Abstract
Antipathes galapagensis is a prevalent habitat-forming black coral in subtidal ecosystems of the Galápagos Marine Reserve (GMR). Despite their ecological importance and status as a CITES-regulated order, little is known about their depth distribution, population structure and ecology in the GMR. Surveys were conducted in 2021 and 2022 at 9 sites in the central Galápagos Archipelago to investigate how black coral densities, occupancy, size, habitat utilization, and epizoan overgrowth varied between 2.0 and 20.0 m depth. The shallowest black corals occurred at 3.4 m depth, one of the shallowest occurrences of an Antipathes spp. in the world. Coral density increased with depth, with a maximum density of 5.2 colonies per m2 observed across the depth range surveyed. Occupancy modeling also yielded curves with increasing probabilities of black coral presence with depth at all sites. Colony height increased with depth at 8 out of 9 sites and was positively correlated with coral density at 6 of 9 sites. Overall, 47% of colonies surveyed occupied cryptic habitats and 53% were attached to exposed substrate, but black coral habitat usage patterns varied with depth at 5 of 9 sites. At these sites, colonies on shallower transects (5.0, 10.0 m depth) more frequently displayed cryptic habitat usage while colonies along the deeper transects (15.0, 20.0 m) were more often exposed. In general, coral density, probability of occupancy, height, and exposed habitat utilization increased with depth, while the average degree of overgrowth and number of epibiont taxa were unrelated to depth. Five hypotheses regarding factors potentially limiting the shallow (upper) depth distribution of A. galapagensis—ranging from negative impacts of the physical environment to high predation on exposed substrates—are presented for future testing. These results provide a comprehensive ecological characterization of Galápagos black coral populations that can be used to assess the impact of future environmental change and applied to management decisions for this key marine foundation species in the GMR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Black corals (Order: Antipatharia) are distributed from the tropics to the poles and are represented by approximately 273 species (Wagner et al. 2012a; Brugler et al. 2013; Opresko 2020). Primarily a deep-water group, 75% of antipatharians have distributions restricted to depths below 50 m (Grigg 1965; Cairns 2007; Wagner et al. 2012a). To date, the shallowest reported populations of black corals occurred at 4.0 m depth in both the New Zealand fiords and the northeastern waters of Hong Kong (Grange 1985; Chui and Ang 2015). Black corals are known to be important foundation species (Dayton 1972; Ellison et al. 2005; Bo et al. 2009; Bosch et al. 2023), providing spatially heterogeneous habitat for species-rich communities living on or between the branches of their tree-like skeletons (Parrish et al. 2002; Sala et al. 2003; Buhl-Mortensen and Mortensen 2004; Boland and Parrish 2005; Love et al. 2007; Tazioli et al. 2007; Bo et al. 2012; Tapia-Guerra et al. 2021). They also represent a major type of marine animal forest within the mesophotic zone (> 30 m to 150 m depth) (Rossi et al. 2017; Bo et al. 2019; Bosch et al. 2023).
As slow-growing and long-lived organisms, black corals can be highly sensitive to disturbance without selective management strategies or an understanding of regional population structure and dynamics (Grange 1985; Grange and Singleton 1988; Grigg 1993; Wagner et al. 2012a). Black coral beds have been listed as Vulnerable Marine Ecosystems (FAO 2009; Cannas et al. 2019) that are threatened and declining (OSPAR 2008), requiring special protection under the Convention of Biological Diversity (UNEP 2007; Aguilar and Marín 2013). Furthermore, all black coral species were listed in CITES Appendix II in 1981 due to their lucrative commercial value and continued overexploitation in certain regions (Grigg 2001; Tsounis et al. 2010; Todinanahary et al. 2016; CITES 2022).
Antipathes galapagensis (Deichmann 1941) has a broad geographic range spanning the tropical and subtropical Eastern Pacific (Lavorato et al. 2021). In the Eastern Tropical Pacific (ETP), the reported Antipathes galapagensis depth distribution ranges from 10 to 90 m, with records indicating the presence of the species down to at least 200 m (Kerstitch 1989; Hickman 2008; Bo et al. 2012; Lavorato et al. 2021). Antipathes galapagensis occurs at depths of 10 to 40 m off coastal Ecuador (Machalilla National Park, Isla de la Plata), where it attains maximum densities of 3.2 colonies per m2 at 25 m depth (Bo et al. 2012). Historically, A. galapagensis was also an important target of the coral jewelry industry in the ETP. Persistent harvesting pressure led to the Mexican government designating endangered status for the coral in 2019 (NOM-059-SEMARNAT 2010) and, in the Galápagos, collection threatened populations into the 1990s (Martinez and Robinson 1983; Romero 1997). Despite the historic harvesting pressure, A. galapagensis beds in the Galápagos Archipelago likely represent some shallowest and densest known along their established and predicted geographic range (Lavorato et al. 2021).
Despite their role as foundation species and threatened status, there are limited studies of antipatharian population ecology and distribution likely due to the inaccessibility of populations that predominantly occur in deep water. Here, we took advantage of the uniquely shallow distribution of black corals in the central Galápagos Archipelago to investigate their vertical zonation, population structure, habitat utilization, and associated sessile invertebrate and algal epibiont communities. Our main study objectives were to: (1) define the upper, shallow limit of the depth distribution of A. galapagensis in the central Galápagos; (2) quantify patterns of black coral density, occupancy, colony size, habitat utilization, and epibiotic communities across environmental gradients; and (3) construct hypotheses concerning the mechanisms limiting the upper depth limit of black corals.
Materials and methods
Study sites
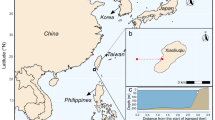
This research was conducted between 2.0 and 20.0 m depth at nine sites within the central Galápagos Marine Reserve (GMR, Fig. 1). Sites were selected based on prior knowledge of black coral colonies in the area and were sampled opportunistically from July–August 2021 during concurrent subtidal ecological monitoring efforts in the GMR (Witman et al. 2010). A maximum working depth of 20.0 m was selected due to logistical constraints of SCUBA diving, and all study sites were characterized by vertical rock walls or sloping ledges with narrow terraces across the entire depth range surveyed (Witman et al. 2010; Castelló y Tickell et al. 2022). Temperature profiling was conducted directly adjacent to each site in January 2020 (five of nine sites), July–August 2021 (all nine sites), and January 2022 (five of nine sites) using a SonTek CastAway® Conductivity Temperature Depth (CTD) (Fig. 2). Casts were deployed up to 80.0 m depth during each time point to characterize differences in vertical water temperature gradients (Fig. 2) in the zone where black coral populations were studied.
Population surveys
Band transects and surveys for occupancy modeling were used to quantify the population ecology of black corals along the depth gradient. Band transects were 5.0 m long by 1.0 m wide and were sampled at 4 depths: 5.0 m, 10.0 m, 15.0 m, and 20.0 m. At each depth, replicate band transects were surveyed along a line laid out horizontally across the substrate along the depth contour. Each transect was separated from its nearest neighbor by at least 0.5 m to achieve spatial independence among replicates. Replicate transects were surveyed at each depth to assess the degree of patchiness within Galápagos black coral populations, and though the number of replicate band transects varied by site, it did not change between depths within each site (Table S1).
A total of 656 A. galapagensis colonies were surveyed on band transects across the GMR. Along each band transect, all A. galapagensis encountered were counted and the height of each colony was measured from colony base to the end of the longest branch tip. Density data (colonies / m2) from these transects represent a common currency for comparing the black coral densities measured here to complementary studies. Additionally, patterns of black coral habitat utilization were determined by scoring each coral as either cryptic, with the colony base attached to the substrate in a hole or crevice, or exposed, with the colony base attached on open substrate (Fig. 3). The percentage of each colony overgrown by sessile invertebrate and algal epibionts was visually estimated as 0%, 20%, 40%, 60%, 80%, or 100 percent overgrowth. Any encrusting epibionts (invertebrates, algae) encountered were identified into 11 functional groups: ascidians, crustose coralline algae, ahermatypic corals, green filamentous algae, hydroids, mollusks, polychaete worms, red filamentous algae, sponges, tunicates, and zoanthids. The term commensals (Buhl-Mortensen et al. 2010) is used to refer to the epibiotic invertebrate and algal species associated with black coral habitat, as the facultative or obligate nature of their interaction with A. galapagensis is unknown. For consistency, all overgrowth estimation and epibiont identification were performed by one observer (M. Agarwal).
Occupancy modeling involved sampling large numbers of small quadrats for the presence or absence of black corals (Bailey et al. 2014). To build a depth distribution model for A. galapagensis via occupancy modeling, small square areas (15.0 cm by 15.0 cm) were surveyed every 30 cm along a 5.0-m transect. Within each square, the presence or absence of a black coral colony was noted. Occupancy transects at each site were conducted from 2.0 to 20.0 m depth at finer depth intervals than the band transects (Table S1), generating continuous data for fitting curves that defined the shape of the depth distribution as the probability of a space being occupied by a black coral at any given depth.
Statistical analyses
Both nonparametric and parametric approaches were used to determine how black coral populations varied across sites and depths in the GMR. Replicate transects were pooled across depths within each site, and Kruskal–Wallis tests were used to analyze differences in median coral density and the number of commensal functional groups encrusting coral colonies. Post hoc Dunn tests identified pairwise differences when the overall Kruskal–Wallis statistic was significant. Coral height data was Box–Cox-transformed (λ = 0.4) to satisfy assumptions of normality (Shapiro–Wilks test; p = 0.057) and homogeneity of variance (Levene’s test; p = 0.0733), and ANOVA and Tukey-HSD post hoc tests were performed to analyze differences in mean coral sizes. Linear regression models and Pearson’s correlation coefficient were utilized to analyze the relationship between coral density and size. Fisher’s exact tests and pairwise Fisher’s exact tests were used to analyze black coral overgrowth and habitat utilization. Variation about the means is reported throughout the paper as ± standard error (SE) in parentheses following the mean.
Site occupancy modeling of animal distributions can use approaches similar to logistic regression (MacKenzie et al. 2002). The occupancy model created here for black coral distributions at each site assumes binary logistic relationships between depth and the presence or absence of a coral, following the binomial density function:
where \(Y_{i}\), a positive detection of a black coral colony, is a function of the probability of coral colony occurrence, \(\psi_{i}\), and depth, \(D_{i}\). Here, the binomial relationship for coral occupancy is represented as:
Positive black coral detection (\(Y_{i}\)) is therefore a product of the probability of coral colony occurrence (\(\psi_{i}\)) and the probability of coral colony observation (\(p_{i}\)) across survey depths (\(D_{i}\)). Thus, a zero detection probability occurred when there was no colony present or no coral colony observed in each 15.0 cm-by-15.0 cm survey box. An encounter with a black coral was deemed as “likely” when the occupancy detection probability was greater than 0.3, following the assumption that A. galapagensis is a patchily distributed species (Lavorato et al. 2021). All analyses of data and models were carried out in R version 4.2.2, and significance was set at 5% (α = 0.05) (R Core Team 2020).
Results
Coral density
Antipathes galapagensis density ranged from 0 to 5.2 colonies per 1.0 m2, with significant differences in median colony density between sites (Kruskal-Wallis; H(8) = 24.1, p = 0.00217). The highest average coral density occurred at Roca Cousins (1.98 土 0.59 colonies per 1.0 m2), while the lowest population density was found at Gordon (0.45 土 0.18 colonies per 1.0 m2). A. galapagensis density varied significantly with depth at all sites (Kruskal-Wallis), with the exception of Guy Fawkes (Table 1). Average coral density was generally lowest at the shallowest depths surveyed and increased significantly and nonlinearly with depth (Fig. 4). After correcting for multiple comparisons, Dunn tests revealed that the majority of significant differences in coral densities with depth stemmed from differences between coral densities at the shallowest transect (5.0 m depth) and at the two deepest transects (15.0 m and 20.0 m) (Table S2).
Changes in mean black coral A density and B untransformed colony height with depth within the central GMR. Error bars denote the standard error of the mean. Sites with stars (*) indicate locations where each black coral population metrics varied significantly with depth as determined via Kruskal–Wallis tests for density and Tukey HSD tests for colony height
Moderate variation about mean coral densities (Fig. 4), as well as large differences in density values between adjacent transects within each depth category (Fig. S1.), signified that the distributional pattern for A. galapagensis is relatively patchy across the depth range examined at all sites. The densities of A. galapagensis varied by as much as 800% between 5.0 m2 transects, though less variation between subsequent replicates was observed at the shallowest depth (5.0 m), as very few corals were found overall (Fig. S1.). Deeper (10.0 m, 15.0 m, 20.0 m), there was more variation in density values within each depth, often exceeding a 50% change between consecutive transects (Fig. S1). Transects where black corals were present often contained high colony abundances, but transects surveyed outside of those areas had reduced density values. The high magnitude of difference in density values between adjacent transects—especially at depths where black corals were common—reflects the patchiness of the population and indicates considerable heterogeneity in coral abundance between replicates. This suggests that the degree of separation between transects (0.5 m) was sufficient to capture variability in black coral densities within the depth range examined and that replicates represent different, independent samples of the subtidal habitat.
Occupancy
Occupancy modeling revealed that the probability of detecting a black coral colony on any 0.0225 m2 area of rock substrate increased with depth at all 9 sites (Fig. 5), corroborating the observed pattern of increased coral density along the depth gradient from transect-based sampling (Table 1, Fig. 5). The shallowest depth that black corals were found was 3.4 m at Guy Fawkes. At all other sites, the shallow distribution limit ranged from 6.0 to 13.5 m depth. These observed shallow limits corresponded to low coral occupancy probabilities (2.4 to 14.8%) under the models (Table 2). The depth at which the model predicts a “likely” (≥ 30%) probability of black coral occupancy also varied by site (Table 2). Interestingly, site-specific depths at which black corals became “likely” as indicated by the model occurred remarkably close to the depths at which diver-surveyed band transects also showed increases in population density. This alignment between model outputs and in situ band transect-based surveys suggests that the occupancy model was effective in predicting black coral distribution patterns across the depth gradient at all sites.
Colony height
Antipathes galapagensis colonies ranged from 4.0 to 140.0 cm tall. Average colony height varied significantly between sites (ANOVA; F[8, 647] = 7.77, p = 5.51e−10). Corals at Guy Fawkes were the largest (64.1 土 2.6 cm), and corals at Gordon were the smallest (35.9 土 2.7 cm). At nearly all sites, colony height increased significantly with depth (ANOVA, Fig. 4, Table 1, Table S3). The Gardner site was the sole exception. As with coral density, significant pairwise differences in mean colony height occurred most often between the shallow transects (i.e., 5.0 and 10.0 m) and their deeper counterparts (i.e., 15.0 and 20.0 m) (Tukey HSD, Table S3).
When pooled at the site level, black coral height was positively correlated with coral density at six locations: Baltra (Pearson’s r (51) = 0.4, p = 0.0039), Champion (Pearson’s r (63) = 0.29, p = 0.021), Daphne (Pearson’s r (142) = 0.37, p = 0.0000055), Gordon (Pearson’s r (27) = 0.5, p = 0.0075), Roca Cousins (Pearson’s r (119) = 0.22, p = 0.017), and Rocas Beagle (Pearson’s r (39) = 0.43, p = 0.0066) (Fig. 6). Significant linear regression models for these sites predicted that transects containing higher numbers of black corals also contained corals that were larger. Transect depth was not integrated into correlation analysis due to a paucity of replicated density estimates (Figure S1) available for each site-depth combination.
Positive relationships between black coral density and height, with linear regressions and Pearson’s correlation coefficient (r) displayed for each significant relationship (p < 0.05). Black coral height was positively correlated with coral density at six sites: Baltra (Pearson’s r(51) = 0.4, p = 0.0039), Champion (Pearson’s r(63) = 0.29, p = 0.021), Daphne (Pearson’s r(142) = 0.37, p = 0.0000055), Gordon (Pearson’s r(27) = 0.5, p = 0.0075), Roca Cousins (Pearson’s r(119) = 0.22, p = 0.017), and Rocas Beagle (Pearson’s r(39) = 0.43, p = 0.0066)
Overgrowth
Epibionts were found overgrowing black corals at all sites. There were significant differences in the amount of overgrowth per coral colony between sites (Fisher’s Exact test; p = 0.0005, Table S4). Colonies living at Champion and Pinzon had the highest overgrowth per colony, with an average of 19.0 土 2.9% and 14.9 土 2.9% of each colony overgrown by commensals, respectively. Baltra (2.45 土 1.3%) and Gardner (2.8 土 1.2%) had the lowest proportions of average overgrowth per colony. Despite differences in the degree of colony overgrowth between sites, Fisher’s exact tests revealed that degree of overgrowth did not vary with depth within each site, with the exception of Guy Fawkes (Table S4). At Guy Fawkes, the average proportion of overgrowth by epibionts and algae on black coral colonies increased with depth.
Epibiont community composition
When commensal organisms were found overgrowing black coral colonies, the most common functional groups were sponges (on 20.7% of corals), red filamentous algae (on 10.2% of corals), crustose coralline algae (on 6.2% of corals), and hydroids (on 5.11% of corals). All other epibiont and algal functional groups were identified on less than 5.0% of encrusted A. galapagensis colonies. The median number of commensal functional groups overgrowing black coral colonies varied across study sites (Kruskal–Wallis; H(8) = 46.4, p < 0.001). Rocas Beagle contained corals hosting the highest average number of commensal functional groups per coral colony (2.7 土 0.35 functional groups) and corals at Gordon had the fewest average epibiont groups per coral (1 土 0). Importantly, sites with the highest estimated degree of overgrowth did not necessarily have the most varied epibiont communities in terms of the number of unique functional group identities. The number of epibiont functional groups overgrowing black corals was also unrelated to the depth of colonies at 8 out of 9 sites (Table 1, Table S5). At Daphne, the one site with differences in the number of functional groups between depths, the median number of epibiont groups overgrowing black coral colonies significantly decreased with depth (Kruskal–Wallis; H(2) = 17.3, p < 0.001).
Habitat utilization
Habitat use by A. galapagensis, qualified as either cryptic or exposed based on the locality of the colony base (Fig. 3), varied within the GMR. Overall, 47% of colonies surveyed were classified as cryptic and 53% were exposed. Daphne had the lowest mean proportion of exposed colonies across transects (0.097 土 0.044) and Baltra had the highest mean proportion of exposed colonies (0.89 土 0.067). A Fisher’s exact test revealed significant differences in habitat utilization of black coral colonies across sites (p = 0.005, n = 649).
As with metrics of abundance and size, black coral habitat usage varied with depth. The proportion of exposed colonies was positively correlated with depth at five sites: Roca Cousins, Rocas Beagle, Guy Fawkes, Pinzon, and Champion (Table S6). At these sites, a higher proportion of colonies living along shallower transects (5.0, 10.0 m depth) displayed cryptic habitat usage, where the majority of each colony’s branches and base were tucked into the habitat matrix. Along the deeper transects (15.0, 20.0 m depth) at these sites, the opposite was true; colonies were attached to exposed substrate rather than within crevices or holes. These results indicate that black coral habitat usage changes significantly across the depth gradient, where black corals occupy cryptic habitats closer to the surface and more exposed habitats as depth increases.
Discussion
Characterizing how populations vary across environmental gradients is an important requirement for understanding the fundamental niches of organisms and their response to changing climate conditions (Hutchinson 1957; McGill et al. 2006). In the subtidal zone, depth is a proxy for changing environmental factors such as light, temperature, water flow, and physical disturbance (Witman and Dayton 2001). In terms of antipatharian populations, there is limited information about the drivers of black coral distributions, especially regarding the upper, shallow depth limit that bounds the extent of their vertical range (Kahng et al. 2010). We addressed this knowledge gap by presenting an ecological characterization of shallow-water (2.0–20.0 m) black coral populations in the central Galápagos Archipelago.
The observed upper limit to the depth distribution of A. galapagensis populations at 3.4 m is shallower than previously published estimates for this species in the region (Lavorato et al. 2021). At some sites, the occupancy model predicted a high probability of detection at depths of less than 5.0 m, indicating that black coral distributions across the central Galápagos Archipelago are among the shallowest in the world (Grange 1985; Tazioli et al. 2007; Risk et al. 2009; Williams and Grottoli 2010; Chui and Ang 2015). As the depth range of this species extends from the shallow subtidal to mesophotic depths (Bo et al. 2012, 2019), A. galapagensis populations likely provide vertical connectivity in habitat structure and biodiversity between shallow and deep-water benthic communities (Bo et al. 2012). Considering that black corals create benthic and pelagic coupling by feeding on plankton in the water column and building biogenic habitats (Kahng et al. 2014), future investigation should focus on the role of antipatharians in influencing nutrient and energy fluxes across depth gradients.
Surveys also revealed prominent differences in A. galapagensis population metrics—namely colony density, size, and habitat usage—across the depth gradient. Black coral recruitment and persistence are impacted by a host of factors that may lead to differential coral survival across depths, which in turn may set shallow distributional limits and explain ecological patterns (Wagner et al. 2012a). We found that black corals living along the shallowest edge of their depth distributions were typically less abundant, smaller, and occupied more cryptic habitats than black corals living deeper. Conversely, deeper populations were larger, more densely aggregated, and less cryptic. The highest density of black corals reported here—4.0 colonies per 1.0 m2 at 20 m depth at Rocas Cousins—is higher than the maximum density of A. galapagensis reported off coastal Ecuador (Machalilla National Park, 3.2 colonies per 1.0 m2, Bo et al. 2012), and is greater than any Antipathes spp. population density reported in a review of black coral ecology by Wagner et al. (2012a). Given the increasing density of black corals with depth, it is also likely that maximal A. galapagensis densities are higher than the values reported here from 20.0 m depth, but sampling would require advanced diving or submersible technology.
Black coral populations also showed a density-dependent relationship at the site level: higher coral densities were correlated with larger colony sizes. Since both colony height and density increase with depth, it is possible that the same environmental factor linked to depth drives both trends. This pattern has been previously documented for Caribbean black corals, where Oakley (1988) found that Antipathes colonies in high-density beds were thinner and taller than corals in scattered, less dense aggregations. The mechanism underlying these patterns may be explained by intraspecific competition for plankton, where larger and taller colonies achieve higher plankton capture than smaller, shorter colonies. Alternatively, the positive density and height relationship may indicate an Allee effect, where positive increases in density correspond to positive increases in fitness (Stephens et al. 1999). Aggregations of individuals could provide benefits to the population through the consolidation of reproductive potential, since antipatharian black corals have been shown to be highly philopatric with short dispersal distances (Miller 1998). Aggregations of colonies might also provide a feeding advantage to black coral polyps if colony branches are able to slow water velocity or form eddies around polyps, as has been shown in other antipatharian species (Warner 1981). Reduced current velocity and small-scale turbulence would thereby enhance the frequency of direct prey interception by polyps, increasing the success of particulate capture. While both mechanisms (i.e., intraspecific competition, Allee effects) potentially explain the density-colony height pattern documented here, they cannot be disentangled without further research as increases to both colony density and height with depth may suggest similar forces driving the observed pattern. Colonies at depth could also be older, explaining their larger size. Though the growth rate of A. galapagensis is unknown, a positive correlation between coral colony height and age has been established for other antipatharian species (Grange 1985; Grigg 1976).
The lack of difference in the degree of coral overgrowth by epibionts across the depth gradient may indicate that, regardless of depth, there is a maximum epibiont load that a black coral can support before it is negatively impacted by the commensal communities it hosts. Epibiotic load is particularly problematic for shallow corals because epibionts increase drag (Witman and Suchanek 1984; Wahl 2008), which would increase colony dislodgement in turbulent, high velocity environments such as those in the shallow GMR (Lamb et al. 2020). At Guy Fawkes, the only site with significant differences in overgrowth across depth, overgrowth was higher along deeper transects, potentially indicating that colonies with high levels of overgrowth in shallow environments are simply unable to persist.
Globally, black corals are characterized as important refugia for biodiversity due to the biogenic habitat that they create (Buhl-Mortensen and Mortensen 2004; Bosch et al. 2023). In the Galápagos and other areas of the Eastern Tropical Pacific, A. galapagensis is known to harbor diverse assemblages of vertebrates and invertebrates (de Ridder and Holthuis 1979; Humann and DeLoach 2003; Reimer and Fujii 2010). Here, we show that A. galapagensis colonies host a wide variety of algal and sessile invertebrate functional groups as epibionts, indicating its role as a foundation species in the GMR. Future taxonomic sampling of black coral commensal species would provide a refined understanding of the role that A. galapagensis plays as a foundation species and would indicate the specific ecosystem services that black coral beds in the GMR provide. Despite the patchiness in coral densities observed between replicate transects within each depth classification, A. galapagensis was still the most conspicuous biological habitat provider across all sites surveyed, creating alternative, architecturally complex habitat in an otherwise exposed and space-limited environment (Witman and Smith 2003).
With some exceptions, there were disproportionate increases in black coral density and height, as well as shifts in habitat usage, between 10.0 and 15.0 m depth. These breaks, and the restriction of black coral populations at the shallow end (< 5.0 m depth) of their distribution, could be explained by five logical hypotheses: high water temperatures (H1), hydrodynamic forces (H2), light levels (H3), predation on exposed colonies (H4), or restricted larval dispersal or recruitment (H5).
Evaluating each hypothesis in turn (H1–H5), we find limited evidence for H1, that high water temperatures in shallow water prevent A. galapagensis from living at depths above 5.0 m. The temperature-depth profiles sampled during the warmest season in the GMR (January, Fig. 2.) indicate temperatures ranging from 23 to 29°C, which seem unlikely to cause black coral mortality. Kahng et al. (2012) hypothesize that sustained water temperatures less than 22°C set the deep distributional limit of Antipathes in Hawaii by preventing gametogenesis and inhibiting reproduction, but the converse—that high water temperatures limit coral reproduction at shallow depths—appears unlikely given that warm water exposure is positively correlated with reproductive duration, recruitment levels, and fecundity in many warm water anthozoans, including antipatharians (Tsounis et al. 2006; Gori et al. 2007; de Putron and Ryland 2009; Wagner et al. 2012b). Further, Godefroid et al. (2024) show that thermal performance curves and oxygen consumption rates for antipatharians collected from Madagascar at 25.0 m do not decline until temperatures exceed 33°C. Daily average sea surface temperatures of this magnitude have not been recorded for the Galápagos (Liu et al. 2014; NOAA Coral Reef Watch 2018), so limitation of black coral distributions by water temperature alone appears unlikely.
Hydrodynamic forces such as drag could limit the penetration of black corals into the shallow zone (H2), as dislodged colonies at less than 5.0 m depth were found at four sites (Pinzon, Roca Cousins, Champion, and Gardner; unpublished observations). Grigg (1965) observed that waves and surge at shallow sites in Hawaii caused black coral tissue loss and mortality via abrasion by particulate matter. Negative effects of high drag and abrasion on large, shallow black corals may help explain their shorter stature and the dominant pattern of colonies tucked into crevices and urchin bore holes. Since current velocity, turbulence, and wave surge decrease with depth (Denny 1988), black coral colonies of the same projected area would experience higher drag forces, and consequently higher dislodgement, in shallow habitats compared to deeper ones. Epibionts also increase drag on the basal organisms they are attached to (Witman and Suchanek 1984; Wahl 1996), suggesting that the epibiont overgrowth documented here would exacerbate the negative effects of high hydrodynamic forces on black corals at shallow depths.
Light exposure could additionally influence the observed depth distribution of black corals (H3; Laverick et al. 2020). Many invertebrate larvae are photonegative (Thorson 1964), preferentially selecting shaded microhabitats for settlement and avoiding the higher light levels generally characteristic of shallow depths (Young and Chia 1984). In a review of black coral ecology, Wagner et al. (2012a) state that black corals typically occur in shaded habitats such as caves or overhangs when they occur at shallow depths (< 50 m), which is consistent with the high prevalence of cryptic habitat occupancy at depths of less than 10.0 m found here. Oakley (1988) and Grigg (1976) reported similar findings, where black corals were more abundant on the shaded side of a shipwreck and within the interstices of the reef, respectively. Strong light intensity has been theorized to limit antipatharians at the larval stage, potentially explaining cryptic settlement patterns (Grigg 1965; Oakley 1988). Assuming that light levels are lower in crevices or holes created by urchin (Eucidaris galapagensis) bioerosion than on exposed rock surfaces in the GMR, we hypothesize that the likely photonegative larval behavior of A. galapagensis is a mechanism underlying greater occupancy of cryptic habitats by black coral colonies at shallow depths. If this is established, there may be a facultative interaction between sea urchin behavior and black coral settlement.
Although we observed six species of fish representing five families biting the branch tips of A. galapagensis during field surveys (Fig. S2.), fish predation on black corals was uncommon and is unlikely to explain their absence from shallow depths or patterns of habitat occupancy. Fish predation does, however, expose the axial skeleton of A. galapagensis, opening up substrate for colonization by algae and invertebrates, and potentially serving as a point of entry for pathogenic microbes (unpublished observations). Green sea turtles (Chelonia mydas) have been documented feeding on A. galapagensis in the Gulf of California (Seminoff et al. 2002), but this interaction was never observed at our study sites, despite high abundances of green sea turtles in the GMR (Carrión-Cortez et al. 2010). However, high grazing and predation pressure by sea urchins (Eucidaris galapagensis, Lytechinus variegatus) on exposed substrates in the Galápagos subtidal (Glynn et al. 1979; Brandt et al. 2012) could eliminate recruiting or juvenile black corals on shallow, exposed rock surfaces (H4).
The scarcity of black corals above 5.0 m depth could also be caused by a failure of larval dispersal or recruitment to the shallow zone (H5). The high-velocity, turbulent environment typical of the shallow subtidal is known to restrict the ability of invertebrate larvae to settle on exposed surfaces, favoring their attachment in reduced flow microhabitats (Reidenbach et al. 2009). It stands to reason then that the microhabitats within crevices and urchin-bored holes may provide a physical refuge from high hydrodynamic forces, which could promote juvenile black coral settlement and persistence and produce the observed pattern of cryptic habitat usage at shallow depths. Miller (1998) also found that black corals are highly philopatric, so if hydrodynamic forces in an area are unsuitable for either larval settlement or colony persistence, it is unlikely that black coral larvae from other sources (i.e., deeper, or populations in close geographic proximity) would colonize the area. As opposed to the high-density populations of black corals living at depths with high larval dispersal potential, a lack of black coral larvae reaching shallow substrates could thus contribute to the persistence of low-density black coral populations at shallow depths.
The pattern of greater occupancy of cryptic habitats at shallow depths at the majority of study sites provides important insight into the likely mechanisms driving black coral distributions. It is highly probable that there are synergisms between the hypothesized drivers, and disentangling these factors is necessary to understand the mechanisms driving the structure of black coral populations. For example, regardless of whether it is an active (i.e., larval microhabitat selection) or passive process, black corals in crevices or urchin-excavated holes may achieve refuge from high hydrodynamic forces or predation on exposed substrates, enhancing their potential for persistence at shallow depths.
In conclusion, black coral forests in the central Galápagos Archipelago create foundational habitats that are limited in distribution by depth ceilings but that support a range of epibiont functional groups. Capitalizing on uniquely shallow populations of A. galapagensis, we defined clear patterns where black coral density, occupancy, height, and habitat utilization changed between sites and with depth, and outlined multiple hypotheses framing the proximal drivers of these patterns for future testing. These results provide an ecological characterization of black coral populations throughout the central Galápagos Archipelago that can be used to manage these biodiverse habitats and assess the impact of future environmental change.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Aguilar R, Marín P (2013) Mediterranean deep-sea corals: reasons for protection under the Barcelona Convention. Oceana: 1–18
Bailey LL, MacKenzie DI, Nichols JD (2014) Advances and applications of occupancy models. Methods Ecol Evol 5:1269–1279
Bo M, Bavestrello G, Canese S, Giusti M, Salvati E, Angiolillo M, Greco S (2009) Characteristics of a black coral meadow in the twilight zone of the central Mediterranean Sea. Mar Ecol Prog Ser 397:53–61
Bo M, Lavorato A, Camillo CGD, Poliseno A, Baquero A, Bavestrello G, Irei Y, Reimer JD (2012) Black Coral Assemblages from Machalilla National Park (Ecuador). Pac Sci 66:63–81
Bo M, Montgomery AD, Opresko DM, Wagner D, Bavestrello G (2019) Antipatharians of the Mesophotic Zone: Four Case Studies. Mesophotic Coral Ecosystems. Springer, Cham, pp 683–708
Boland RC, Parrish FA (2005) Description of Fish Assemblages in the Black Coral Beds off Lahaina, Maui, Hawai’i. Pac Sci 59:411–420
Bosch NE, Espino F, Tuya F, Haroun R, Bramanti L, Otero-Ferrer F (2023) Black coral forests enhance taxonomic and functional distinctiveness of mesophotic fishes in an oceanic island: implications for biodiversity conservation. Sci Rep 13:4963
Brandt M, Witman JD, Chiriboga AI (2012) Influence of a dominant consumer species reverses at increased diversity. Ecology 93:868–878
Brugler MR, Opresko DM, France SC (2013) The evolutionary history of the order Antipatharia (Cnidaria: Anthozoa: Hexacorallia) as inferred from mitochondrial and nuclear DNA: implications for black coral taxonomy and systematics. Zool J Linn Soc 169:312–361
Buhl-Mortensen L, Mortensen PB (2004) Symbiosis in Deep-Water Corals. Symbiosis
Buhl-Mortensen L, Vanreusel A, Gooday AJ, Levin LA, Priede IG, Buhl-Mortensen P, Gheerardyn H, King NJ, Raes M (2010) Biological structures as a source of habitat heterogeneity and biodiversity on the deep ocean margins: Biological structures and biodiversity. Mar Ecol 31:21–50
Cairns SD (2007) Deep-water corals: an overview with special reference to diversity and distribution of deep-water scleractinian corals. Bull Mar Sci 81:311–322
Cannas R, Follesa MC, Cau A, Cau A, Friedman K (2019) Global Report on the Biology, Fishery and trade of Precious Corals. FAO Fish Aquac Circ No 1184 Rome: FAO:
Carrión-Cortez JA, Zárate P, Seminoff JA (2010) Feeding ecology of the green sea turtle (Chelonia mydas) in the Galapagos Islands. J Mar Biol Assoc U K 90:1005–1013
Castelló Y, Tickell S, Low NHN, Lamb RW, Brandt M, Witman JD (2022) Distribution and feeding ecology of sea stars in the Galápagos rocky subtidal zone. J Exp Mar Biol Ecol 553:151754
Chui APY, Ang PO (2015) Extensive mortality of black corals Antipathes curvata along a depth gradient in Hong Kong, China. Mar Biodivers 45:631–632
CITES (2022) Appendices I, II, and III. https://cites.org/eng/app/appendices.php
Dayton P (1972) Toward an understanding of community resilience and the potential effects of enrichments to the benthos at McMurdo Sound, Antarctica. Proc Colloq Conserv Probl Antarct 81–96
Deichmann E (1941) Coelenterates collected on the presidential cruise of 1938. 99:1–17
Denny M (1988) Biology and the Mechanics of the Wave-Swept Environment. Princeton University Press
Ellison AM, Bank MS, Clinton BD, Colburn EA, Elliott K, Ford CR, Foster DR, Kloeppel BD, Knoepp JD, Lovett GM, Mohan J, Orwig DA, Rodenhouse NL, Sobczak WV, Stinson KA, Stone JK, Swan CM, Thompson J, Von Holle B, Webster JR (2005) Loss of foundation species: consequences for the structure and dynamics of forested ecosystems. Front Ecol Environ 3:479–486
FAO (2009) International Guidelines for the Management of Deep-sea Fisheries in the High Seas. Rome FAO
Glynn PW, Wellington GM, Birkeland C (1979) Coral Reef Growth in the Galápagos: Limitation by Sea Urchins. Science 203:47–49
Gori A, Linares C, Rossi S, Coma R, Gili J-M (2007) Spatial variability in reproductive cycle of the gorgonians Paramuricea clavata and Eunicella singularis (Anthozoa, Octocorallia) in the Western Mediterranean Sea. Mar Biol 151:1571–1584
Godefroid M, Vandendriessche M, Todinanahary GGB, Ransquin I, Dubois P (2024) Thermal sensitivity of black corals (Antipatharia: Hexacorallia): Comparisons between sympatric species from a thermally fluctuating site in Madagascar and between allopatric congenerics. Sci Total Environ 908:168311
Grange KR (1985) Distribution, standing crop, population structure, and growth rates of black coral in the southern fiords of New Zealand. N Z J Mar Freshw Res 19:467–475
Grange KR, Singleton RJ (1988) Population structure of black coral, Antipathes aperta, in the southern fiords of New Zealand. New Zealand Journal of Zoology 15:481–489
Grigg RW (1965) Ecological Studies of Black Coral in Hawaii. Pac Sci 19:244–260
Grigg RW (1976) Fishery Management of Precious and Stony Corals in Hawaii.
Grigg RW (1993) Precious Coral Fisheries of Hawaii and the U.S. Pacific Islands Marine Fisheries Review 55:50–60
Grigg RW (2001) Black Coral: History of a Sustainable Fishery in Hawai’i. Pac Sci 55:291–299
Hickman CP (2008) A field guide to corals and other radiates of Galapagos: an illustrated guidebook to the corals, anemones, zoanthids, black corals, gorgonians, sea pens, and hydroids of the Galapagos Islands. Sugar Spring Press, Lexington, Va
Humann P, DeLoach N (2003) Reef Fish Identification: Galápagos. New World Publications,
Hutchinson GE (1957) Concluding Remarks. Cold Spring Harb Symp Quant Biol 22:415–427
Kahng S, Copus J, Wagner D (2014) Recent advances in the ecology of mesophotic coral ecosystems (MCEs). Curr Opin Environ Sustain 7:72–81
Kahng S, Wagner D, Lantz C, Vetter O, Gove J, Merrifield M (2012) Temperature related depth limits of warm-water corals. Proc 12th Int Coral Reef Symp
Kahng SE, Garcia-Sais JR, Spalding HL, Brokovich E, Wagner D, Weil E, Hinderstein L, Toonen RJ (2010) Community ecology of mesophotic coral reef ecosystems. Coral Reefs 29:255–275
Kerstitch A (1989) Sea of Cortez marine invertebrates: A guide for the Pacific coast, Mexico to Ecuador.
Lamb RW, Smith F, Witman JD (2020) Consumer mobility predicts impacts of herbivory across an environmental stress gradient. Ecology 101:e02910
Laverick JH, Tamir R, Eyal G, Loya Y (2020) A generalized light-driven model of community transitions along coral reef depth gradients. Glob Ecol Biogeogr 29:1554–1564
Lavorato A, Stranges S, Bonilla HR (2021) Potential Distribution and Environmental Niche of the Black Corals Antipathes galapagensis and Myriopathes panamensis in the Eastern Tropical Pacific. Pac Sci 75
Liu G, Heron S, Eakin C, Muller-Karger F, Vega-Rodriguez M, Guild L, De La Cour J, Geiger E, Skirving W, Burgess T, Strong A, Harris A, Maturi E, Ignatov A, Sapper J, Li J, Lynds S (2014) Reef-Scale Thermal Stress Monitoring of Coral Ecosystems: New 5-km Global Products from NOAA Coral Reef Watch. Remote Sens 6:11579–11606
Love MS, Yoklavich MM, Black BA, Andrews AH (2007) Age of Black Coral (Antipathes dendrochristos) Colonies, with Notes on Associated Invertebrate Species. Bull Mar Sci 80:10
MacKenzie DI, Nichols JD, Lachman GB, Droege S, Andrew Royle J, Langtimm CA (2002) Estimating site occupancy rates when detection probabilities are less than one. Ecology 83(8):2248–2255
Martinez P, Robinson E (1983) Investigacion sobre la explotacion del coral negro (Antipathes panamensis) en las islas Galapagos, Ecuador. Instituto Nacional de Pesca (INP). Bol Cient Tec 6:107–123
McGill BJ, Enquist BJ, Weiher E, Westoby M (2006) Rebuilding community ecology from functional traits. Trends Ecol Evol 21:178–185
Miller KJ (1998) Short-distance dispersal of black coral larvae: inference from spatial analysis of colony genotypes. Mar Ecol Prog Ser 9
NOAA Coral Reef Watch (2018) NOAA Coral Reef Watch Version 3.1 Daily 5km Satellite Regional Virtual Station Time Series Data for Galapagos, Ecuador. NOAA Coral Reef Watch, College Park, Maryland, USA
NOM-059-SEMARNAT (2010) NORMA Oficial Mexicana NOM-059-SEMARNAT-2010, Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo. Diario Oficial de la Federación, 30 de diciembre de 2010. https://www.dof.gob.mx/normasOficiales/4254/semarnat/semarnat.htm
Oakley SG (1988) Settlement and growth of Antipathes pennacea on a shipwreck. Coral Reefs 7:77–79
Opresko DM (2020) New species of black corals (Cnidaria: Anthozoa: Antipatharia) from the New Zealand region, part 2. N Z J Zool 47:149–186
OSPAR (2008) Case Reports for the OSPAR List of threatened and/or declining species and habitats. OSPAR Commission
Parrish FA, Abernathy K, Marshall GJ, Buhleier BM (2002) Hawaiian Monk Seals (Monachus schauinslandi) Foraging in Deep-Water Coral Beds. Mar Mammal Sci 18:244–258
de Putron SJ, Ryland JS (2009) Effect of Seawater Temperature on Reproductive Seasonality and Fecundity of Pseudoplexaura porosa (Cnidaria: Octocorallia): Latitudinal Variation in Caribbean Gorgonian Reproduction. Invertebr Biol 128:213–222
R Core Team (2020) R: A language and environment for statistical computing.
Reidenbach MA, Koseff JR, Koehl MAR (2009) Hydrodynamic forces on larvae affect their settlement on coral reefs in turbulent, wave-driven flow. Limnol Oceanogr 54:318–330
Reimer J, Fujii T (2010) Four new species and one new genus of zoanthids (Cnidaria, Hexacorallia) from the Galapagos Islands. ZooKeys 42:1–36
de Ridder C, Holthuis LB (1979) Pontonides sympathes, a new species of commensal shrimp (Crustacea, Decapoda, Pontoniinae) from Antipatharia in the Galapagos Islands. Zool Meded 54:101–110
Risk M, Sherwood O, Nairn R, Gibbons C (2009) Tracking the record of sewage discharge off Jeddah, Saudi Arabia, since 1950, using stable isotope records from antipatharians. Mar Ecol Prog Ser 397:219–226
Romero XM (1997) Ecuador’s vanishing black corals. Aquat J Shedd Aquar 26:21–25
Rossi S, Bramanti L, Gori A, Orejas C (2017) An Overview of the Animal Forests of the World. In: Rossi S, Bramanti L, Gori A, Orejas C (eds) Marine Animal Forests. Springer International Publishing, Cham, pp 1–26
Sala E, Aburto-Oropeza O, Paredes G, Thompson G (2003) Spawning Aggregations and Reproductive Behavior of Reef Fishes in the Gulf of California. Bull Mar Sci 72:21
Seminoff JA, Resendiz A, Nichols WJ (2002) Diet of East Pacific Green Turtles (Chelonia mydas) in the Central Gulf of California, México. J Herpetol 36:447–453
Stephens PA, Sutherland WJ, Freckleton RP (1999) What Is the Allee Effect? Oikos 87:185–190
Tapia-Guerra JM, Asorey CM, Easton EE, Wagner D, Gorny M, Sellanes J (2021) First Ecological Characterization of Whip Black Coral Assemblages (Hexacorallia: Antipatharia) in the Easter Island Ecoregion. Southeastern Pacific Front Mar Sci 8:755898
Tazioli S, Bo M, Boyer M, Rotinsulu H, Bavestrello G (2007) Ecological Observations of Some Common Antipatharian Corals in the Marine Park of Bunaken (North Sulawesi, Indonesia). Zool Stud 15
Thorson G (1964) Light as an ecological factor in the dispersal and settlement of larvae of marine bottom invertebrates. Ophelia 1:167–208
Todinanahary G, Terrana L, Lavitra T, Eeckhaut I (2016) First records of illegal harvesting and trading of black corals (Antipatharia) in Madagascar. Madag Conserv Dev 11
Tsounis G, Rossi S, Aranguren M, Gili J-M, Arntz W (2006) Effects of spatial variability and colony size on the reproductive output and gonadal development cycle of the Mediterranean red coral (Corallium rubrum L.). Mar Biol 148:513–527
Tsounis G, Rossi S, Grigg R, Santangelo G, Bramanti L, Gili J-M (2010) The Exploitation and Conservation of Precious Corals. 52
UNEP (2007) Report of the expert workshop on ecological criteria and biogeographic classification systems for marine areas in need of protection.
Wagner D, Luck D, Toonen R (2012a) The Biology and Ecology of Black Corals (Cnidaria: Anthozoa: Hexacorallia: Antipatharia). Adv Mar Biol 63:67–132
Wagner D, Waller RG, Montgomery AD, Kelley CD, Toonen RJ (2012b) Sexual reproduction of the Hawaiian black coral Antipathes griggi (Cnidaria: Antipatharia). Coral Reefs 31:795–806
Wahl M (1996) Fouled snails in flow:potential of epibionts on Littorina littorea to increase drag and reduce snail growth rates. Mar Ecol Prog Ser 138:157–168
Wahl M (2008) Ecological lever and interface ecology: epibiosis modulates the interactions between host and environment. Biofouling 24:427–438
Warner GF (1981) Species Descriptions and Ecological Observations of Black Corals (Antipatharia) from Trinidad. Bull Mar Sci 31:147–163
Williams B, Grottoli AG (2010) Stable nitrogen and carbon isotope (δ15N and δ13C) variability in shallow tropical Pacific soft coral and black coral taxa and implications for paleoceanographic reconstructions. Geochim Cosmochim Acta 74:5280–5288
Witman J, Dayton P (2001) Rocky subtidal communities. Marine Community Ecology. Sinauer Press, pp 339–366
Witman JD, Smith F (2003) Rapid community change at a tropical upwelling site in the Galápagos Marine Reserve. Biodivers Conserv 12:25–45
Witman JD, Brandt M, Smith F (2010) Coupling between subtidal prey and consumers along a mesoscale upwelling gradient in the Galápagos Islands. Ecol Monogr 80:153–177
Witman JD, Suchanek TH (1984) Mussels in flow: drag and dislodgement by epizoans. Mar Ecol Prog Ser 16:259–268
Young CM, Chia FS (1984) Microhabitat-associated variability in survival and growth of subtidal solitary ascidians during the first 21 days after settlement. Mar Biol 81:61–68
Acknowledgements
This work was funded by the United States National Science Foundation (OCE-1623867, OCE-2035354) and by an award from the Galápagos Conservancy, Inc. to J.D. Witman. It was also supported by awards to M. Agarwal from the Brown University International Undergraduate Teaching and Research Award, the Brown University Voss Fellowship in Environmental Science, and the Women Divers Hall of Fame. The authors thank field assistants (F. Rivera, M. Zeff, C. Munson), boat captains (N. Ibarra, W. Aguirre, S. Aguirre), coordinators (H. Jager, M. Romoleroux, and staff of the Charles Darwin Foundation), and the Dirección del Parque Nacional Galápagos for issuing research permits (PC-79-20 & PC-54-21). This publication is contribution number 2619 of the Charles Darwin Foundation for the Galapagos Islands.
Funding
National Science Foundation, OCE-1623867, Jon Witman, OCE-2035354, Jon Witman, Galapagos Conservancy, Institute at Brown for Environment and Society, Brown University, Brown University, Women Divers Hall of Fame.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Agarwal, M., Lamb, R.W., Smith, F. et al. Distribution and ecology of shallow-water black corals across a depth gradient on Galápagos rocky reefs. Coral Reefs 43, 733–745 (2024). https://doi.org/10.1007/s00338-024-02497-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-024-02497-6