Abstract
ZnO thin films were grown by metal-organic chemical vapor deposition on Zn- and O-polar surfaces of ZnO substrate. The effect of Zn- and O-polar substrate on the surface morphology and opto-thermal proprieties has been studied. Hall-measurements were used to determine the carrier concentration of the deposited films. Photothermal deflection spectroscopy (PDS) was used to determine the optical absorption spectrum and the gap energy by comparing experimental amplitude of the photothermal signal to the corresponding theoretical one. Thermal conductivity and diffusivity were also deduced from the photothermal deflection measurements. The found values were very low due to the thermal resistivity of the layer–substrate interface.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
ZnO has gained increasing attention due to its interesting applications in electronics and optoelectronics, especially as transparent conducting oxide (TCO), and also as nanostructured material. Its large exciton binding energy (60 meV) makes it highly attractive for applications as blue and ultraviolet light emitters [1]. Several growth techniques such as metal-organic chemical vapor deposition (MOCVD) [2], sputtering [3], pulsed laser deposition [4] and molecular beam epitaxy [5] have been employed. Among them, MOCVD method provides the advantage of growing high-quality films due to its versatility in controlling the various thermodynamic interactions. Good knowledge of the vibrational properties of this material could help to understand the effect of the substrate on deposited ZnO films. The key factor to assure the performance of semiconductor devices is their good crystalline qualities.
Microstructure, morphology and surface roughness affect strongly the properties of the thin solid film. The appearance of defects due to lattice mismatch introduced by the substrate, largely affects the optical and vibrational properties of ZnO films [6]. Sapphire substrates are commonly used to elaborate ZnO films [7], but the best results have been reported for homoepitaxially grown films [8]. Moreover, in the case of C-axis ZnO substrate, we can distinguish between the O- and Zn-terminated faces. This polarity could strongly affect the quality of the deposited layer. Characterization of thermal properties of thin films is very important for electronic, photoelectronic and microelectronic sciences and technologies. Different photothermal techniques have become popular for the characterization of thin films such as photoacoustic (PA) technique [9], transient thermal grating (TTG) method [10] and piezoelectric technique [11].
In this work, photothermal deflection spectroscopy (PDS) measurements have been used for investigating optical and thermal properties of semiconductors. PDS is a powerful technique to study opto-thermal properties of ZnO samples with high transparency. The advantage of the PDS is the sensitivity to a very small optical absorption in a highly transparent sample. We have obtained the optical absorption spectrum, gap energy, thermal conductivity and thermal diffusivity of the ZnO layer by comparing the experimental amplitude and phase of the photothermal signal to the corresponding theoretical ones.
The effect of substrate polarization on surface morphology, optical and thermal properties of ZnO thin films was studied. Hall-measurements were used to determine the carrier concentration of the deposited films.
2 Experiment
ZnO thin films were grown using metal-organic chemical vapor deposition (MOCVD) in a horizontal reactor. Diethylzinc and tertiary-butanol were used as zinc and oxygen sources, respectively; organometallic bubblers were fixed at 12 °C for diethylzinc and 28 °C for tert-butanol. The carrier gas was helium. The growth temperature was 420 °C and the pressure was 50 torr. ZnO layers were grown on ZnO substrates. The thickness of the deposed ZnO layer was about 3.5 μm for the two ZnO layers, much thinner than the substrate: 0.4 mm.
The morphologies have been studied using a JEOL840 scanning electron microscope (SEM).
The photothermal deflection spectroscopy experimental device is describe elsewhere [12] and depicted in Fig. 1. A monochromatic light beam coming out of a monochromator illuminated with 250 W halogen lamp and modulated with a mechanical chopper at a frequency f is focused on the sample surface. The absorption of the pump beam by the sample generates a thermal wave that propagates in the sample and in the deflecting fluid (CCl4 or air), leading to the deflection of a probe laser beam (He–Ne) skimming the sample surface. This deflection is detected with a photodetector linked to a lock in amplifier giving us the amplitude and phase of the measured PDS signal; the principle of this technique is shown in Fig. 2.
The normalized amplitude is obtained from the ratio of the amplitude measured for a given semiconductor sample to the reference amplitude obtained for a carbon black sample. The probe beam deflection is given by Ψ=L/n(dn/dT)σ f T 0 [13], where n is the fluid refractive index, z 0 the distance between probe beam axis and the sample surface, L the sample length; \(\sigma_{f} = (1 + j)/\mu_{f}, \mu_{f} = \sqrt{D_{f}/\pi F}\) is thermal diffusion length in the fluid, f the frequency of the heating beam and D f the thermal diffusivity. We notice that the deflection is proportional to the complex temperature expression T 0 at the sample surface.
This temperature T 0 is calculated by solving the one-dimensional heat equation in the different media: the sample, the backing and the surrounding fluid.
The obtained surface temperature T 0 is given by

The thermal and optical parameters that appear in the above expression are given in Ref. [14].
3 Results and discussion
3.1 Zn- and O-polar substrate surface effect
Figures 3(a) and (b), shows the top view SEM images of ZnO thin films grown on Zn-polar (S1) and O-polar (S2) ZnO substrates, respectively. The morphology of O-polar ZnO surface (sample S2) shows grains with different sizes. In contrast, the film morphology of Zn polar surface (sample S1) is different: it constitutes of random platelets resembling rose; this morphology has also been observed by Fan et al. [15].
3.2 Photothermal deflection measurements
In Fig. 4, the amplitude curves of photothermal signal obtained for the samples S1 and S2 are drawn. The amplitude shows two saturations zones corresponding respectively to high and low optical absorption coefficient. For energy higher than the gap energy (λ<0.37 μm), the sample is optically opaque (maximum amplitude) and the amplitude saturates. Then it decreases toward a second saturation when the sample becomes transparent. For the sample S1 we notice that the amplitude is higher in the transparency region.
3.2.1 Absorption spectrum
The comparison of experimental curves of amplitude versus wavelength to theoretical amplitude versus absorption coefficient in Fig. 5 allows obtaining the optical absorption spectrum for the two samples shown in Fig. 6.
We notice that for sample S1, ZnO(O) has a relatively higher absorption in the transparency region than for S2. This is probably due to the contribution of free carriers to optical absorption. Indeed, Hall effect measurements show that free carrier concentration is higher for S1 than for S2 (Table 1).
3.2.2 Determination of the gap energies
In order to obtain the gap energy E g from optical spectrum, we have used the Tauc method [15]. For excitation energies where E=hν is higher than E g, the quantity (αE)n should have linear variations with E, following the Tauc law: (αE)n=β(E−E g), where α is an absorption coefficient and β is a constant, and case n=2 is for direct band gap semiconductors such as ZnO. It is found that values of the optical energy gaps decrease from 3.38 eV for the sample ZnO (O) to 3.34 eV for the sample ZnO(Zn), as shown in Fig. 7. The change from O- to Zn-polar surface causes a slight blue shift of gap energy. As discussed in the Raman spectra by Souissi et al. [6], the disorder and lattice defects are the main reason of this shift in gap energy. It is also known that the energy band broadening (blue shift) would be caused by the lifting of the Fermi level into the conduction band of the degenerated semiconductor. This phenomenon is well known as the Burstein–Moss effect [16]. It may be attributed to intrinsic donor defects such as donor vacancies and Zn interstitials.
4 Photothermal properties
Theoretical simulations consist of varying simultaneously the values of thermal conductivity and diffusivity in order to fit theoretical curves to experimental ones [17]. The best values of the two parameters for each ZnO samples are reported in Table 1.
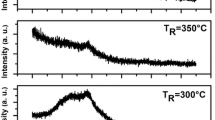
Figure 8 presents the curves of the amplitude of the photothermal signal versus square root of frequency, for the two ZnO layers. These curves have the same behavior, but we notice small differences in the slope at low and high frequencies, and also small shifts of the maximum. From the fitting of the theoretical curves obtained by the model given in expression (1) to experimental amplitude, we have obtained the values of thermal diffusivity and conductivity for each ZnO layer, reported in Table 1.
The average thermal diffusivity of the ZnO samples is calculated from the values given in Table 1. The obtained value D=7.9×10−2 cm2 s−1 agrees with the diffusivity D=8.1×10−2 cm2 s−1 received by other authors [10].
We notice that thermal diffusivity depends on the substrate polarization. Indeed, the roughness of ZnO(O) is more pronounced than that of ZnO(Zn) (Fig. 7), which leads to reduction of thermal diffusivity.
Thermal conductivity (k) is a kinetic property determined by the contributions from the rotational electronic and vibration degrees of freedom.
Thermal conductivity generally decreases with increasing carrier concentration, because the decrease in the lattice component of k due to increased phonon scattering from both impurities and free electrons outweighs the increase in the electronic contribution to k [18].
In our case, analysis of the experimental data reveals that thermal conductivity decreases from S1 to S2; this deviation may be explained by the zinc excess and the deficiency of oxygen in interstitials sites. The presence of zinc creates additional phonon scattering centers, and results in increasing of thermal resistance.
5 Conclusion
ZnO thin films are grown by metal-organic chemical vapor deposition on Zn- and O-polar surfaces of ZnO substrate. The effect of the substrate on the crystalline structure, surface morphology and opto-thermal properties is investigated by different techniques such as SEM, Hall effect measurements and phothermal deflection spectroscopy. The polarization of ZnO substrate causes a slight blue shift of gap energy that may be attributed to intrinsic donor defects and leads to a reduction of thermal diffusivity and conductivity at the same time, which can be explained by the excess of zinc and the deficiency of oxygen in interstitials sites.
References
D.C. Look, Mater. Sci. Eng. B 80, 383 (2001)
A. Markouki, A. Lusson, F. Jomard, A. Sayari, P. Galtier, M. Oueslati, V. Sallet, Journal of Crystal Growth 312, 3063 (2010)
B.J. Kwon, H.S. Kwack, S.K. Lee, Y.H. Cho, D.K. Hwang, S.J. Park, Appl. Phys. Lett. 91, 061903 (2007)
H. Kim, A. Cepler, M.S. Osofsky, R.C.Y. Auyeung, A. Piqué, Appl. Phys. Lett 90, 203508 (2007)
Y.S. Jung, W.K. Choi, O.V. Kononenko, G.N. Panin, J. Appl. Phys. 99, 013502 (2006)
A. Souissi, C. Sartel, A. Sayari, A. Meftah, A. Lusson, P. Galtier, V. Sallet, M. Oueslati, Solid State Communications 152, 794–797 (2012)
A. Souissi, A. Marzouki, A. Sayari, V. Sallet, A. Lusson, M. Oueslati, Journal of Raman Spectroscopy 42, 1574–1577 (2011)
M. Losurdo, M.M. Giangregorio, P. Capezzuto, G. Bruno, G. Malandrino, M. Blandino, Super. and Micros. 38, 291 (2005)
T. Terasako, T. Yamanaka, S. Yura, M. Yagi, S. Shirakata, Thin Solid Films 519(5), 1546–1551 (2010)
X. Dong, M. Di, Z. Shu-Yi, L. Ai-Hua, K. Wasa, Chin. Phys. Lett. 25(1), 176 (2008)
T. Kakenoa, K. Sakaia, H. Komakia, K. Yoshinoa, H. Sakemib, K. Awaic, T. Yamamotod, T. Ikaria, Materials Science and Engineering B 118, 70–73 (2005)
F. Saadallah, N. Yacoubi, F. Genty, C. Alibert, Journal of Applied Physics 94(8), 5041–5048 (2003)
A.R. Warrier, T. Sebastian, C. Sudha Kartha, K.P. Vijayakumar, J. Appl. Phys. 107, 073701 (2010)
S.E. Aw, H.S. Tan, C.K. Ong, J. Phys: Condens. Matter 3, 8213 (1991)
J. Tauc, R. Grigorovici, A. Vancu, Phys. Status Solidi 15, 627 (1966)
F.K. Shan, G.X. Liu, W.J. Lee, G.H. Lee, I.S. Kim, B.C. Shin, Y.C. Kim, J. Cryst. Growth 277, 284 (2007)
T. Ghrib, N. Yacoubi, F. Saadallah, Sensors and Actuators A 135, 346–354 (2007)
D.I. Florescu, V.M. Asnin, F.H. Pollak, R.J. Molnar, C.E.C. Wood, J. Appl. Phys. 88, 3295 (2000)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Ktifa, S., Souissi, A., Saadallah, F. et al. Photothermal investigation Study of ZnO thin films: effects of Zn and O polar substrate. Appl. Phys. A 114, 559–563 (2014). https://doi.org/10.1007/s00339-013-7614-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00339-013-7614-6