Abstract
Cerium-doped 1% Ce:Gd2Y1Ga2.7Al2.3O12(GYGAG) single crystal samples grown via Czochralski method were annealed under air, O2 and N2 atmospheres from 350 to 1400 °C. The X-ray excited luminescence spectra, energy spectra and UV as well as thermally stimulated luminescence (TSL) spectra were performed comparatively on “as-grown” and thermally annealed samples. It was found that the luminescence efficiency after annealing in air and O2 was significantly enhanced compared to the non-annealed samples and this phenomenon was suggested to be caused by the existence of some oxygen vacancies in the Ce:GYGAG crystals. And the oxygen vacancies in the as-grown GYGAG crystals can be effectively eliminated by means of annealing in O2 containing atmosphere without changing the luminescence mechanism. From the TSL curves before and after annealing, three traps within 77–650 K were found to be related to oxygen vacancies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cerium and Praseodymium ion plays an important role in scintillation crystals as activators [1,2,3,4]. Ce3+ and Pr3+-doped Lu3Al5O12 garnet crystals display a series of promising scintillation properties [5,6,7,8]. However, scintillation performance of doped LuAG is strongly degraded by traps lying bellow the bottom of conduction band, and those shallow traps were proved to be related to the anti-site defects [9,10,11]. Interestingly, further studies on intrinsic defects occurring in the LuAG host [12, 13] motivated the search for new garnet-based scintillator materials. It has been reported that Ga3+ admixture to Ce:LuAG diminishes the trapping effects [14] due to the burying shallow traps into conduction band as a result of the lowering of the conduction band edge with Ga admixture [15]. In contrast to the effects of Ga3+, the admixture of Gd3+ into Ce:Y3Al5O12 can increase the conduction band edge and lower the position of the Ce 5d1 level [16, 17]. Thus, in the frame of so-called “band-gap engineering” and “5d-level positioning” strategies [18,19,20], a family of (Lu, Y, Gd)3(Ga, Al)5O12 scintillators were developed, of which 1%Ce:Gd3Ga3Al2O12 crystal has high light yield up to 46,000 Ph/MeV [18] and 1%Ce:Gd2Y1Ga2.7Al2.3O12 crystal demonstrates excellent properties with its light yield 65,000 Ph/MeV in our previous work [20].
Thermal annealing treatment is always applied to scintillators to improve their overall scintillation quality. Systematical annealing experiments of Ce:Lu2Si2O7 crystals under different temperatures and different atmospheres were performed by Feng et al. [20], they found a strong increase of luminescence intensity of Ce:LPS annealed in air above 1400 °C, which was due to vacancies’ filling by diffusing oxygen atoms. Drozdowski et al. [21,22,23] annealed Pr:LuAlO3 and Pr:Lu3Al5O12 crystals in hydrogen and air atmosphere, respectively, and a great yield enhancement was achieved in these crystals. And the positive influence of annealing in air at high temperatures are explained by the creation of an alternative fast radiative pathway through stable Ce4+ centers and diminishing concentration of oxygen vacancies [24].
However, little attention has been put on the effect of annealing at elevated temperatures on (Y, Gd)3(Ga, Al)5O12 scintillators, which present great light yield improvement compared to the LuAG crystals. In this work, the thermal annealing effects on Ce:Gd2Y1Ga2.7Al2.3O12(GYGAG) crystals in different atmospheres were studied and the positive effects were explained. The scintillation properties before and after annealing were compared and discussed. The results of correlated Absorption spectra, X-ray excited Radioluminescence, Thermoluminescence (TSL) and UV-excitation and emission spectra measurements, performed on the “as-grown” and annealed samples, have been analyzed.
2 Materials and experiment
The CeO2, Y2O3, Ga2O3, Gd2O3 and α-Al2O3 raw powders of 99.99% purity were used in this study as starting material. They were weighed according to the stoichiometric ratio of Gd2Y1Ga3Al2O12:1% Ce and were calcinated at 1500 °C before crystal growth. The crystal was grown by the Czochralski method using iridium crucible, and a Ø1-inch boule one was attained. Details about the growth parameters were presented in [20]. The samples with dimensions of 5 × 5×1 and 10 × 10 × 2 mm3 were cut from the crystal ingot and polished carefully before annealing. The first annealing treatment was performed on two big samples in air at 350, 700, 1000, 1100, 1200, 1300 and 1400 °C, each for 6 h. The small 5 × 5×1 mm3 samples were annealed at 1200 °C in the following atmospheres: 100% N2, Ar, O2 and air. The heating and cooling rate in the annealing process was about 100 °C/h.
The measurement of X-ray excited luminescence (XEL) was performed by using a home-made equipment. Optical absorption spectra were measured at RT by using a Shimadzu UV-2501 PC spectrophotometer. The UV-excited emission spectra and fluorescence decays were recorded on the Perkin-Elmer LS50B and FLS-920 spectrofluorometer, respectively.
The thermal stimulated luminescence (TSL) curves of Ce:GYGAG crystals were determined with a FJ-427A1 thermal spectrometer with a linear heating rate of 1 K/s from 313 to 763 K and 0.15 K/s from 77 to 300 K. Prior to two dimensional TSL experiment, the samples were irradiated with X-ray for 90 s. Meanwhile, three dimensional TSL curves (the TSL as a function of both the temperature and the wavelength, shortened as 3D TSL) were measured at the as-grown and annealed samples. The samples were exposed to X-ray for 240 s prior to 3D TSL experiment.
3 Results and discussion
A sample of Ce:GYGAG with dimension of 10 × 10 × 2 mm3 was sequentially annealed in air at a series of temperature from 350 to 1400 °C for 6 h, the XEL spectra and their integrated intensities at different annealing temperature are shown in Fig. 1. It is clearly found that the scintillation efficiency could be enhanced after annealing and the biggest (around 260% as-grown) was attained at 1100 °C, but it significantly decreases after annealing at higher temperature (>1200 °C), and the same results was found under O2 annealing. Such phenomenon is similar to the Lu0.8Sc0.2BO3:Pr [25], which is attributed to the removal of the oxygen vacancies and the decrease of the scintillation efficiency after annealing at a higher temperature is caused by the oxidization of praseodymium ions. While the phenomenon is different from the other Ce3+-doped oxide scintillators [21, 26], whose scintillation efficiency is enhanced after annealing, but keep stable even at high temperature (1400 °C).To prove that O2 containing atmosphere exerts critical effects on the scintillation efficiency of the crystals after annealing, another sample was annealed in nitrogen atmosphere, as shown in Fig. 2. Contrast to the annealing process in air, the scintillation efficiency of the crystal decreased after annealing in nitrogen atmosphere. It is interesting that the scintillation efficiency does not keep consistent, but fluctuates at different annealing temperature. The same phenomenon is found in argon annealing atmosphere. As the instrument was calibrated each time before testing, it seems that annealing temperature has some impacts on the scintillation efficiency regardless of the ambient atmosphere, although the mechanism is unclear. The 137Cs pulse height spectrum of Ce:GYGAG crystals before and after annealing at 1200 and 1400 °C in air and nitrogen atmosphere are presented in Fig. 3. In the air atmosphere, one observes the biggest photoelectron yield at 1200 °C, while the photoelectron yields both decrease at 1200 and 1400 °C after annealing in nitrogen compared to the as-grown. This phenomenon is consistent with the results of the X-ray excited radioluminescence spectra. As mentioned above, it is proposed that the removal of oxygen vacancies plays a dominant role in the air-annealing process (lower than 1200 °C). On the other hand, the decrease of the XEL intensity after 1200 °C can be tentatively explained by the Ce3+ oxidation to stable Ce4+. In the O2 contained atmosphere, the possible remedy of oxygen vacancies can be described as follow:
The other indirect evidence of the oxidization process and the remedy of oxygen vacancies after high temperature air-annealing will be presented in the following paragraph.
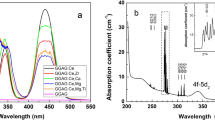
Optical absorption spectra of as-grown and air-annealed Gd2Y1Ga3Al2O12:1%Ce crystals are plotted in Fig. 4. Thermal etching would occur when the sample was annealed at 1400 °C (the pictures in Fig. 4), so after annealing, the samples’ surfaces were polished before each test. Three peaks as in [27] can be observed: the 4f-5d1,2 transitions of Ce3+ center at 342 and 438 nm, the Gd3+ 4f-4f transition 8S7/2-6I3/2 at 274 nm. Compared with the as-grown sample, the intensity of 342 and 438 nm peak of the annealed ones decrease slightly. The possible explanation is the accumulation of Ce4+ while annealing, which leads to the reduction of the stable Ce3+ and accordingly the decrease of 4f-5d1,2 transitions intensity. Such phenomenon is consistent with the divalent cation Mg2+and Ca2+ co-doped LuAG:Ce [28] and LYSO:Ce [29]. Here we can also find the increasing intensity of broadband absorption 200–350 nm after air-annealing, and the band can be attributed to the Ce4+-O2− charge transfer(CT) absorption [28,29,30,31]. On the other hand, the Ce4+-O2− charge transfer(CT) absorption band in our experiment does not show the same features with that given in the literature, it may be due to that the samples co-doped with divalent ions lead to more amount of stable Ce4+, thus more stronger absorption around 260 nm in the Ca2+, Mg2+, B2+ co-doped hosts [21, 28, 29].
To get better knowledge of the depth of deep traps in the sample, the Thermally Stimulated Luminescence (TSL) curves of GYGAG:Ce before and after annealing in air for 6 h at 1200 °C are shown in Fig. 5. The general form of TSL intensity I, as a function of temperature T, can be described by the following equation [32]:
In the above equation, n 0 is the concentration of trapped charges at t = 0, Et the energy level of the trap, κB the Boltzmann constant, l the kinetic order, s the frequency factor, and β is the heating rate. Because Eq. (2) cannot be directly used to fit the experimental data, a modified one (3) [21] was adopted in our fitting process,
According to Eq. (3), all the TSL data were fitted using ORIGIN 8 software. After several trials to fit the experimental curves we found that the most optimum fitting model consists of four peaks for TSL curves at around 350, 398, 422 and 496 K, which are labeled as 1#, 2#, 3# and 4#, respectively, as shown in Fig. 5. The calculated TSL parameters are listed in Table 1. The slight shifts of the peaks after air-annealing are believed to be caused by non-ideal heat transfer between the samples and the heating plate [33]. Theoretically, when the crystal sample is annealed under air at high temperature, the oxygen in air would diffuse into the crystal lattice and fill the oxygen vacancies, so that there should be great decrease even disappearance in intensity of one or more peaks. After air-annealing at 1200 °C, the intensity of 3# and 4# peaks decrease a lot, thus both of them are considered oxygen-vacancy-related traps.
Glow curves of 1%Ce:GYGAG before and after annealing are presented in Fig. 6, revealing the existence of several shallow traps. One can easily notice that there are two main traps at very low temperature, i.e., 1# (103 K) and 2# (114 K), but the curves cannot be appropriately fitted with Eq. (3), so it is very difficult to determine an exact number of traps and accurate values of their depths and frequency factors. On the other hand, it can be clearly seen that the peak 2# declines a lot after air-annealing at 1200 °C whilst it keeps the same after N2-annealing. The possible explanation for this is that shallow trap 2# is related to oxygen vacancies. Figure 7 shows the 3D TSL curves and their corresponding contour plots of the Ce:GYGAG crystal (a) before and (b) after air-annealing at 1200 °C. The two contours show the sample’s luminescent wavelength ranging both between 500 and 700 nm (characteristic of Ce3+ 5d-4f transition) before and after annealing. Figure 8 shows that the photoluminescence spectra of the Ce:GYGAG sample before and after air-annealing process at various temperature and it can be found that the excitation and emission peaks do not shift after the annealing process. From the data in the Figs. 7 and 8, we conclude that the Ce3+ ions are the recombination centers for the detrapped charge carriers, which is applicable for the traps corresponding to TSL peaks 1#, 2#, 3# and 4#.
4 Conclusions
The results presented in this paper indicate that thermal annealing, mostly in 100% air and O2 (below 1200 °C), can be successfully utilized to improve the scintillation performance of 1%Ce:GYGAG by increasing its scintillation efficiency. However, the luminescence efficiency of the crystal will decline after air-annealing above 1200 °C due to the dominant role of the oxidation of Ce3+ to Ce4+. It is proposed that the enhanced scintillation efficiency of the as-grown 1%Ce:GYGAG after air-annealing is caused by the removal of oxygen vacancies inevitably present in the as-grown crystals. There are three traps, a shallow and two deeper ones monitored by TSL, which are related to the oxygen vacancies. Deeper study of the influence of these traps on the scintillation mechanism and characteristics (e.g., light yield, energy resolution, decay time) are definitely needed. With the removed drawbacks mentioned above the, Ce:GYGAG scintillator would join the group of the brightest oxide scintillators known today.
References
J. Pejchal, M. Nikl, E. Mihóková, J.A. Mareš, A. Yoshikawa, H. Ogino, K.M. Schillemat, A. Krasnikov, A. Vedda, K. Nejezchle, V. Múčka, J. Phys. D Appl. Phys. 42, 5 (2009)
E.V.D van Loef, P. Dorenbos, C.W.E van Eijk, K.W Krämer, Nucl. Instr. Method. Phys. Res. A 486, 254 (2002)
L. Pidol, B. Viana, A. Kahn-Harari, B. Ferrand, P. Dorenbos, C.W.E. van Eijk, Nucl. Instr. Method. Phys. Res. A 537(1–2), 256 (2005)
J. Glodo, E. van Loef, R. Hawrami, W.M. Higgins, A. Churilov, U. Shirwadkar, K.S. Shah, IEEE. Trans Nucl. Sci. 58, 333 (2011)
M. Nikl, H. Ogino, A. Krasnikov, A. Beitlerova, A. Yoshikawa, T. Fukuda, Phys. Stat. Sol. (a) 202(1), 4 (2005)
C. Dujardin, C. Mancini, D. Amans, G. Ledoux, D. Abler, E. Auffray, P. Lecoq, D. Perrodin, A. Petrosyan, K.L. Ovanesyan, J. Appl. Phys. 108(1), 1 (2010)
J.A. Mares, A. Beitlerova, M. Nikl, N. Solovieva, C. D’Ambrosio, K. Blazek, P. Maly, K. Nejezchleb, F. de Notaristefani, Radiat. Meas. 38(4–6), 353 (2004)
T. Kato, J. Kataoka, T. Nakamori, T. Miura, H. Matsuda, K. Sato, Y. Ishikawa, K. Yamamura, N. Kawabata, H. Ikeda, G. Sato, K. Kamada, Nucl. Instr. Meth. A 638(1), 83 (2011)
M. Nikl, A. Vedda, M. Fasoli, I. Fontana, V.V. Laguta, E. Mihokova, J. Pejchal, J. Rosa, K. Nejezchleb, Phys. Rev. B 76, 195121 (2007)
W. Chewpraditkul, L. Swiderski, M. Moszynski, T. Szczesniak, A. Syntfeld-Kazuch, C. Wanarak, P. Limsuwan, I.E.E.E. Trans, Nucl. Sci. 56, 3800 (2009)
M. Nikl, E. Mihokova, J. Pejchal A. Vedda, Yu. Zorenko, K. Nejezchleb, Phys. Status Solidi B. 242(14), 119 (2005)
W. Drozdowski, K. Brylew, M.E. Witkowski, A.J. Wojtowicz, P. Solarz, K. Kamada, A. Yoshikawa, Opt. Mater. 36, 1665 (2014)
D. Robbins, B. Cockayne, B. Lent, C.N. Duckworth, J.L. Glasper, Phys. Rev. B 19(2), 1254 (1979)
M. Nikl, J. Pejchal, E. Mihokova, J.A. Mares, H. Ogino, A. Yoshikawa, T. Fukuda, A. Vedda, C. D’Ambrosio, Appl. Phys. Lett. 88(14), 141916 (2006)
M. Nikl, E. Mihokova, J. Pejchal, A. Vedda, M. Fasoli, I. Fontana, V.V. Laguta, V. Babin, K. Nejezchleb, A. Yoshikawa, H. Ogino, G. Ren, Trans. Nucl. Sci. 55, 1035 (2008)
M. Kottaisamya, P. Thiyagarajan, J. Mishra, M.S. Ranachandra, Rao. Mater. Res. Bulletin 43, 1657 (2008)
W. Jennifer, G. Gundiah, A.K. Cheetham, Chem. Phys. Lett. 441, 250 (2007)
K. Kamada, T. Endo, K. Tsutumi, T. Yanagida, Y. Fujimoto, A. Fukabori, A. Yoshikawa, J. Pejchal, M. Nikl, Cryst. Growth Des. 11(10), 4484 (2011)
K. Kamada, T. Yanagida, J. Pejchal, M. Nikl, T. Endo, K. Tsutumi, Y. Fujimoto, A. Fukabori, A. Yoshikawa, J. Phys. D Appl. Phys. 44, 505104 (2011)
C. Wang, W. Yuntao, H. Li, X. Chen, J. Shi, G. Ren, Nucl. Inst. Meth. Phys. Res. A 820, 8 (2016)
H. Feng, D. Ding, H. Li, S. Lu, S. Pan, X. Chen, G. Ren, J. Appl. Phys. 103, 0831091 (2008)
W. Drozdowski, A.J. Wojtowicz, D. Wis´niewski, T. Lukasiewicz, J. Kisielewski. Opt. Mat. 28,102 (2006)
W. Drozdowski, K. Brylew, M.E. Witkowski, A.J. Wojtwicz, K. Kamada, T. Yanagida, A. Yoshikawa, Radiat. Measurements 56, 80 (2013)
M. Nikl, V. Babin, J.A. Mares, K. Kamada, S. Kurosawa, A. Yoshikawa, J. Tous, J. Houzvicka, K. Blazek, J. Lumin. 169, 539 (2016)
W. Drozdowski, K. Brylew, A. Chruscinska, K. Kamada, T. Yanagida, A. Yoshikawa, Opt. Mater. 34, 1975 (2012)
Y.T. Wu, G.H. Ren, D.Z. Ding, F. Yang, S.K Pan. Solid State Sci. 14, 635 (2012)
D.Z. Ding, H. Feng, G.H. Ren, M. Nikl, L.S. Qin, S.K. Pan, F. Yang, I.E.E.E. Trans, Nucl. Sci. 57, 1272 (2010)
Y.T. Wu, M. Nikl, V. Jary, G.H. Ren, Chem. Phys. Lett. 574, 56 (2013)
M. Nikl, K. Kamada, V. Babin, J. Pejchal, K. Pilaronva, E. Mihokova, A. Beitlerova, K. Bartosiewicz, S. Kurosawa, A. Yoshikawa, Cryst. Growth Des. 14, 4827 (2014)
W. Chewpraditkul, C. Wanarak, T. Szczesniak, M. Moszynski, V. Jary, A. Beitlerova, M. Nikl. Opt. Mater. 35, 1679 (2013)
Y.T. Wu, F. Meng, Q. Li, M. Koschan. C.L. Melcher, Phys. Rev. Appl. 2, 044009 (2014)
D.W. Cooke, B.L. Bennett, E.H. Farnum, W.L. Hults, R.E. Muenchausen, J.L. Smith, Appl. Phys. Lett. 70, 3594 (1997)
T.M. Piters, R. Melendrez, W. Drozdowski, Radiat. Prot. Dosim. 84(1–4), 127 (1999)
Acknowledgements
This work was supported by the National Nature Science Foundation of China (Grant No. 51202276), Nature Science Foundation of Shanghai (Grant No. 14520710400), and Open Fund of the State Key Laboratory of Crystal Material (Grant No. KF1305).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wang, C., Ding, D., Wu, Y. et al. Effect of thermal annealing on scintillation properties of Ce:Gd2Y1Ga2.7Al2.3O12 under different atmosphere. Appl. Phys. A 123, 384 (2017). https://doi.org/10.1007/s00339-017-0997-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-017-0997-z