Abstract
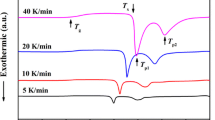
Binary Si12.5Te87.5 glass was prepared using the melt-quench technique. Differential scanning calorimetry measurements of the obtained glass measured at different heating rates (10 ≤ α ≤ 70 K/min) have shown three, one endo- and two exothermic, peaks. The glass transition kinetics have been analyzed using the isoconversional (model-free) methods in addition to the model-fitting method. The analysis of the present data shows that the glass transition kinetics are not constant values but vary with the transformed extent (x) and hence with temperature of the specimen. Non-linear decrease of E with increase in the transformed extent could be attributed to a complicated mechanism. Based on the peak shape of n(α) relation, one concludes that two competing mechanisms are working together during transformation of the solid glass to supercooled liquid state. A good agreement between the experimental and the reconstructed (x–T) curves confirms the validity of the applied models.









Similar content being viewed by others
References
S.N. Zhang, T.J. Zhu, X.B. Zhao, Phys. B 403, 3459 (2008)
L.A. Kulakova, B.A. Matveev, B.T. Melekh, J. Non-Cryst. Solids 266–269, 969 (2000)
G.E.A. Bartsch, H. Bromme, T. Just, J. Non-Cryst. Solids 18, 65 (1975)
N. Jaussaud, P. Toulemonde, M. Pouchard, A. San Miguel, P. Gravereau, S. Pechev, G. Goglio, C. Cros, Solid State Sci. 6, 401 (2004)
A. Schlieper, R. Blachnik, J. Alloys Compd. 235, 237 (1996)
S. Asokan, G. Parthasarathy, E.S.R. Gopal, J. Non-Cryst. Solids 86, 48 (1986)
S. Asokan, G. Parthasarathy, G.N. Subbanna, E.S.R. Gopal, J. Phys. Chem. Solids 47, 341 (1986)
M.K. Gauer, I. Dézsi, U. Gonser, G. Langouche, H. Ruppersberg, J. Non-Cryst. Solids 109, 247 (1989)
R.D. Vengrenovitch, S.V. Podolyanchuk, I.A. Lopatniuk, M.O. Stasik, S.D. Tkachova, J. Non-Cryst. Solids 171, 243 (1994)
S.R. Elliott, Physics of Amorphous Materials, 2nd edn. (Longman Scientific and Technical, Essex, 1990)
G.W. Scherer, Relaxation in Glasses and Composites (Wiley, New York, 1986)
J. Jackle, Rep. Prog. Phys. 49, 171 (1986)
C.A. Agnell, W. Sichina, Ann. N.Y. Acad. Sci. 279, 53 (1976)
M.H. Cohen, G.S. Grest, Phys. Rev. B 21, 4113 (1980)
P. Tuinstra, P.A. Duine, J. Sietsma, A. van den Beukel, Acta Metall. Mater. 7, 2815 (1995)
M. Abu El-Oyoun, Mater. Chem. Phys. 131, 495 (2011)
A.A. Al-Ghamdi, M.A. Alvi, S.A. Khan, J. Alloys Compd. 509, 2087 (2011)
A.M. Abd Elnaeimm, K.A. Aly, N. Afify, A.M. Abousehlly, J. Alloys Compd. 491, 85 (2010)
F. Abdel-Wahab, Phys. B 5, 1053 (2011)
A.A. Elabbar, J. Alloys Compd. 476, 125 (2009)
A.A. Abu-Sehly, Thermochim. Acta 501, 103 (2010)
Abdalla A. Elabbar, Phys. B 403, 4328 (2008)
A.A. Elabbar, J. Alloys Compd. 476, 125 (2009)
H.E. Kissinger, J. Res. Nat. Bur. Stand. 57, 217 (1956)
H.E. Kissinger, Anal. Chem. 29, 1702 (1957)
T. Akahira, T. Sunose, Res. Rep. Chiba Inst. Technol. 16, 22 (1971)
J.H. Flynn, L.A. Wall, J. Res. Natl. Bur. Stand. Sect. A 70, 487 (1966)
T. Ozawa, Bull. Chem. Soc. Jpn. 38, 1881 (1965)
S. Vyazovkin, J. Comput. Chem. 18, 393 (1997)
V.M. Gorbachev, J. Therm. Anal. 8, 349 (1975)
A.A. Joraid, Thermochim. Acta 456, 1 (2007)
B. Jankovic, J. Chem. Eng. 139, 128 (2008)
P. Budrugeac, A.L. Petre, E. Segal, J. Comput. Chem. 47, 123 (1996)
N. Sbirrazzuoli, Y. Girault, E. Elegant, Thermochim. Acta 293, 25 (1997)
P. Budrugeac, D. Homentcovschi, E. Segal, Therm. Anal. Calorim. 66, 557 (2001)
S. Vyazovkin, Anal. Chem. 74, 2749 (2002)
W. Lu, B. Yan, W. Huang, J. Non-Cryst. Solids 351, 3320 (2005)
B. Jankovic, B. Adnadevic, J. Jovanovic, Thermochim. Acta 452, 106 (2007)
S. Vyazovkin, C.A. Wight, J. Phys. Chem. A 101, 8279 (1997)
J.A. Kennedy, S.M. Clark, Thermochim. Acta 307, 27 (1997)
H.C. Yang, H.C. Eun, Y.Z. Cho, H.S. Lee, I.T. Kim, Thermochim. Acta 484, 77 (2009)
S. Vyazovkin, J. Therm. Anal. Calorim. 83, 45 (2006)
S. Vyazovkin, W. Linert, Chem. Phys. 193, 109 (1995)
S. Vyazovkin, A.I. Lesnikovich, Thermochim. Acta 128, 297 (1988)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moharram, A.H., Abu El-Oyoun, M. Glass transition kinetics of the binary Si12.5Te87.5 alloy. Appl. Phys. A 116, 311–317 (2014). https://doi.org/10.1007/s00339-013-8122-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-013-8122-4