature was increased to 1000 °C. The narrower transition width with the increasing sintering temperature implies more complete MgB2 formation consistent with the increment of Jc (which will be discussed in the following section).
Temperature dependence of normalized DC susceptibility for a different weight percentages of (1.5Mg + 2B) addition and sintered at 700 °C for 1 h, b 30 wt.% (1.5Mg + 2B) added samples sintered at different temperatures for 1 h. Plots of δχ/δT versus T for the samples c with different weight percentages of (1.5Mg + 2B) addition and sintered at 700 °C for 1 h, d 30 wt.% (1.5Mg + 2B) added samples sintered at different temperatures for 1 h
3.3.2 Critical current density, J c, and pinning force
Figure 6 shows the M–H loops of the samples measured at 20 K after zero-field cooling. Flux jump is not apparent as evidenced from the loops. The width of the loops, ΔM is larger reflecting increment of Jc as the addition level and the sintering temperature increases. Figure 7 shows the dependency of Jc on the applied magnetic fields from 0 to 3 T at 20 K. Self-field Jc for the 0 wt.% added (1.5Mg + 2B) sample is 3.0 kA cm−2. When 10 wt.% of (1.5 Mg + 2 B) was introduced into the sample, self-field Jc increased slightly to 3.8 kA cm−2. A sudden increase in self-field Jc (10.0 kA cm−2) was observed in the sample added with 30 wt.% (1.5Mg + 2B). The value of self-field Jc continued to increase to 13.1 kA cm−2 with an addition of 50 wt.% (1.5Mg + 2B). As shown in Fig. 7a, a significant enhancement of Jc is seen over the field range 0–3 T after the addition of 30 wt.% of (1.5Mg + 2B). The increase in Jc can be associated with a reduction in the dimensions of voids between the particles, thereby resulting in increased number of contact points among the grains which in turn improved the intergranular grain coupling [15, 16]. Apart from this, in situ formation of MgB2 also helped in connecting the ex situ MgB2 grains. The enhanced Jc versus field for both the 30 wt.% and 50 wt.% added samples coincide with the appearance of the double-step transition (Fig. 5a) manifesting the presence of a higher fraction of defects for flux pinning as a result of the increased addition of (1.5Mg + 2B).
For the 30 wt.% (1.5Mg + 2B) added samples, self-field Jc (20 K) increased from 10.0 kA cm−2 to 20.7 kA cm−2 when the sintering temperature was raised from 700 to 1000 °C, respectively. However, at a higher field, the Jc value of the sample sintered at 800 °C is lower than that sintered at 700 °C (Fig. 7b). This could be due to lower defect density as indicted by the smaller ΔTc of the sample (Table 3). Consequently, flux pinning contributed by the defects was reduced and thus decreased Jc. Moreover, the Jc also showed a stronger dependency on the applied field. As the sintering temperature was increased to 1000 °C, Jc was enhanced, because grain coupling was further reinforced through solid-state self-sintering [15] in addition to voids filling and grain connectivity assisted by in situ formation of MgB2. This is consistent with the narrower transition width, ΔTc (Fig. 5b) indicating improved homogeneity leading to enhanced grain coupling of the sample. It is noteworthy that Jc of the sample sintered at 1000 °C is the highest despite its lowest fraction of MgB2 (68.0%) (Table 2). This indicates that the Jc can be further increased by optimization of heat treatment and material processing conditions. By doing so, it is anticipated the grain coupling can be further strengthened while reducing the impurities considerably. On the other hand, it should be noted that non-superconducting phases can also serve as flux pinning centers. Table 4 compares Jc’s of the best sample from this study and that of other reported work on the addition of Mg into in situ and ex situ MgB2.
Figure 8 shows normalized flux pinning force, fp = Fp/Fp,max as a function of reduced field, h = H/Hirr at 20 K. The irreversibility field, Hirr was determined from the M-H loops using the criterion of Jc = 100 A cm−2 [47, 48]. Curve fitting was undertaken using the software Origin in order to better estimate the peak value of h, hpeak. As shown in Table 5, hpeak for the sample without addition is 0.34 indicating the dominant pinning mechanism of normal core [49] which may originate from the non-superconducting inhomogeneity in the commercial MgB2 powder [50]. The inhomogeneity was worsened after the sintering process [50]. As reported by Tanaka et al., their sintered ex situ MgB2 (homemade) with better homogeneity showed hpeak ≈ 0.2 indicating predominant grain boundary pinning similar to that obtained for the in situ MgB2 [15, 30]. With the addition of 10 wt.% and 30 wt.% (1.5Mg + 2B), hpeak was changed to 0.35 and 0.24, respectively. For the 30 wt.% added sample, hpeak ≈ 0.24 suggests that other forms of pinning are active in addition to that from the grain boundaries. However, hpeak increased to 0.36 for the 50 wt.% added sample probably because of excessive addition of (1.5Mg + 2B). For the samples added with 30 wt.% of (1.5Mg + 2B) and sintered at 700 °C, 800 °C, and 1000 °C, the values of hpeak are 0.24, 0.32, and 0.29, respectively. Again, the increase in the hpeak implies that the pinning landscape has changed toward that of normal core.
Normalized pinning force Fp/Fp,max as a function of reduced magnetic field H/Hirr at 20 K for a different weight percentages of (1.5Mg + 2B) addition and sintered at 700 °C for 1 h, b 30 wt.% (1.5Mg + 2B) added samples sintered at different temperatures for 1 h. Solid line is the fitting curve using fp = Ahp(1-h)q
We now turn to understand more about how Jc is influenced by the pinning mechanism. Looking at Fig. 7a, b, we notice that the sample added with 30 wt.% of (1.5Mg + 2B) (hpeak ≈ 0.24) and sintered at 700 °C has the weakest field dependency on Jc among others. This could be due to the presence of grain boundary pinning [51] in addition to other forms of pinning in the sample (as reflected in its largest ΔTc). The absence of grain boundary pinning may also explain the slightly lower in-field Jc of the 50 wt.% added sample (hpeak ≈ 0.36) than that of the 30 wt.% added one. For instance, Jc (20 K) at 1 T and 2 T are 2.1 kA cm−2 and 0.4 kA cm−2, respectively, for the 50 wt.% added sample as compared with 2.5 kA cm−2 and 0.6 kA cm−2, respectively, for the 30 wt.% added sample. Similar phenomenon can also be seen in the 30 wt.% added sample sintered at 700 °C and 800 °C (hpeak ≈ 0.32) (Fig. 8b). Jc (20 K) at 1 T and 2 T of the latter are 1.6 kA cm−2 and 0.1 kA cm−2, respectively. For the 30 wt.%, added sample sintered at 1000 °C (hpeak ≈ 0.29), its highest Jc among the samples is attributed to its reinforced grain coupling via solid-state self-sintering [15] as discussed before. Taken all, our results are in agreement with the previous finding that the Jc is depends essentially on both the grain coupling and grain boundary pinning strength [51]. Nonetheless, it is a subject of further investigation to elucidate the kinetics of microstructural evolution and its relationship with the pinning mechanism.
4 Conclusion
The role of (1.5Mg + 2B) additions and heat treatment conditions on Tc and Jc of ex situ MgB2 bulks were investigated in this work. This was done through studying two set of samples: (i) ex situ MgB2 added with x wt.% (x = 0, 10, 30 and 50) of (1.5Mg + 2B) and sintered at 700 °C for 1 h; (ii) ex situ MgB2 added with 30 wt.% of (1.5Mg + 2B) and sintered at 700 °C, 800 °C, 1000 °C, respectively, for 1 h. XRD patterns showed the increased fraction of MgB4 and MgO for the samples sintered at 1000 °C as a result of decomposition of MgB2. According to the SEM images, the average grain size varied slightly only (0.48–0.56 μm) across the samples. All the samples showed Tc-onset ≈ 38 K as determined inductively from the measurement of temperature dependence of magnetic susceptibility. A double-step transition of Tc was observed as the amount of the addition was increased. Nonetheless, the width of the double-step transition became narrower with the increasing sintering temperature due to improved MgB2 phase formation. Self-field Jc at 20 K was increased from 3.0 kA.cm−2 to 13.1 kA cm−2 upon increasing the addition to 50 wt.%. The Jc was consistently increased to 20.7 kA cm−2 as the sintering temperature was raised to 1000 °C. The increment of the Jc is believed to be due to enhanced grain coupling assisted by in situ formation of MgB2, which filled the voids and connected the MgB2 grains. As the sintering temperature was increased, solid-state self-sintering took place and resulted in further strengthening of the grain coupling.
References
J. Nagamatsu, N. Nakagawa, T. Muranaka, Y. Zenitani, J. Akimitsu, Nature 410, 63–64 (2001)
Y. Slimani, E. Hannachi, A. Ekicibil, M.A. Almessiere, F.B. Azzouz, J Alloys Compd. 781, 664–673 (2019)
M.R. Koblischka, A. Koblischka-Veneva, X. Zeng, E. Hannachi, Y. Slimani, Curr. Comput.-Aided Drug Des. 10, 986 (2020)
E. Hannachi, K.A. Mahmoud, Y. Slimani, M.I. Sayyed, Ceram. Int. 48, 24355–24362 (2022)
E Hannachi, Y Slimani, MI Sayyed, KA Mahmoud, Ceram. Int. 48, 31902-31908 (2022)
M.R. Koblischka, Y. Slimani, A. Koblischka-Veneva, T. Karwoth, X.L. Zeng, E. Hannachi, M. Murakami, Materials 13, 5018 (2020)
C. Buzea, T. Yamashita, Supercond. Sci. Technol. 14, R115–R146 (2001)
D.C. Larbalestier, L.D. Cooley, M.O. Rikel, A.A. Polyanskii, J. Jiang, S. Patnaik, X.Y. Cai, D.M. Feldmann, A. Gurevich, A.A. Squitieri, M.T. Naus, C.B. Eom, E.E. Hellstrom, R.J. Cava, K.A. Regan, N. Rogado, M.A. Hayward, T. He, J.S. Slusky, P. Khalifah, K. Inumaru, M. Haas, Nature 410, 186–189 (2001)
A. Yamamoto, H. Tanaka, J.I. Shimoyama, H. Ogino, K. Kishio, T. Matsushita, Jpn. J. Appl. Phys. 51, 010105 (2012)
D. Larbalestier, A. Gurevich, D.M. Feldmann, A. Polyanskii, Nature 414, 368–377 (2001)
M. Muralidhar, M. Higuchi, M. Jirsa, P. Diko, I. Kokal, M. Murakami, IEEE Trans. Appl. Supercond. 27, 6201104 (2017)
S.S. Arvapalli, M. Miryala, M. Murakami, J. Supercond. Nov. Magn. 32, 1891–1895 (2019)
J.H. Kim, S.X. Dou, J.L. Wang, D.Q. Shi, X. Xu, M.S.A. Hossain, Supercond. Sci. Technol. 20, 448–451 (2007)
Z.S. Gao, Y.W. Ma, D.L. Wang, X.P. Zhang, S. Awaji, K. Watanabe, Chin. Phys. Lett. 27, 3–5 (2010)
H. Tanaka, A. Yamamoto, J.I. Shimoyama, H. Ogino, K. Kishio, Supercond. Sci. Technol. 25, 117401 (2012)
S. Mizutani, A. Yamamoto, J.I. Shimoyama, H. Ogino, K. Kishio, Supercond. Sci. Technol. 27, 114001 (2014)
A. Malagoli, V. Braccini, C. Bernini, G. Romano, M. Vignolo, M. Putti, C. Ferdeghini, Supercond. Sci. Technol. 23, 025032 (2010)
M.K. Ben Salem, Y. Slimani, E. Hannachi, A. Hamrita, F.B. Azzouz, M.B. Salem, AIP Conf Proc. 1569, 423–426 (2013)
N.M. Hapipi, S.K. Chen, A.S. Halim, M.M.A. Kechik, K.B. Tan, K.P. Lim, O.J. Lee, J. Supercond. Nov. Magn. 32, 1191–1198 (2019)
Y. Slimani, M.A. Almessiere, E. Hannachi, M. Mumtaz, A. Manikandan, A. Baykal, F.B. Azzouz, Ceram. Int. 45, 6828–6835 (2019)
S.K. Chen, M. Maeda, A. Yamamoto, S.X. Dou, in Vortices and nanostructured superconductors. ed. by A. Crisan, Ed. Switzerland (Springer Series in Materials Science, Switzerland, 2017), pp.65–108
W.X. Li, R. Zeng, J.L. Wang, Y. Li, S.X. Dou, J. Appl. Phys. 111, 07E135 (2012)
A.V. Pan, S. Zhou, H. Liu, S. Dou, Supercond. Sci. Technol. 16, 639–644 (2003)
P. Kováč, M. Reissner, T. Melišek, I. Hušek, S. Mohammad, J. Appl. Phys. 106, 013910 (2009)
D.X. Chen, R.B. Goldfarb, J. Appl. Phys. 66, 2489–2500 (1989)
M. Miryala, S.S. Arvapalli, P. Diko, M. Jirsa, M. Murakami, Adv. Eng. Mater. 22, 1900750 (2020)
P. Kováč, I. Hušek, T. Melišek, J.C. Grivel, W. Pachla, V. Štrbík, R. Diduszko, J. Homeyer, N.H. Andersen, Supercond. Sci. Technol. 17, L41–L46 (2004)
J.H. Kim, S.X. Dou, D.Q. Shi, M. Rindfleisch, M. Tomsic, Supercond. Sci. Technol. 20, 1026–1031 (2007)
K.Y. Tan, K.L. Tan, K.B. Tan, K.P. Lim, S.A. Halim, S.K. Chen, J. Supercond. Nov. Magn. 24, 2025–2029 (2011)
N.M. Hapipi, M. Miryala, S.K. Chen, S.S. Arvapalli, M. Murakami, M.M.A. Kechik, K.B. Tan, O.J. Lee, Ceram. Int. 46, 23041–23048 (2020)
B.B. Sinha, M.B. Kadam, M. Mudgel, V.P.S. Awana, H. Kishan, S.H. Pawar, Physica. C. 470, 25–30 (2010)
Y. Guo, W. Zhang, D. Yang, R. Yao, J. Am. Ceram. Soc. 95, 754–759 (2012)
S. Brutti, A. Ciccioli, G. Balducci, G. Gigli, P. Manfrinetti, A. Palenzona, Appl. Phys. Lett. 80, 2892–2894 (2002)
G.A.B. Matthews, S. Santra, R. Ma, C.R.M. Grovenor, P.S. Grant, S.C. Speller, Supercond. Sci. Technol. 33, 1–23 (2020)
G. Balducci, S. Brutti, A. Ciccioli, G. Gigli, P. Manfrinetti, A. Palenzona, M.F. Butman, L. Kudin, J. Phys. Chem. Solids 66, 292–297 (2005)
D.G. Hinks, J.D. Jorgensen, H. Zheng, S. Short, Physica. C. 382, 166–176 (2002)
M. Shahabuddin, N.S. Alzayed, S. Oh, S. Choi, M. Maeda, S. Hata, Y. Shimada, M.S.A. Hossain, J.H. Kim, AIP Adv. 4, 017113 (2014)
E.W. Collings, M.D. Sumption, M. Bhatia, M.A. Susner, S.D. Bohnenstiehl, Supercond. Sci. Technol. 21, 103001 (2008)
H. Zhang, L. Li, Y. Zhao, Y. Zhang, J. Phys. Conf. Ser. 871, 012057 (2017)
R. Zeng, L. Lu, W.X. Li, J.L. Wang, D.Q. Shi, J. Horvat, S.X. Dou, M. Bhatia, M. Sumption, E.W. Collings, J.M. Yoo, M. Tomsic, M. Rindfleisch, J. Appl. Phys. 103, 083911 (2008)
H. Zhang, Y. Zhao, Y. Zhang, J. Supercond. Nov. Magn. 28, 2711–2714 (2015)
F. Wu, J. Low Temp. Phys. 177, 157–164 (2014)
S.K. Chen, A. Serquis, G. Serrano, K.A. Yates, M.G. Blamire, D. Guthrie, J. Cooper, H. Wang, S. Margadonna, J.L. MacManus-Driscoll, Adv. Funct. Mater. 18, 113–120 (2008)
J. Kortus, O.V. Dolgov, R.K. Kremer, A.A. Golubov, Phys. Rev. Lett. 94, 027002 (2005)
R. Zeng, L. Lu, J.L. Wang, J. Horvat, W.X. Li, D.Q. Shi, S.X. Dou, M. Tomsic, M. Rindfleisch, Supercond. Sci. Technol. 20, L43–L47 (2007)
S.S. Arvapalli, M. Miryala, M. Murakami, Adv. Eng. Mater. 21, 1900497 (2019)
M. Eisterer, Phys. Rev. B 77, 144524 (2008)
M. Muralidhar, N. Kenta, M.R. Koblischka, M. Murakami, Phys. Status Solidi Appl. Mater. Sci. 212, 2141–2145 (2015)
D. Dew-Hughes, Philos. Mag. 30, 293–305 (1974)
W. Pachla, A. Presz, R. Diduszko, P. Kovác, I. Husek, Supercond. Sci. Technol. 15, 1281–1287 (2002)
M. Teruo Matsushita, A.Y. Kiuchi, J. Shimoyama, K. Kishio, Supercond Sci. Technol. 21, 015008 (2008)
Acknowledgements
This research was funded by Universiti Putra Malaysia through the Putra Grant Scheme (Project Code: UPM.RMC.800-3/3/1/GPB/2020/9691400). N. M. Hapipi would like to acknowledge financial support from School of Graduate Studies, Universiti Putra Malaysia, through Graduate Research Fellowship (GRF) scholarship. This work was partly supported by Shibaura Institute of Technology (SIT) International Research Centre for Green Electronics and special funds for education and research budget code: 721MA56383.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest among themselves.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hapipi, N.M., Chen, S.K., Shaari, A.H. et al. Excess Mg in situ powder addition for enhancing critical current density of ex situ MgB2. Appl. Phys. A 128, 913 (2022). https://doi.org/10.1007/s00339-022-06060-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-022-06060-4