Abstract
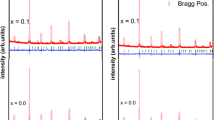
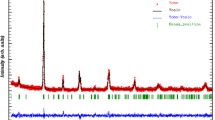
We have investigated the effect of Al doping on physical properties of \({\rm Pr}_{0.67}^{3+}{\rm Sr}_{0.33}^{2+}{\rm Mn}_{0.67-x}^{3+}{\rm Al}_{x}^{3+}{\rm Mn}_{0.33}^{4+}{\rm O}_{3}^{2-}\) manganites synthesized using the solid-state reaction method at high temperature. Rietveld refinement of XRD patterns revealed that all samples crystallize in an orthorhombic structure with Pnma space group. Magnetization measurements show that all samples exhibit a paramagnetic–ferromagnetic phase transition at the Curie temperature T C which decreases from 282 to 240 K when increasing Al content from x = 0.025 to 0.1, respectively. Electrical properties of samples have been investigated using admittance spectroscopy technique in 102–106 Hz and 100–320 K, frequency and temperature ranges, respectively. All samples exhibit a metallic behavior below the metal–semiconductor transition temperature (T M–Sc) and a semiconductor behavior above T M–Sc. The total conductance curves for our samples are found to obey Jonscher power law (G(ω) = G dc + Aω n). The activation energy (E a) increases with increasing Al content from 34.44 meV for x = 0.025 to 43.18 meV for x = 0.1. From AC conductance study, we deduced the binding energy (W m) at 100 K. Its values decrease with Al content increases.






Similar content being viewed by others
Notes
The average grain size may be estimated by using an intercept method, described as follows [20]: straight lines all the same lengths are drawn through several micrographs that show the grain structure. The grains intersected by each line segment are counted; the line length is then divided by an average of the number of grains intersected, taken over all the line segments. The average grain size is found by dividing this result by the linear magnification of the micrographs.
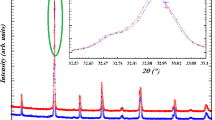
The Goldschmidt tolerance factor (t G) is a dimensional criterion which takes into account the size of the ions for characterize the different structure derived from the perovskite structures. According to this criterion, the ideal cubic structure is observed for t G = 1, the limits of stability of the perovskite phase (more or less distorted) being defined by 0.89 < t G < 1.02 [21, 22]. In particular, the rhombohedral distortions occur for 0.96 < t G < 1 and for t G < 0.96, the distortions are orthorhombic [22].
References
S. Jin, T.H. Tiefel, M. Mc. Cormak, R.A. Fastnacht, R. Ramesh, L.H. Chen, Science 264, 413 (1994)
A. Moreo, S. Yunoki, E. Dagotto, Science 283, 2034 (1999)
L. Millar, H. Taherparvar, N. Filkin, P. Slater, J. Yeomans, Solid State Ion. 179, 32 (2009)
V.N. Krivoruchko, M.A. Marchenko, Y. Melikhov, Phys. Rev. B 82, 064419 (2010)
M. Pekala, V. Drozd, J. Alloys Compd. 456, 30 (2008)
J. Zaanen, G.A. Sawatzky, J.W. Allen, Phys. Rev. 55, 418 (1985)
G.H. Jonker, J.H. Van Santen, Physica 16, 337 (1950)
C. Zener, Phys. Rev. 82, 403 (1951)
M. Muroi, R. Street, P.G. McComik, J. Appl. Phys. 87, 3424 (2000)
M. Medarde, J. Mesot, P. Lcorre, S. Rosenkranz, P. Fischer, K. Gobrecht, Phys. Rev. B 52, 9248 (1995)
J. Blasco, J. Garcia, J.M. de Teresa, M.R. Ibarra, J. Perez, P.A. Algarabel, C. Marquina, C. Ritter, Phys. Rev. B 55, 8905 (1997)
A. Mellergard, R.L. McGreevy, S.G. Eriksson, J. Phys. Condens. Matter 12, 4975 (2000)
N. Kallel, S. Kallel, A. Hagaza, M. Oumezzine, Phys. B 404, 285 (2009)
S.K. Barik, C. Krishnamoorthi, R. Mahendiran, J. Magn. Magn. Mater. 323, 1015 (2011)
Pengyue Zhang, Hangfu Yang, Suyin Zhang, Hongliang Ge, Sihao Hua, Phys. B 410, 1 (2013)
C.P. Reshmi, S. Savitha Pillai, K.G. Suresh, M. Raama Varma, Solid State Sci. 19, 130 (2013)
H. Rahmouni, B. Cherif, M. Baazaoui, K. Khirouni, J. Alloys Compd. 575, 5 (2013)
R.D. Shannon, C.T. Prewitt, Acta Crystallogr. Sect. B 25, 925 (1969)
J. Rodriguez-Carvajal, Fullprof 2000–2005, Laboratoire Leon Briouillon (CEA-CNRS)
W.D. Callister Jr, Fundamentals of Materials Science and Engineering, 5th edn. (Wiley, London, 2000)
J.M.D. Coey, M. Viret, S. Von Molnar, Adv. Phys. 48, 167 (1999)
Joël Cibert, Jean-François Bobo, Ulrike Lüders, C. R. Physique 6, 977 (2005)
V.M. Goldschmit, Geochem Verteil Elem 7, 8 (1927)
S. Hcini, S. Zemni, A. Triki, H. Rahmouni, M. Boudard, J. Alloys Compd. 509, 1394 (2011)
A. Guinier, in: X. Dunod (Ed.), Theorie et Technique de la Radiocristallographie, 3rd ed. (1964) 482
C. Vázquez-Vázquez, M.C. Blanco, M.A. López-Quintela, R.D. Sánchez, J. Rivas, S.B. Oseroff, J. Mater. Chem. 8, 991 (1998)
E. Tka, K. Cherif, J. Dhahri, E. Dhahri, J. Alloys Compd. 509, 8047 (2011)
H. Terashita, J. Neumeier, J. Phys. Rev. B 71, 134402 (2005)
P.G. Radaelli, G. Iannone, M. Marezio, H.Y. Hwang, S.W. Cheong, J.D. Jorgensen, D.N. Argyriou, Phys. Rev. B 56, 8265 (1997)
P.A. Joy, C. Raj Sankar, S.K. Date, J. Phys. Condens. Matter 14, L663 (2002)
H. Rahmouni, M. Nouiri, R. Jemai, N. Kallel, F. Rzigua, A. Selmi, K. Khirouni, S. Alaya, J. Magn. Magn. Mater. 316, 23–28 (2007)
A.K. Jonscher, Universal Relaxation Law (Chelsea Dielectics Press, London, 1996)
N.F. Mott, E.A. Davis, Electronic Process in Non Crystalline Materials (Clarendon Press, Oxford, 1979)
K. Funke, Prog. Solid State Chem. 22, 111–195 (1993)
S.R. Elliott, AC conduction in amorphous chalcogenide and pnictide semiconductors. Adv. Phys. 36, 135–218 (1987)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dhahri, A., Dhahri, J., Hcini, S. et al. Influence of Al substitution on physical properties of Pr0.67Sr0.33Mn1−x Al x O3 manganites. Appl. Phys. A 120, 247–253 (2015). https://doi.org/10.1007/s00339-015-9161-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-015-9161-9