Abstract
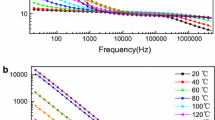
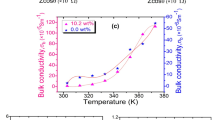
Polarisation processes and charge transport in polyvinylidene fluoride (PVDF) with a small amount (0.01–10 wt%) of the ionic liquid (IL) 1-ethyl-3-methylimidazolium nitrate (\(\hbox {[EMIM]}^+[\hbox {NO}_3]^-\)) are investigated by means of dielectric spectroscopy. The response of PVDF that contains more than 0.01 wt% IL is dominated by a low-frequency relaxation which shows typical signatures of electrode polarisation. Furthermore, the \(\alpha _{{\rm a}}\) relaxation, related to the glass transition, disappears for IL contents of more than 1 wt%, which indicates that the amorphous phase loses its glass-forming properties and undergoes structural changes. The DC conductivity is determined from the low-frequency limit of the AC conductivity and from the dielectric loss peak related to the electrode polarisation. DC conductivities of \(10^{-10}\) to \(10^{-2}\,\hbox {S}/\hbox {m}\) are obtained—increasing with IL content and temperature. The dependence of the DC conductivity on the IL content follows a power law with an exponent greater than one, indicating an increase in the ion mobility. The temperature dependence of the DC conductivity shows Vogel–Fulcher–Tammann behaviour, which implies that charge transport is coupled to polymer chain motion. Mobile ion densities and ion mobilities are calculated from the DC conductivity and the dielectric loss related to electrode polarisation, with the results that less than one per cent of the total ion concentration contributes to the conductivity and that the strong increase in conductivity with temperature is mainly caused by a strong increase in ion mobility. This leads to the conclusion that in particular the ion mobility must be reduced in order to decrease the DC conductivity.










Similar content being viewed by others
References
A.J. Lovinger, in Developments of Crystalline Polymers, vol. 1, ed. by D.C. Basset (Springer, Berlin, 1982), pp. 195–273
A.J. Lovinger, Science 220(4602), 1115–1121 (1983)
R.G. Kepler, R.A. Anderson, Adv. Phys. 41, 1–57 (1992)
T. Furukawa, Phase Transit. 18, 143–211 (1989)
T. Furukawa, Key Eng. Mater. 92–93, 15–30 (1994)
T. Furukawa, Adv. Colloid Interface 71–72, 183–208 (1997)
K. Tashiro, in Ferroelectric Polymers, ed. by H.S. Nalwa (Marcel Dekker Inc, New York, 1995), pp. 63–181
V.V. Kochervinskii, Russ. Chem. Rev. 65(10), 865–913 (1996)
X. He, K. Yao, Appl. Phys. Lett. 89, 112909 (2006)
F. Wang, A. Lack, Z. Xie, P. Frübing, W. Wirges, R. Gerhard, in 2011 Annual Report, Conference on Electrical Insulation and Dielectric Phenomena (National Academy of Sciences, Washington, DC, 2011), pp. 710–713
F. Wang, A. Lack, Z. Xie, P. Frübing, A. Taubert, R. Gerhard, Appl. Phys. Lett. 100, 062903 (2012)
C. Xing, M. Zhao, L. Zhao, J. You, X. Cao, Y. Li, Polym. Chem. 4, 5726–5734 (2013)
C. Liang, Z. Mai, Q. Xie, R. Bao, W. Yang, B. Xie, M. Yang, J. Phys. Chem. B 118, 9104–9111 (2014)
P. Frübing, F. Wang, M. Wegener, Appl. Phys. A 107, 603–611 (2012)
M. Lada, Appl. Phys. Lett. 93, 143308 (2008)
R. Kalbitz, P. Frübing, R. Gerhard, D.M. Taylor, in Proceedings of the 10th IEEE International Conference on Solid Diel. (ICSD) 2010, IEEE Cat. No. CFP10ICS-PRT, (2010). doi:10.1109/ICSD2010.5567939:410
J.R. Macdonal. Phys. Rev. 91, 412 (1953)
A. Serghei, Phys. Rev. B 80, 184301 (2009)
S. Emmert, M. Wolf, R. Gulich, S. Krohns, S. Kastner, P. Lunkenheimer, A. Loidl, Eur. Phys. J. B 83, 157–165 (2011)
A.K. Jonscher, Dielectric Relaxation in Solids (Chelsea Dielectrics Press, London, 1983), p. 76
D. Rollik, S. Bauer, R. Gerhard-Multhaupt, J. Appl. Phys. 85, 3282–3288 (1999)
J.C. Dyre, J. Appl. Phys. 64, 2456–2468 (1988)
J.C. Dyre, T.B. Schrøder, Rev. Mod. Phys. 72(3), 873–892 (2000)
According to DIN 53482, Solef™ PVDF Design & Processing Guide, (2015), p. 43. http://www.solvay.com
R.J. Klein, S. Zhang, S. Dou, B.H. Jones, R.H. Colby, J. Runt, J. Chem. Phys. 124, 144903–8 (2006)
J.R. Macdonald, Phys. Rev. 91, 412 (1953)
R. Coelho, Rev. Phys. Appl. 18, 137–146 (1983)
R. Coelho, J. Non-Cryst. Solids 131–133, 1136–1139 (1991)
U.H. Choi, M. Lee, S. Wang, W. Liu, K.I. Winey, H.W. Gibson, R.H. Colby, Macromolecules 45, 3974–3985 (2012)
J.R. Sangoro, A. Serghei, S. Naumov, P. Galvosas, J. Kärger, C. Wespe, F. Bordusa, F. Kremer, Phys. Rev. E 77, 051202–4 (2008)
J. Sangoro, C. Iacob, A. Serghei, S. Naumov, P. Galvosas, J. Kärger, C. Wespe, F. Bordusa, A. Stoppa, J. Hunger, R. Buchner, F. Kremer, J. Chem. Phys. 128, 214509–5 (2008)
J. Sangoro, C. Iacob, A. Serghei, C. Friedrich, F. Kremer, Phys. Chem. Chem. Phys. 11, 913–916 (2009)
J.R. Sangoro, F. Kremer, Acc. Chem. Res. 45, 525–532 (2012)
J.L. Barton, Verr. Réfract. 20, 328 (1966)
T. Nakajima, in Annual Report CIEDP, (1971), p. 168
H.J. Namikawa, J. Non-Cryst. Solids 14, 88 (1974)
H.J. Namikawa, J. Non-Cryst. Solids 18, 173 (1975)
C. Tsonos, A. Kanapitsas, A. Kechriniotis, N. Petropoulos, J. Non-Cryst. Solids 358(14), 1638–1643 (2012)
N. Nishimura, H. Ohno, Polymer 55, 3289–3297 (2014)
P. Frübing, M. Wegener, R. Gerhard-Multhaupt, A. Buchsteiner, W. Neumann, L. Brehmer, in Proceedings of the SPIE 4017, (1999), pp. 26–28, ISBN 0-8194-3643-7
M. Wegener, S. Bauer, R. Gerhard-Multhaupt, in Proceedings of the 10th International Symposium on Electrets, (1999), pp. 643–646, IEEE Cat. Nr. 99CH36256, ISBN 0-7803-5025-1
J.F. Scott, J. Phys. Condens. Mater. 20, 021001 (2008)
Acknowledgments
The authors are grateful to Prof. Andreas Taubert, Institute of Chemistry, University of Potsdam for valuable comments and to the European Union for partial financial support within the European Fund for Regional Development (EFRD). F. Wang would like to acknowledge a visiting scholarship provided by the State Key Laboratory of Power Transmission Equipment and System Security and New Technology in Chongqing University, China.
Author information
Authors and Affiliations
Corresponding author
Appendix: Calculation of the total ion density and of the CH2–CF2 dipole concentration
Appendix: Calculation of the total ion density and of the CH2–CF2 dipole concentration
Under the assumption that ions do not penetrate into crystallites, the total ion concentration within the film is given by
where \(c\equiv m_\mathrm{c}/m_\mathrm{film}\) is the crystallinity (\(m_\mathrm{c}\) mass of crystalline portion, \(m_\mathrm{film}\) mass of the film).
For a film containing 1 wt% IL, \(m_\mathrm{IL}=1.0\) g, \(m_\mathrm{PVDF}=99.0\) g.
The density of the IL is not exactly known and is difficult to determine because [EMIM][\(\hbox {NO}_3\)] is hygroscopic. However, for an estimate, it can be assumed that the density is not far from the densities of other related ILs, which lie between 1100 and 1500 kg/m\(^3\) (IoLiTec GmbH, Germany). The density of the solid PVDF homopolymer is \(\rho _\mathrm{PVDF}=1780\,\hbox {kg}/\hbox {m}^{-3}\). Thus, for low IL contents, \(\rho _\mathrm{film}\approx \rho _\mathrm{PVDF}\) is a reasonable approximation.
The crystallinity of the film increases by addition of the IL to the solution: for the 5 wt% film, \(c=47\) and for the pure PVDF film, \(c=36\) % were obtained, respectively [10].
By use of \(c=0.36,\,c=0.40\) and \(c=0.47\) for the 0.1, 1 and 5 wt% IL films, respectively, total ion concentrations in the amorphous fraction of \(n_\mathrm{tot}=9.65\times 10^{24},\,1.03\times 10^{26}\) and \(5.83\times 10^{26}\,\hbox {m}^{-3}\), respectively, are calculated.
The total \(\hbox {CH}_2\)–CF2 dipole concentration is
With the density \(\rho _\mathrm{PVDF}=1780\,\hbox {kg}/\hbox {m}^{-3}\) and the molar mass \(m_\mathrm{mol, unit VDF}=0.064\) kg/mol, \(n_{\rm d}=1.67\times 10^{28}\,\hbox {m}^{-3}\) is obtained.
\(n_{\rm d}\gg n_\mathrm{ion}\), even for 5 wt% IL.
Rights and permissions
About this article
Cite this article
Frübing, P., Wang, F., Kühle, TF. et al. AC and DC conductivity of ionic liquid containing polyvinylidene fluoride thin films. Appl. Phys. A 122, 79 (2016). https://doi.org/10.1007/s00339-015-9540-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-015-9540-2