Abstract
A new approach for manufacturing hollow polyimide (PI) microspheres is presented. The method is based on cavitation bubbles and small CO2 gas bubble which can generate uniform microspheres. When liquid PI was irradiated by a 355- nm nanosecond pulsed laser in a gas pressure chamber, microspheres ranging from 25 to 35 μm and smaller spheres attached to the microspheres ranging from 5 μm to submicron dimensions were generated in the liquid. Microspheres ranging from 25 to 35 μm were fabricated by laser-induced formation. The spheres’ morphology was influenced by the pressure in the chamber. When the pressure is high in the chamber, non-uniform microspheres are produced due to flow caused by the laser-induced shockwave and change of the carbon dioxide concentration in the liquid. Smaller spheres with 5 μm to submicron dimensions attached onto the microspheres were fabricated by a gas bubble template method and laser-induced formation. The resulting shell of hollow PI microspheres was about 4 μm, and the sphere inside has a porous structure. Our results indicate that the proposed laser-induced formation technology in a gas pressure chamber can be used to prepare polymer spheres by controlling the gas pressure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Controlling particle size and shape is necessary for a variety of applications such as drug delivery carriers and low dielectric constant materials because the properties of particles vary depending on their size and shape [1, 2]. While there has been active research into methods of fabricating nanometal particles and nanoceramic particles in liquid using a laser, few groups have investigated the fabrication of polymer-based particles in liquid using a laser [3, 4]. Among polymers, polyimide (PI) is well known for its excellent properties, including a low dielectric constant, good biocompatibility, outstanding mechanical properties, thermal properties, and chemical resistance [5]. As such, if it were possible to easily and quickly fabricate PI particles, they could be utilized in a variety of applications. A few groups have studied the fabrication of PI spheres. Lin et al. [6] prepared submicron PI particles whose dimensions could be controlled by the cooling rate through precipitation in a mixed solvent of water and N-methyl-2-pyrrolidone (NMP). Unfortunately, the precipitation method does not produce uniform sizes. Zhao et al. [7, 8] researched polyimide nanoparticles that were fabricated using the reprecipitation method with poly(acrylic acid) (PAS1) and poly(sodium-4-styrenesulfonate) (PSS) as porogens to generate nanopores on the particles’ surface. Further, they fabricated hollow PI nanoparticles using poly(vinylpyrrolidone) (PVP) and poly(methyl methacrylate) (PMMA) as porogens [2]. Watanabe et al. [9] reported on core–shell polystyrene/polyimide particles prepared by dispersion polymerization of styrene. Yan et al. [10] fabricated hierarchical PI hollow spheres via a gas bubble-templated transimidization. Heating PMDA-AP and DAT generated small gas bubbles, by evaporating the AP. The generated gas bubbles have a very small diameter and hence have high surface energy. As a result, the small gas bubbles act as nuclei, and monomers become attached to them. However, reprecipitation, dispersion polymerization, and gas bubble template methods all require the use of a second material.
As an alternative approach to address some of the above-mentioned methods, we present a laser-based method to fabricate PI spheres in a liquid. When short pulses of a laser beam are irradiated into the liquid, the laser beam generated plasma that rapidly heats the liquid and then the bubbles nucleate and a shockwave is generated. The bubbles, which have high internal pressure, expand and collapse [11, 12].
In this paper, we report a new method to form micro-PI spheres and submicron PI spheres in a liquid PI by different approaches at pressures of 20 and 30 bar. We describe the correlation between PI sphere morphology and pressure, based on a cavitation bubble and gas bubbles at high pressures.
2 Experimental
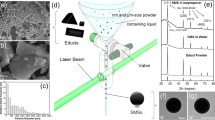
The liquid PI used in this experiment was VTEC™ PI-1388 which is polyamic acid suspended in NMP solvent. The laser in the experiment was a Series 3500 UV Laser from DPSS Lasers, Inc. This is a Nd:YVO4 355-nm nanosecond pulse laser with an average power of 2.0 W, a pulse duration of 20 ns, an average pulse repetition rate of 30 kHz, and a TEM00 beam mode. A galvano-scanner system and an F-θ lens were used to control the direction of the laser beam and to focus the beam on the material. Figure 1a shows a schematic diagram of the laser system.
The liquid PI was contained in a tungsten bowl and placed in a gas chamber (Fig. 1a), and the experiment was conducted under CO2 gas at 20 and 30 bar in the chamber. After pressurization, a square area of the liquid PI was irradiated by a pulsed laser in a grid pattern, producing irradiated dots separated by intervals of 0.01 mm, for number of 40,000 irradiation points at 0.3 J/cm2. After decompressing the chamber, the irradiated liquid PI was cured at 130 °C for 20 min in an oven. For scanning electron microscopy (SEM) measurements, the precured PI blocks were picked off the tungsten bowl and the bottom surface of the sample was coated with platinum.
3 Results and discussion
The microspheres fabricated by a 355-nm pulsed laser in these experiments could be divided into two kinds of spheres, one type with uniform spheres ranging from 25 to 35 μm, and the other with sphere sizes ranging from 5 μm to submicron dimensions. Figure 2 shows the SEM micrographs of microspheres produced under 30 bar pressure. Some smaller microspheres are attached to the larger microspheres. The size of the PI spheres is not uniform (Fig. 2b, c, d). However, under 20 bar pressure conditions, the size of the PI spheres is uniform (Fig. 3a, b).
The mechanism underlying the fabrication of PI microspheres has not yet been clarified, but the fabrication method can be described in two ways: via a bubble template and via a cavitation bubble. The bubble template method is as follows [13]. CO2 gas enters the liquid PI due to the shockwaves generated between the medium and compressed CO2 gas. Containing much air makes more stable over time and results in a greater quantity of CO2 bubbles.
Very small CO2 bubbles have a high surface energy, CO2 bubbles are surrounded by polyamic acid, and a curing process then takes place when gas bubbles surrounded by the monomer are irradiated by the laser. Figure 2b, c shows microspheres fabricated by the bubble template. The spheres are not uniform because the CO2 bubbles in the liquid increase per the laser beam irradiation and are not stable.
A cavitation bubble is generated when the laser beam energy absorbed by the medium overcomes the optical threshold. When a liquid is irradiated, rapid liquid heating occurs and plasma is generated. A cavity abruptly expands in the liquid, and the inflating cavity rapidly displaces the free surface of the liquid. The heat simultaneously cures cavitation bubble, and PI spheres are fabricated before the cavitation bubble collapses. The cavitation bubble in the water collapses within microseconds, but the cavitation bubble in the liquid PI is transformed into the PI spheres due to high temperature induced by the laser beam. The PI spheres then sink in the liquid toward the bottom surface due to their density. However, the produced microspheres cannot adhere to other surfaces; in other words, the solvent NMP cannot attach to the microspheres due to its surface energy and it does not decompose the PI. The microspheres thus are not dissolved in the solvent and remain in the liquid.
In addition, they also do not adhere to the tungsten bowl used in the experiments. After curing at 130 °C for 20 min in the oven, the produced microspheres are visible at the bottom of the PI blocks because the density of the microspheres is higher than the liquid PI. Therefore, when processing the liquid PI by the laser, it is not possible to distinguish the PI spheres from the irradiated liquid PI.
The spheres were produced under two other conditions, 20 bar and 30 bar pressure. The spheres produced under 20 bar pressure are generally uniform (Fig. 3a), whereas the spheres produced under 30 bar pressure are diverse (Fig. 2b, c, d). We present two reasons. The first involves the laser-induced shockwave. The shockwave value was larger when the pressure in the chamber was high. The shockwave vibrates the surface of the medium. Due to its repetition rate of 30 kHz, flow continuously occurs on the surface of the liquid. This causes the formation of non-uniform microspheres at 30 bar. The second involves CO2 gas in the liquid PI. After the laser processing under 30 bar pressure, the pressure changes from 30 to 28 bar because the higher pressure causes CO2 to disperse in the liquid PI. CO2 bubbles in the liquid are unstable due to change of the pressure and the number of the bubbles per the laser beam irradiation. However, in the case of 20 bar pressure, the resulting change in pressure is inadequate.
Hemispheres were observed with an optical microscope to examine their internal space, which was found to have a porous morphology (Fig. 4a). The shell thickness is about 4 μm. We also observed the microspheres using an optical microscope (Fig. 4b). White circles are observed at the sphere centers because they are not solid spheres. The fabricated spheres can be applied to ultra low-k dielectric materials. It is well known that the dielectric constants of porous structures can be reduced by replacing the polymer with air, because the dielectric constant of air is unity [2].
Figure 5 shows a 2-μm particle attached the Fig. 2d’s sphere. Patrascioiu et al. [14] studied the simultaneous time-resolved imaging of bubble evolution. When the cavitation bubble collapses, smaller bubbles are generated. The smaller spheres are then attached to the bigger spheres as can be seen in Figs. 2d and Fig. 5.
Magnification of area in Fig. 2d denoted by the red rectangle
4 Conclusion
We proposed a novel strategy to fabricate polyimide spheres using a pulsed laser at 355 nm in liquid. Uniform polyimide microspheres were successfully fabricated using a 355-nm pulsed laser under 20 bar pressure. Uniform microspheres ranging from 25 to 35 μm were fabricated by laser-induced formation and attached microspheres with size below 5 μm were fabricated. The spheres’ morphology is influenced by the pressure in the chamber. When the pressure is high in the chamber, non-uniform microspheres are produced due to the flow caused by the laser-induced shockwave and carbon dioxide concentration change in the liquid. The resulting hollow PI microspheres have potential application as ultralow-k materials. In summary, irradiated liquid PI will provide a new route to realize ultralow-k materials.
References
J.A. Champion, Y.K. Katare, S. Mitragotri, J. Control. Release 121(1), 3 (2007)
G. Zhao, T. Ishizaka, H. Kasai, M. Hasegawa, T. Furukawa, H. Nakanishi, H. Oikawa, Chem. Mater. 21(2), 419 (2008)
F. Mafuné, J.Y. Kohno, Y. Takeda, T. Kondow, H. Sawabe, J. Phys. Chem. B 105(22), 5114 (2001)
C.L. Sajti, R. Sattari, B.N. Chichkov, S. Barcikowski, J. Phys. Chem. C 114(6), 2421 (2010)
M. Ghosh, Polyimides: Fundamentals and Applications (Marcel Dekker, New York, 1996)
T. Lin, K.W. Stickney, M. Rogers, J.S. Riffle, J.E. McGrath, H. Marand, R.M. Davis, Polymer 34(4), 772 (1993)
G. Zhao, T. Ishizaka, H. Kasai, H. Oikawa, H. Nakanishi, Chem. Mater. 19(8), 1901 (2007)
G. Zhao, T. Ishizaka, H. Kasai, M. Hasegawa, H. Nakanishi, H. Oikawa, Polym. Adv. Technol. 20(1), 43 (2009)
S. Watanabe, K. Ueno, K. Kudoh, M. Murata, Y. Masuda, Macromol. Rapid Commun. 21(18), 1323 (2000)
Y. Yan, L. Chen, X. Li, Z. Chen, X. Liu, Polym. Bull. 69(6), 675 (2012)
T. Juhasz, G.A. Kastis, C. Suarez, Z. Bor, W.E. Bron, Lasers Surg. Med. 19(1), 23 (1996)
P.K. Kennedy, D.X. Hammer, B.A. Rockwell, Prog. Quantum Electron. 21(3), 155 (1997)
T. Makuta, S. Takada, H. Daiguji, F. Takemura, Mater. Lett. 63(8), 703 (2009)
A. Patrascioiu, J.M. Fernández-Pradas, A. Palla-Papavlu, J.L. Morenza, P. Serra, Microfluid. Nanofluid. 16(1–2), 55 (2014)
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2015R1D1A3A01016057) and the National Research Foundation of Korea (NRF) Grant funded by the Korean Government (MSIP) (No. 2015R1A5A7036513).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ma, YW., Kang, MS., Park, C. et al. Fabrication of polyimide spheres using a pulsed laser at 355 nm. Appl. Phys. A 122, 481 (2016). https://doi.org/10.1007/s00339-016-9995-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-016-9995-9