Abstract
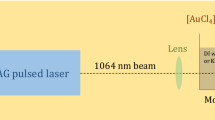
Spherical Ge nanoparticles with diameters of 20–80 nm were fabricated by laser ablation of a Ge single crystal in water and in aqueous HCl using sub-picosecond laser pulses (1040 nm, 700 fs, 100 kHz, and a pulse energy of 10 µJ). We found that the as-synthesized nanoparticles suffered rapid oxidization followed by dissolution when laser ablation was conducted in pure water. In contrast, oxidation of Ge nanoparticles produced in dilute HCl and stored intact was minimal, and colloidal dispersions of the Ge nanoparticles remained stable up to 7 days. It was elucidated that dangling bonds on the surfaces of the Ge nanoparticles were terminated by Cl, which inhibited oxidation, and that such hydrophilic surfaces might improve the dispersibility of nanoparticles in aqueous solvent.






Similar content being viewed by others
References
S. Baricikowski, G. Compagnini, Phys. Chem. Chem. Phys. 15, 3022 (2013)
V. Amendola, M. Meneghetti, Phys. Chem. Chem. Phys. 15, 3027 (2013)
V. Amendola, M. Meneghetti, Phys. Chem. Chem. Phys. 11, 3805 (2009)
F. Mafuné, J. Kohno, Y. Takeda, T. Kondow, H. Sawabe, J. Phys. Chem. B 104, 9111 (2000)
A.A. Ruth, J.A. Young, Colloids Surf. A Physicochem. Eng. Asp. 279, 121 (2006)
H.S. Nalwa, Nanostructured materials and nanotechnology, Concise edn. (Academic Press, San Diego, 2002)
K.V. Anikin, N.N. Melnik, A.V. Simakin, G.A. Shafeev, V.V. Voronov, A.G. Vitukhnovsky, Chem. Phys. Lett. 366, 357 (2002)
R.A. Ganeev, M. Baba, A.I. Ryasnyansky, M. Suzuki, H. Kuroda, Appl. Phys. B 80, 595 (2005)
N.G. Semaltianos, S. Logothetidis, W. Perrie, S. Romani, R.J. Potter, M. Sharp, P. French, G. Dearden, K.G. Watkins, Appl. Phys. A 94, 641 (2009)
H. Wang, A. Pyatenko, K. Kawaguchi, X. Li, Z. Swiatkowska-Warkocka, N. Koshizaki, Angew. Chem. Int. Ed. 49, 6361 (2010)
Y. Jiang, P. Liu, Y. Liang, H.B. Li, G.W. Yang, Appl. Phys. A 105, 903 (2011)
R. Intartaglia, K. Bagga, M. Scotto, A. Diaspro, F. Brandi, Opt. Mater. Exp. 2, 510 (2012)
Y. Maeda, Phys. Rev. B 51, 1658 (1995)
S. Sato, T. Ikeda, K. Hamada, K. Kimura, Solid State Commun. 149, 862 (2009)
D.C. Lee, J.M. Pietryga, I. Robel, D.J. Werder, R.D. Schaller, V.I. Klimov, J. Am. Chem. Soc. 131, 3436 (2009)
D.A. Ruddy, J.C. Johnson, E.R. Smith, N.R. Neale, ACS Nano 4, 7459 (2010)
Z.C. Holman, U. Kortshagen, Phys. Status Solidi RRL 5, 110 (2011)
S. Okamoto, Y. Kanemitsu, Phys. Rev. B 54, 16421 (1996)
M. Zacharias, P.M. Fauchet, Appl. Phys. Lett. 71, 380 (1997)
S. Takeoka, M. Fujii, S. Hayashi, K. Yamamoto, Phys. Rev. B 58, 7921 (1998)
L.M. Wheeler, L.M. Levij, U.R. Kortshagen, J. Phys. Chem. Lett. 4, 3392 (2013)
C.Y. Chien, W.T. Lai, Y.J. Chang, C.C. Wang, M.H. Kuo, P.W. Li, Nanoscale 6, 5303 (2014)
S. Sun, Y. Sun, Z. Liu, D. Lee, S. Peterson, P. Pianetta, Appl. Phys. Lett. 88, 021903 (2006)
J. Israelachivili, Intermolecular and surface forces, 2nd edn. (Academic Press, London, 1992)
V.A. Gavva, T.V. Kotereva, V.A. Lipskiy, A.V. Nezhdanov, Opt. Spectrosc. 120, 255 (2016)
E.G. Barbagiovanni, D.J. Lockwood, P.J. Simpson, L.V. Goncharova, Appl. Phys. Rev. 1, 011302 (2014)
J.F. Scott, Phys. Rev. B 1, 3488 (1970)
M.F. Ehman, K. Vedam, W.B. White, J.W. Faust Jr., J. Mater. Sci. 6, 969 (1971)
L.P. Lindeman, M.K. Wilson, Spectrochim. Acta 9, 47 (1957)
R.J.H. Clark, C.J. Willis, Inorg. Chem. 10, 1118 (1970)
I.R. Beattie, P.J. Jones, G. Reid, M. Webster, Inorg. Chem. 37, 6032 (1998)
Acknowledgements
The authors would like to thank Dr. Satoshi Fujita, Aisin Seiki Co. Ltd., and IMRA America, Inc. for allowing the use of the femtosecond laser and helpful technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study received no funding.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hamanaka, Y., Iwata, M. & Katsuno, J. Fabrication of oxidation-resistant Ge colloidal nanoparticles by pulsed laser ablation in aqueous HCl. Appl. Phys. A 123, 425 (2017). https://doi.org/10.1007/s00339-017-1044-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-017-1044-9