Abstract
Black corals are important components of mesophotic and deep-water marine habitats. Their presence at great depths (e.g., 50 to 200 m) makes accessibility difficult, limiting our understanding of the associated biodiversity. Amphipods dominate vagile epifauna in marine habitats around the world, fulfilling important ecosystem functions. However, there are no studies on amphipods exclusively associated with black corals, including relationships between their ecological patterns (e.g., abundances) and the size of coral colonies. We investigated the epifaunal composition and abundance associated with black coral colonies of Antipathella wollastoni in the subtropical eastern Atlantic Ocean. In total, 1,736 epifaunal individuals were identified, of which 1,706 (98.27%) were amphipods, belonging to 6 taxa. We identified and described a new amphipod genus and species within the Stenothoidae family, Wollastenothoe minuta gen. nov., sp. nov., which outnumbered the amphipod assemblage (86.15%) and provided a complete taxonomic key of Stenothoidae family including this new finding. For the first time, the association between an amphipod species and a black coral was described, including a strong correlation between coral colony size and amphipod abundances. This study demonstrates that epifauna associated with mesophotic black corals remains largely undescribed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Corals are key components of sublittoral ecosystems not only in tropical (Spalding et al. 2001), but also subtropical (Czechowska et al. 2020), temperate, and cold ecosystems (Orejas et al. 2009; Bo et al. 2014; Buhl-Mortensen et al. 2018). While there are an increasing number in the studies on the classes of Scleractinia and Octocorallia, inhabiting various depth zones, Antipatharia (Cnidaria: Anthozoa: Hexacorallia) has received relatively less attention (Bo et al. 2012). Antipatharians, commonly known as black corals, encompasses over 300 accepted species (Molodtsova and Opresko 2023) and, under favorable environmental conditions, can form dense aggregations, creating “coral forests” (a type of animal forests, sensu Rossi et al. 2017) or “coral gardens” (sensu Hall-Spencer and Tasker 2006). These communities have some structural and functional similarities with terrestrial forests, with the main difference that they are dominated by animals instead of plants (Rossi et al. 2017). Their colony morphology varies from branched, bush-like, and feather-like to whip types (Wagner et al. 2012). The dense canopies they form can change local physical conditions and generate a three-dimensional habitat, which provide shelter to associated species and, ultimately, increase biodiversity (Freiwald et al. 2004; Buhl-Mortensen et al. 2010; De Clippele et al. 2019). One of the main components of the visible black coral forests-associated biodiversity is accounted by epifauna (i.e., crustaceans, polychaetes and molluscs, Lavelle 2012; Wagner et al. 2012), which can find habitat (Herler 2007), food (Angel 1990; Bo et al. 2012), and protection against predators (Lavelle 2012).
Amphipods are one of the most abundant and diverse taxa of marine macro-invertebrates (Arfianti et al. 2018), as well as a diverse and important component of deep-sea habitats worldwide (Arfianti and Costello 2020a, b). Amphipods include species with different trophic strategies (e.g., detritivores, omnivores, carnivores, and herbivores), and they are predated by other crustaceans, polychaetes, and fishes (Guerra-García et al. 2014; Jiménez Prada et al. 2015). Moreover, they play an important role in marine food webs, by directly or indirectly recycling nutrients and linking different trophic levels (Karlson et al. 2007; Havermans and Smetacek 2018).
The amphipod family Stenothoidae includes marine benthic species ranging from the shallow subtidal to depths over 3,000 m (Krapp-Schickel 2015; Krapp-Schickel and Vader 2015). Several species of Stenothoidae have received attention from the scientific community due to their biological association (i.e., commensalism) with marine sessile cnidaria, such as anemones (Vader and Krapp-Schickel 1996; Auster et al. 2011) and hydrozoans (Lewis 1992), on which they find food, nesting grounds, and shelter (Marin and Sinelnikov 2017). However, to date, there are no studies on amphipods and their ecological interactions with black corals.
In the present study, we investigated the epifaunal composition and abundance in mesophotic forests created by the black coral Antipathella wollastoni (Gray 1857) from Lanzarote Island over time (Canary Islands, eastern Atlantic Ocean). Identification of sampled specimens has been performed in a robust taxonomic framework and resulted in the description of a new amphipod genus and species within the Stenothoidae family, for which a complete key to genera is provided. Then, we described the first biological association between an amphipod species (i.e., Wollastenothoe minuta gen. nov., sp. nov.) and a black coral species. Specifically, the relationship between coral colony size and amphipod abundances was tested for both the entire amphipod assemblage and the new described species, suggesting an exclusive association.
Material and methods
Study region
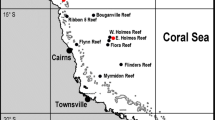
The study was conducted in the Southeastern coast of Lanzarote Island (Canary Islands, eastern Atlantic Ocean) at Puerto del Carmen (28º55′26.81″ N, 13º 39′ 12.61″ W) (Fig. 1a, b). The site was selected based on previous records of A. wollastoni in the shallower limits of its depth distribution range (ca. 60 m depth, Fig. 1c, d) (Bianchi et al. 2000; Czechowska et al. 2020). In this area, local topography is characterized by narrow rocky shelves and steep slopes, typical of oceanic volcanic islands (Acosta et al. 2005; Tuya et al. 2021). Local hydrography is complex, with NE trade winds affecting shallow subtidal habitats by generating wind waves and near-bottom turbulence (Mann and Lazier 2013). The high nutrient load transported by currents may affect the upper limit of black coral distribution, by either providing food or physically smothering the corals (Wagner et al. 2012; Czechowska et al. 2020).
Sampling design and collection of samples
Epifauna was collected in mesophotic monospecific forests of A. wollastoni at three times during 2021: T1 (February), T2 (April), and T3 (October). At each time, 10 colonies spanning between 40 and 180 cm (total height from the seafloor to the upper tip of the colony) were selected randomly, for a total of 30 colonies. All sampling was carried out at the same depth (ca. 60 m), taking care to not sample twice the same area. Sampling was carried out by two divers using mixed-gas rebreather diving. On each colony, samples of associated epifauna were collected by sucking up on their top branches through a vacuum cleaner for 20 s (Fig. 2). The temperature was monitored between February and October 2021 by a temperature data logger (Hobo data-logger Pedant Temp-Light, Onset Computer Corporation, USA; sampling frequency 15 min) positioned next to the black corals.
Specimen processing
Collected epifauna was directly brought to the laboratory after surfacing in sealed containers filled with seawater. Then, each sample was carefully inspected, rinsed, and sorted to separate epifauna from marine debris and coral mucus. Each organism was then stored in 96% ethanol and identified to the lowest taxonomical level. Identifications were implemented by using a stereoscopic microscope (Leica, EZ4W, Wetzlar, Germany). The taxonomic guide provided by Barnard and Karaman (1991), Ruffo (1998), and specific references from the Stenothoidae family (i.e., Krapp-Schickel 2000, 2011, 2015) was used for amphipod identification. Specifically, 68 specimens of the new amphipod genus/species were dissected in alcohol and mounted on microscope slides using dimethylhydantoin–formaldehyde resin for morphological description. Appendages were observed under a Nikon SMZ800N stereomicroscope and a Nikon Eclipse Ci microscope and photographed with a Nikon DS-Fi2 camera. Body length (BL) was measured with NIS-Elements Analysis software from the anterior margin of the head to the posterior end of the telson. Drawings were carried out from pictures using Inkscape software (v.0.48). For scanning electron microscope (SEM) observations, 12 specimens were dehydrated in a graded ethanol series, critical point dried, using carbon dioxide as a medium, mounted on stubs, coated with gold for 60 s sputter coated with gold, and photographed with a Hitachi tabletop microscope TM3030Plus.
DNA sequencing
DNA extraction was performed on a pool of 23 ethanol-preserved specimens using the MagAttract DNA Extraction Kit (Qiagen) following the manufacturer's protocol. Folmer’s 658-bp fragment of the first subunit of cytochrome c oxidase mitochondrial gene (COI) was amplified using primers LCO1490 and HCO2198 (Folmer et al. 1994). In addition, a fragment of the 28S rRNA gene (28S) was amplified using primers from Verovnik et al. (2005). All amplifications were performed using DreamTaq Green PCR Master Mix (ThermoScientific). For COI, the PCR protocol involved an initial denaturation period at 94 °C for 3 min, followed by 50 cycles of denaturation at 94 °C for 30 s, annealing at 45 °C for 1 min, and elongation at 72 °C for 1 min. For 28S, the PCR protocol comprised an initial denaturation period at 94 °C for 3 min, then 40 cycles of denaturation at 94 °C for 20 s, annealing at 50 °C for 50 s, and elongation at 72 °C for 1 min. PCR products were checked on 1% agarose gel, and subsequently, 1–2 µL of each PCR product (depending on band intensity) was pooled (together with other PCR products from unrelated research projects). The pool was then cleaned up using the InnuPREP PCRpure Kit (Innuscreen) and used to prepare a SKQ-LSK114 library that was sequenced on a FLO-FLG114 Nanopore Flongle flow cell featuring R10.4.1 pore proteins. The resulting reads (1264 for COI and 1932 for 28S) were assembled using amplicon_sorter (Vierstraete and Braeckman 2022).
Phylogenetic analyses
For the COI marker, all Stenothoidae sequences available in GenBank on 19 November 2023 were collected and complemented with sequences of Iphimedia obesa and Gitana sarsi as outgroups. Alignment was performed by hand in MEGA11 (Tamura et al. 2021) taking into account the amino acid translation of the sequences, followed by maximum likelihood phylogenetic analysis using IQtree2 (Minh et al. 2020) with automatic model selection (Kalyaanamoorthy et al. 2017) and 100,000 ultrafast boostraps (Hoang et al. 2018). The resulting Newick tree was displayed, re-rooted, and turned into PDF in MEGA11.
Statistical analyses
All modeling and testing were conducted using R (Rstudio Team 2022). Mixed effects generalized linear models (GLMs) were fitted to univariate responses, including the total abundance of amphipods and the abundance of the new genus and species, Wollastenothoe minuta gen. nov., sp. nov. GLMs were fitted by means of the ‘lme4’ (Bates et al. 2014) and ‘lmerTest’ packages (Kuznetsova et al. 2017) considering time (three levels) as a random factor and the size of colonies of A. wollastoni as a covariate. Models were fitted using a ‘negative binomial’, as the family distribution of residuals, with a ‘log’ link function. For all fitted GLMs, diagnosis plots of residuals and Q-Q plots were visually inspected to check the appropriateness of the fitted models (Harrison et al. 2018). We realized simple linear regressions tested whether the total abundance of amphipod and the abundance of W. minuta gen. nov., sp. nov., were predicted by the colony size.
Results
Taxonomy
Class Malacostraca Latreille 1806
Order Amphipoda Latreille 1816
Suborder Senticaudata Lowry & Myers 2013
Family Stenothoidae Boeck 1871
Genus Wollastenothoe Gouillieux & Navarro-Mayoral gen. nov.
urn:lsid:zoobank.org:act:5FFE2706-D2F1-4EB9-B6A8-B341FF6D10D5.
Type species. Wollastenothoe minuta Gouillieux & Navarro-Mayoral gen. nov., sp. nov., here designated.
Diagnosis of the new genus
Body dorsally smooth. Head without rostrum. Antenna 1 article 1 not nasiform; accessory flagellum with 1 article. Mandible palp with 1 article, molar process conical. Maxilla 1 palp with 2 articles. Gnathopod 1 and 2 subchelate, subequal. P5 basis rectolinear without posterodistal lobe. P6-7 basis widened.
Etymology
The genus name, Wollastenothoe, refers the combination of host name corresponding to the species of black coral (i.e., Antipathella wollastoni) with the genus name Stenothoe belonging to the Stenothoidae family.
Remarks on genus assignation and Stenothoidae diagnosis
Characters of the present new genus agree with the diagnosis of the Stenothoidae family except for the molar process of the mandible. In the present new genus, molar process is slightly developed, conical, whereas the diagnosis for the family Stenothoidae includes a molar process evanescent. This conical shape of the molar process is already present in other genera of Stenothoidae, such as Antatelson, Pseudothaumatelson, Ptychotelson, Raukumara or Thaumatelsonella. According to Horton et al. (2022), 46 genera belong to the family Stenothoidae, and only 12 present a mandibular palp uniarticulate: Ausatelson J.L. Barnard 1972; Metopelloides Gurjanova 1938; Paraprobolisca Ren in Ren & Huang 1991; Prostenothoe Gurjanova 1938; Prothaumatelson Schellenberg 1931; Pseudothaumatelson Schellenberg 1931; Ptychotelson Krapp-Schickel 2000; Stenothoides Chevreux 1900; Stenula J.L. Barnard 1962; Victometopa Krapp-Schickel 2011; Vonimetopa Barnard & Karaman 1987 and Zaikometopa Barnard & Karaman 1987. Wollastenothoe gen. nov. can be distinguished from Ausatelson, Metopelloides, Prostenothoe, Prothaumatelson, Ptychotelson, Stenula, Vonimetopa and Zaikometopa by the presence of an uniarticulate accessory flagellum (vs absence), with Paraprobolisca and Pseudothaumatelson by pereopod 7 basis expanded (vs rectolinear), with Victometopa by pereopod 5 without posterodistal lobe (vs with) and with Stenothoides by 2-articulate maxilla 1 palp (vs 1-articulate). In the case of Paraprobolisca, the only species of this genus, Paraprobolisca leptopoda Ren in Ren & Huang 1991 was synonymized with Probolisca ovata (Stebbing 1888) by Krapp-Schickel and Koenemann (2006). They based their diagnosis on the morphological similarities of the gnathopods and the mouthparts and considered that the uropod 3 described as being one-articulated in Paraprobolisca leptopoda, a character of the genus, has simply been overlooked and should be two-articulated as for Probolisca ovata. Even if most of species have uropod 3 ramus two-articulated, some have an one-articulated uropod 3 ramus as some Raumahara J.L. Barnard 1972 species. Furthermore, Probolisca ovata presents uropods 1 and 2 peduncles marginally bares, whereas Paraprobolisca leptopoda has uropods 1 and 2 peduncles with many robust setae along outer margin. Thus, based on the original descriptions, we consider Paraprobolisca leptopoda as a valid species that should not be synonymized with Probolisca ovata.
Wollastenothoe minuta Gouillieux & Navarro-Mayoral sp. nov.
urn:lsid:zoobank.org:act:7A70A805-5499-4FC6-93AD-1A8CE17C53DE Description of Wollastenothoe minuta.
Type material.
Holotype: brooding female, BL = 1.38 mm, 1 egg (MNHN-IU-2016–3389). Paratypes: brooding female, BL = 1.21 mm, 1 egg, dissected specimen, 11 slides (MNHN-IU-2016–3388); brooding female, BL = 1.45 mm, 2 eggs, dissected specimen, 11 slides (MNHN-IU-2016–3387); female with oostegites, BL = 1.37 mm, dissected specimen, 12 slides (MNHN-IU-2021–8807). Atlantic Ocean, European waters, Canary Islands, Puerto del Carmen in Lanzarote Island. 28′55′26″81″ N, 13º’39′ 12″61″ W, October 2021, 60 m depth, collected by technical diving and rebreathers with the air-vacuum method on black coral branches. Collectors: Francisco Otero-Ferrer, Lorenzo Bramanti and Lucas Terrana.
Additional material: 5 juveniles, 53 females and 10 specimens sex not determined (MNHN-IU-2016–3359), 12 specimens used for SEM pictures (MNHN-IU-2021–8808). Same data as holotype and paratypes.
Diagnosis
Body length less than 1.5 mm. Antenna subequal, shorter than half length of body. Antenna 1 accessory flagellum with 1 small article. Gnathopod 1 and 2 subchelate, subequal. Pereonite 4 slightly longer than pereonite 3. Coxa 4 ventral margin concave. Coxae 5–7 posterior margin with a notch. P5 basis rectolinear without posterodistal lobe. P6-7 basis widened with posterodistal lobe reaching along half of ischium, merus posterodistal lobe reaching more than half length of carpus. Telson with dorsal spines.
Description based on holotype and paratypes (Figs. 3, 4, 5, 6, 7).
Body (Fig. 6a) dorsally smooth, very compressed laterally. Pereonite 4 slightly longer than pereonite 3. Urosomites free.
Head. Antennae subequal in length, setose. Antenna 1 (Fig. 3aA) peduncle article 1 slightly tapering distally, about 2 times longer than wide; article 2 cylindrical, about half length of article 1; article 3 cylindrical, subequal to article 2; flagellum consists of 4 articles increasing in length, accessory flagellum 1 articulate, very small, with 1 to 3 distal setae. Antenna 2 (Fig. 3bB) peduncular article 3 cylindrical, as long as wide; article 4 and 5 subequal, about 2.8 times longer than article 3; flagellum consists of 5 articles of decreasing length.
Wollastenothoe minuta gen. nov., sp. nov. a, b, e, f, h: Paratype MNHN-IU-2016–3387, BL: 1.37 mm. c, d: Paratype MNHN-IU-2016–3388, BL: 1.21 mm. g: Based on SEM picture, MNHN-IU-2021–8808. a Right antenna 1 with a focus on accessory flagellum; b Right antenna 2; c Left maxilla 1; d Left maxilla 2, e Mandible left; f Mandible right; g Upper lip; h Maxilliped. Scale bars: a, b: 0.1 mm; c–g: 0.01 mm; h: 0.025 mm
Mouthparts. Upper lip (Fig. 3g) cleft at the apex, asymmetric lobes. Mandible (Fig. 3e–f) Palp uni-articulate with 1 or 2 distal setae, about 1.4 to 2.6 longer than wide, partial suture line sometimes visible at the base of the palp; incisor and lacinia mobilis multi-dentate; accessory setarow consists of 3 blades with comb-shaped distal part; molar process slightly developed, conical, finely setose. Lower Lip (Fig. 7c) Lower lip with many setae, inner lobes coalesced, mandibular lobes well developed. Maxilla 1 (Fig. 3c) inner plate inner margin with a single simple setae, outer plate distal part with 6 large stiff robust setae; palp two-articulate, article 1 smooth, about 2 times shorter than article 2; article 2, with three distolateral robust setae, a subdistal simple seta and some little simple setae on distal part and inner plate. Maxilla 2 (Fig. 3d, 7b) inner plate with 4 long robust setae; apical margin rounded and armed with 3 setae; outer plate inner margin with row of 6 simple setae, distal part with 4 long and 1 short robust setae. Maxilliped (Fig. 3h) with reduced outer plate, inner plate with 2 distal simple setae; palp 4-articulate: article 3 the longest, with many very small setae distally; article 4 with lateral row of setae.
Gnathopods. Gnathopod I (Figs. 4a, b, 7e) subchelate, slightly smaller to gnathopod 2; coxa tapering distally, about 1.5 times longer than wide; basis and ischium with few setae; merus posterior and distal margins with dense small simple setae, posterodistal margin with long simple and plumose setae; carpus widening distally, posterodistal corner with long simple and plumose setae; propodus about twice as long as wide, dorsal margin bare except 2 dorsodistal setae, inner face with row of fine and small plumose setae and 2 or 3 long subdorsal setae; propodus palmar edge serrate, 3 stout robust setae at the base of propodus; dactylus with serrate cutting margin and 2 or 3 simple setae, subdistal notch with 4 setae. Gnathopod 2 (Figs. 4c–e, 7d) subchelate; coxa anteroventral margin regularly rounded, 1.8 times longer than wide, posteroventral corner notched; basis to carpus similar to gnathopod 1; propodus about twice as long as wide, dorsal margin bare except 2 dorsodistal setae; propodus palmar edge not serrated except a small part between 4 stout robust setae at the base of propodus; dactylus with 1 or 2 simple setae along cutting margin, not serrate, subdistal setae present without subdistal notch.
Wollastenothoe minuta gen. nov., sp. nov. a, d: Paratype MNHN-IU-2016–3387, BL: 1.37. b, c, e: Based on SEM picture. a Gnathopod 1 right, outer view; b Gnathopod 1 left, propodus and dactylus, inner view; c Gnathopod 1 distal part of dactylus, outer view; d Gnathopod 2 left outer view; e Gnathopod 2 right, propodus and dactylus, outer view. Scale bars: a, b, e: 0.05 mm; c: 0.01 mm; d: 0.1 mm
Pereopods. Pereopods 3–7 subequal in length, with few setae; ischium posterodistal seta absent for pereopods 3–4, present for pereopods 5–7; merus with distal lobe increasing in length, dactylus with dorsal seta. Pereopod 3 (Fig. 5a) coxa elongate, somewhat rectangular, posteroventral corner notched; length ratio of articles from basis to dactylus about 3.3:1:1.9:1.8:2.7:1.5; basis rectolinear, about 3.8 times longer than wide; merus about twice as long as wide, with small distodorsal lobe reaching 0.2 length of carpus; carpus 3.5 as long as wide; propodus elongated, about 5.5 times longer than wide. Pereopod 4 (Fig. 5b) similar in shape to pereopod 3 except for coxa very large, subtriangular, about the same length as the first four segments of the thorax, reaching half-length of ischium, smooth, unarmed, ventral margin slightly concave; length ratio of articles from basis to dactylus about 4.5:1:2.7:1.8:3.3:1.7. Pereopods 5–7 ischium to dactylus similar in shape, merus increasingly expanded. Pereopod 5 (Fig. 5c) coxa mostly developed posteriorly, posterior margin notched with a simple seta inside; length ratio of articles from basis to dactylus about 4.3:1:2.1:1.4:2.8:1.8; basis rectolinear, without posterodistal lobe. Pereopod 6 (Fig. 5d) coxa posterior margin notched with a simple seta inside; length ratio of articles from basis to dactylus about 5:1:2.6:1.6:4:2; basis slightly expanded, about 2 times longer than wide, posterior margin notched with a simple seta inside, posterodistal lobe reaching half-length of ischium. Pereopod 7 (Fig. 5e) coxa rounded, posterior margin notched with a simple seta inside; length ratio of articles from basis to dactylus about 6.3:1:2:1.6:3.8:2.1; basis expanded, slightly longer than wide, posterior margin notched with a simple seta inside, posterodistal lobe reaching two-thirds of ischium length; merus about 1.4 longer than wide, posterodistal lobe reaching 0.7 length of carpus.
Epimeral plates (Fig. 6a) 1–3 smooth, posteroventral corner weakly produced in a blunt lobe, more or less pronounced; posterior margin weakly convex.
Wollastenothoe minuta gen. nov., sp. nov. a: Paratype MNHN-IU-2016–3388. b: Based on SEM picture, MNHN-IU-2021–8808. c, d, e: Paratype MNHN-IU-2016–3388, BL: 1.21 mm. a Posterior part, lateral view; b Telson, dorsal view; c Right uropod 1; d Left uropod 2, distal part of outer ramus broken; e right uropod 3. Scale bars: a: 0.1 mm; b–e: 0.05 mm
Uropods (Figs. 6a, 7f). Uropod 1 (Fig. 6c) biramous; peduncle about 1.2 times longer than rami, with 2 robust setae on outer margin and a distal one, and 1 distal robust seta on inner margin; rami subequal, outer ramus with 1 dorsal robust seta, inner ramus with or without 1 dorsal robust seta. Uropod 2 (Fig. 6d) biramous; peduncle with 2 robust setae on outer margin and 1 distal robust seta on inner margin; rami unequal, inner ramus slightly longer than peduncle, 1.3 times longer than outer ramus, with 1 dorsal robust seta, inner ramus slightly smaller than peduncle, with or without 1 dorsal robust seta. Uropod 3 (Fig. 6e) uniramous, ramus 2-articulate, length ratio of peduncle and articles variable between 1:1:1.1 and 1.4:1:1.2; peduncle with 1 dorsodistal robust seta, ventrodistal corner produced into a small tooth; article 1 with 1 dorsodistal robust seta, ventrodistal corner produced into a small tooth with 1 simple seta, article 2 unarmed, smooth.
Wollastenothoe minuta gen. nov., sp. nov. SEM pictures, MNHN-IU-2021–8808. a Lateral view; b Maxilla 2, left; c Lower lip; d Gnathopod 2, outer face, dactylus and propodus; e Gnathopod 1, inner face, dactylus and propodus; f Urosome, lateral view. Scale bars: a: 0.25 mm; b: 0.01 mm; c: 0.01 mm, d, e: 0.02; f: 0.1 mm
Telson (Fig. 6b) entire, tapering distally, with 1 or 2 lateral robust setae and a small subdistal seta on each side.
Male: unknown.
Color in vivo: Whitish brown.
Type locality. Puerto del Carmen (28º55′26.81″ N, 13º 39′ 12.61″ W), Lanzarote, Canary Islands, Spain.
Etymology. The epithet specific of the species, minuta, refers to its small size.
Key to Genera of Stenothoidae (based on Barnard & Karaman 1991 key with inclusion of recent subsequent new genera descriptions)


Abundance patterns
In total, 1,736 epifaunal individuals were sampled, with amphipods representing a 98.3% of the total epifauna (Table 1); there were 1,721 amphipods belonging to 6 species (Table 1). Significant variation in the total abundance of amphipod was mainly explained by colony size (Pseudo-R2 = 0.85; Table S1), while the random factor time hardly explained variability (Pseudo-R2 = 0.13). The highest abundances of amphipods (mean ± SE) were recorded in T3 (163.3 ± 36.2 ind. colony−1), relative to T1 and T2 (27.6 ± 8.7 and 35.1 ± 9.4 ind. colony−1, respectively; Fig. 8a; Table S2). Wollastenothoe minuta gen. nov., sp. nov., was the most abundant species (Table 1) over time, accounting for 86.6% of amphipods in T1, 59.3% in T2 and 92% in T3 (Fig. 8b). These differences were mostly explained by colony size (Pseudo-R2 = 0.97; Table S1) and, to a lesser extent, by different sampling times (Pseudo-R2 = 0.10). A significant positive correlation between the total abundance of amphipods and the size of the coral colonies was found, i.e., the bigger the colonies, the larger the abundances of amphipods (Fig. 8c). A similar pattern was found for W. minuta gen. nov., sp. nov. (Fig. 8d). The highest abundances found in T3 (October) coincided with the higher average monthly temperature recorded in our study (Fig. S1).
Temporal variation in the total abundance of amphipods (a) and Wollastenothoe minuta gen. nov., sp. nov. (b), at T1 (February), T2 (April) and T3 (October). Relationship between colony size of Antipathella wollastoni and the total abundance of amphipods (c) and W. minuta gen. nov., sp. nov., at different times (d)
The new genus and species were found with other amphipod species belonging to five different families: Caprellidae (i.e., Phtisica marina Slabber 1769), Pleustidae (Buchholz, 1874), Ischyroceridae Stebbing, 1899 (i.e., Jassa Leach, 1814), Lysianassidae (Dana, 1849) and Oedicerotidae (Lilljeborg, 1965).
Phylogeny
Molecular data were successfully obtained at the two independent loci investigated. The COI sequence (GenBank accession number PP595991) was 658 bp length, and molecular phylogenetic analyses were consistent with the position of the new genus and species within the Stenothoidae family (Fig. 9), confirming its distinctiveness from other genera for which COI sequence data are currently available. The closest sequence (p-distance = 0.25) was from a specimen identified as Metopa boeckii G.O. Sars, 1892, but sequences attributed to this species were found at three different places in our Stenothoidae COI tree (with p-distances among them of about 0.25), suggesting that some of these sequences come from misidentified specimens. The 28S sequence fragment (GenBank accession number PP594429) was 1025 bp long—due to the dearth of Stenothoidae 28S sequences currently available in GenBank; no phylogenetic analysis was conducted for this marker.
Maximum-likelihood tree obtained using IQtree2 with 100,000 ultrafast bootstraps showing the relationships between Wollastenothoe minuta gen. nov., sp. nov., and other Stenothoidae COI sequences available in GenBank. Iphimedia obesa (Iphimediidae) and Gitana sarsi (Amphilochidae) were used as outgroups. These sequences attributed to Stenothoidae grouped with Gitana sarsi suggesting that these sequences may come from misidentified specimens, but as the corresponding nodes have low ultrafast bootstrap support values (< 65%), this grouping is not strongly supported
Discussion
This is the first study to date describing the temporal patterns of a new genus and species of amphipod associated with a black coral (i.e., Antipathella wollastoni). Wollastenothoe minuta gen. nov., sp. nov., was the most abundant and recurrent over time amphipod associated with this habitat, and we found that the abundances of this new genus and species were directly related to colony size.
The 46 valid genera of Stenothoidae include around 280 species (Horton et al. 2022), among which the genera Metopa Boeck 1871 and Stenothoe Dana 1852 are represented by more than 40% of the total species. Among these, 17 genera include only one species, and 10 genera are represented by only 2 species (Horton et al. 2022). W. minuta gen. nov., sp. nov., represents the eleventh monotypic genus and the tenth Stenothoid genus in European waters. Its small size (i.e., 1.21—1.45 mm), combined with the difficult accessibility of the mesophotic black coral habitat where the species lives, may explain why this genus had not been yet discovered. Our results suggest a high specificity of W. minuta gen. nov., sp. nov., for A. wollastoni as a host. To date, studies conducted in other habitats from the Canary Islands such as rhodoliths (Otero-Ferrer et al. 2019; Navarro-Mayoral et al. 2020), seagrasses (i.e., Cymodocea nodosa; Navarro-Mayoral et al. 2023), seaweeds (i.e., Caulerpa prolifera; Tuya et al. 2014), or sediments (Riera et al. 2012) have not revealed the presence of this genus and species.
Many species of amphipods live in close association with a wide variety of cnidarians (e.g., sea anemones, gorgonians and corals), through different types of specializations with their hosts (Vader 1983), which vary depending on the family and genus. Amphipods on black corals have been described as opportunistic (Wagner et al. 2012), based on the observations of caprellid amphipods feeding on living tissue of Antipathes sp., which led to the death of the entire colony (Tazioli et al. 2007). However, these observations are limited to caprellids and cannot be extended to all amphipods. Furthermore, it is not clear if the cause of death of Antipathes sp. is directly linked to the presence of amphipods, or to any previous disturbance. In our study with A. wollastoni, we reported a different pattern relative to Tazioli et al. (2007), with large abundances of amphipods that varied over time, while we did not observe any tissue necrosis of colonies throughout the study (almost 1 year). In fact, the most abundant species, W. minuta gen. nov., sp. nov., belongs to Stenothoidae, a family that include some species which tends to establish a biological association and display a strongly specificity with their hosts, as it has been observed with hydroids, for example (Vader and Krapp-Schickel 1996). This fact implies that amphipods spend their entire life cycle on their hosts, where they find shelter and food, such as Stenothoe brevicornis with the sea anemone Actinostola callosa (Vader 1983). Regarding the relationships between corals and amphipods, multiple ecological roles between them have been reported. Stenula nordmanni, for example, was observed in mutualistic relationship with some octocorals (e.g., Gersemia rubiformis, Caulier et al. 2021) and Stenothoe valida in commensalism with some hydrocorals (e.g., Millepora complanate, Lewis 1992). Understanding the interspecific relationships between black corals and amphipods is of great importance, especially in the case of W. minuta gen. nov., sp. nov., which was the dominant species over time in all A. wollastoni colonies. Regarding their population structure, we did not find any males among the dissected individuals. This result was expected, considering that we dissected 68 specimens out of 1496, and other studies have observed a skewed sex ratio toward females in species of the family Stenothoidae, such as Stenothoe valida that exhibited a sex ratio of up to 0.79 for females (Lee and Park 2021).
We found that amphipods were the dominant epifauna on A. wollastoni colonies over time, accounting for 98.3% of the total abundances, with W. minuta gen. nov., sp. nov., as the most abundant species by far (87.6% of the amphipods). Contrary to our results, the few previous studies on epifauna associated with black corals did not report a dominance of amphipods (Bo et al. 2012; Wagner et al. 2012; Deidun et al. 2015; Matamoros-Calderón et al. 2021), except Love et al. (2007), who reported a dominance of the amphipod Ericthonius rubricornis Stimpson 1853 on Antipathes dendrochristos Opresko 2005. However, this result was obtained from a single dead coral colony, and it is not easily comparable with our results, as in this case it is not sure if amphipods may be found in living colonies. Moreover, in our study, the abundances of amphipods were consistently large throughout the study, despite some variation among times. We observed the colonization of the near sea bottom by epibionts, such as sponges (e.g., Axinella spp.), and the presence of ascidians (e.g., Stolonica sp. and Pycnoclavella sp.) on the branches of A. wollastoni, but in the latter case the presence in the colonies was relatively infrequent or rare. Temporal variation in the abundances of amphipods can be attributed to changes in habitat structure throughout varying time scales, which alter habitat complexity via increased occurrence of epiphytes and associated algae (Jacobucci et al. 2009; Navarro-Mayoral et al. 2020). However, unlike other ecosystem engineers, such as seagrasses (e.g., Zostera noltii; Vermaat and Verhagen 1996), the primary habitat generated by A. wollastoni is stable over time and does not experience seasonal changes.
Our results showed that variation in amphipod abundances was correlated by the size of the colonies of A. wollastoni, with a significant (positive) relationship between coral colony size and amphipod abundances, both for the entire amphipod assemblage and for W. minuta gen. nov., sp. nov. Moreover, there is a colony size influence on the high variability of abundances within the times, especially in T3, where a greater difference in heights between the randomly selected colonies led to larger variations in abundances between replicates. Therefore, this suggests that the presence of amphipods is determined by habitat availability (i.e., colony size). Colonies of A. wollastoni dwell on rocky platforms, where they provide three-dimensional complexity to the substratum, offering a variety of microhabitats for several species (Czechowska et al. 2020). When A. wollastoni colonies are dense enough, they form a canopy (marine animal forest, sensu Rossi et al. 2017) that gives shelter and protection against strong currents and predators (Buhl-Mortensen et al. al. 2018). Thus, amphipods can benefit from different habitats provided by black corals, including (1) branches surface, (2) cavities within tissues or skeletons, and (3) free space between branches (Buhl-Mortensen and Mortensen 2004). This is consistent with the relatively large body of research that relates differences in abundances of marine invertebrates, in general, and amphipods in particular, with changes in the availability of habitat (Osman 1977; Aikins and Kikuchi 2001), so a higher abundance is observed in habitats with greater complexity and more available surface. Moreover, at these depths, there is a more limited availability of biogenic habitats compared to shallow waters, e.g., coral reefs, kelp forests, or seagrass meadows (Arfianti and Costello 2020a, b). Thus, the presence of A. wollastoni provides a 3-dimensional habitat for many species.
We found that the highest amphipod abundances were recorded in October (T3), which coincided with the highest temperature recorded. Some amphipods in shallow water environments show a turnover (i.e., growth and reproduction), which it is commonly adjusted to seasonal variation in terms of environmental conditions (e.g., temperature, photoperiod) and food resources (e.g., epiphytic biomass for herbivorous amphipods) (Neuparth et al. 2002; Martins et al. 2002; Navarro-Mayoral et al. 2020; Fernandez-Gonzalez et al. 2021). It is known that the temperature-induced changes in amphipod metabolic rates can lead to important physiological constraints to reproduction and determine of life history patterns of amphipods (Sainte-Marie 1991). For example, under laboratory conditions, Gammarus locusta showed faster individual growth, anticipated age at maturity, and higher population growth rate at a temperature of 20ºC compared to 15ºC (Neuparth et al. 2002). In our case, the difference between the minimum and maximum temperatures was 3.9ºC and controlled studies would be necessary to know exactly the direct relationship between temperature and the abundances of W. minuta gen. nov., sp. nov. Of course, temperature is typically correlated with a range of environmental and ecological processes. For example, an interesting observation was that the highest abundances of W. minuta gen. nov., sp. nov., coincided with a high amount of mucus generated by the colonies (personal observations). Mucus production is a strategy adopted by corals to obtain protection against sedimentation, which also plays a role in the food chain of habitats generated by corals (Galil 1987; Vytopil and Willis 2001; Wee et al. 2019; Fraser et al. 2020). Thus, mucus can catch detritus and phytoplankton that facilitate the feeding of amphipods, as observed in decapod taxa that feed on particles trapped by coral mucus (Galil 1987). Most of the species of the Stenothoid family are detritivorous and/or carnivorous species (Krapp-Schickel 1993; Moore et al. 1994; Sano et al. 2003; Tandberg et al. 2010; Auster et al. 2011; Vázquez-Luis et al. 2013; Guerra-García et al. 2014; Sedano et al. 2020), and W. minuta gen. nov., sp. nov., could take advantage of A. wollastoni secretions for feeding. Therefore, the amount of food available in the form of mucus could also be playing a role in the abundance pattern of amphipods, and specially of W. minuta gen. nov., sp. nov. However, more studies are needed to clarify the biological associations between deep-sea corals and epifauna, particularly focusing on the relationship between this black coral and this new genus and species of amphipod that dominated the associated epifauna community over time. Overall, this study supports that black coral forests in mesophotic depths have enormous potential for overlooked biodiversity and that much of the associated diversity remains undescribed.
Conclusions
We here reported patterns in composition and abundances of epifauna associated with Antipathella wollastoni over time, showing that amphipods were the dominant group. Our results confirmed that marine animal forests host an important, but somehow unknown, associated biodiversity. The new genus and species of the Stenothoidae family seem to be coral specific, displaying a direct relationship with the availability of habitat generated by the coral host. Our study reinforces the necessity to increase research effort in animal forest to improve management and conservation of these key habitats.
References
Aikins S, Kikuchi E (2001) Studies on habitat selection by amphipods using artificial substrates within an estuarine environment. Hydrobiologia 457(1):77–86. https://doi.org/10.1023/A:1012261116232
Angel, M. V. (1990). Life in the benthic boundary layer: connections to the mid-water and sea floor. Philosophical Transactions of the Royal Society of London. Series A, Mathematical and Physical Sciences, 331(1616), 15–28. https://doi.org/10.1098/rsta.1990.0053
Arfianti T, Wilson S, Costello MJ (2018) Progress in the discovery of amphipod crustaceans. PeerJ 6:e5187
Arfianti, T., & Costello, M. J. (2020). The biological, ecological, and ecosystem roles of marine Amphipoda. Encyclopedia of the World's Biomes.
Arfianti T, Costello MJ (2020b) Global biogeography of marine amphipod crustaceans: latitude, regionalization, and beta diversity. Mar Ecol Prog Ser 638:83–94
Auster, P. J., Heinonen, K. B., Watling, L., Parrish-Kuhn, C., Heupel, E., & Lindholm, J. (2011). A rare deepwater anemone and its associates in the Stellwagen Bank National Marine Sanctuary (Gulf of Maine, north-west Atlantic). Marine Biodiversity Records, 4. https://doi.org/10.1017/S175526721100020
Barnard, J.L. (1962). Benthic marine Amphipoda of southern California. 3. Families Amphilochidae, Leucothoidae, Stenothoidae, Argissidae, Hyalidae Pacific Naturalist, 3(3): 116–163.
Barnard JL (1972) Gammaridean Amphipoda of Australia, Part I. Smithsonian Contributions to Zoology 103:1–333
Barnard J.L. & Karaman G.S. (1987). Revisions in classification of gammaridean amphipoda (Crustacea). 3. Proceedings of the Biological Society of Washington, 100, 4, 856–875
Barnard JL, Karaman GS (1991) The families and genera of marine gammaridean Amphipoda (except marine gammaroids). Parts 1 and 2. Records of the Australian Museum, Supplement 13:1–866
Bates, D., Mächler, M., Bolker, B., & Walker, S. (2014). Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:1406.5823. https://doi.org/10.48550/arXiv.1406.5823
Bianchi C, Haroun R, Morri C, Wirtz P (2000) The subtidal epibenthic communities off Puerto del Carmen (Lanzarote, Canariy Islands). Arquipélago 2000(2):145–155
Bo, M., Lavorato, A., Di Camillo, C. G., Poliseno, A., Baquero, A., Bavestrello, G., ... & Reimer, J. D. (2012). Black coral assemblages from Machalilla National Park (Ecuador). Pacific Science, 66(1), 63–81. https://doi.org/10.2984/66.1.4.
Bo, M., Cerrano, C., Canese, S., Salvati, E., Angiolillo, M., Santangelo, G., & Bavestrello, G. (2014). The coral assemblages of an off‐shore deep Mediterranean rocky bank (NW Sicily, Italy). Marine Ecology, 35(3), 332–342. https://doi.org/10.1111/maec.12089
Boeck A (1871) Crustacea Amphipoda borealia et arctica. Forhandlinger I Videnskabs-Selskabet in Christiania 1870:81–280
Buhl-Mortensen LENE, Mortensen PB (2004) Symbiosis in deep-water corals. Symbiosis 37:33–61
Buhl‐Mortensen, L., Vanreusel, A., Gooday, A. J., Levin, L. A., Priede, I. G., Buhl‐Mortensen, P., … & Raes, M. (2010). Biological structures as a source of habitat heterogeneity and biodiversity on the deep ocean margins. Marine Ecology, 31(1), 21–50. https://doi.org/10.1111/j.1439-0485.2010.00359.x.
Buhl-Mortensen, L., Buhl-Mortensen, P., Rungruangsak-Torrissen, K., Schwach, V., Hjort, J., Jakobsen, T., & Toresen, R. (2018). Cold temperate coral habitats. Corals in a Changing World, 9. https://doi.org/10.1007/s10750-014-2116-x.
Caulier G, Hamel JF, Hendrycks EA, Conlan KE, Mercier A (2021) Mutualistic relationship between the amphipod Stenula nordmanni (Stephensen, 1931) and the nephtheid coral Gersemia rubiformis (Ehrenberg, 1834). Symbiosis 85(1):93–104. https://doi.org/10.1007/s13199-021-00800-5
Chevreux, E. (1900). Amphipodes provenant des campagnes de « l’Hirondelle » 1885–1888. omplanat des campagnes scientifiques du Prince Albert I de Monaco. 16: iv + 195 pp.
Czechowska, K., Feldens, P., Tuya, F., Cosme de Esteban, M., Espino, F., Haroun, R., … & Otero-Ferrer, F. (2020). Testing side-scan sonar and multibeam echosounder to study black coral gardens: a case study from Macaronesia. Remote Sensing, 12(19), 3244. https://doi.org/10.3390/rs12193244
De Clippele LH, Huvenne VA, Molodtsova TN, Roberts JM (2019) The diversity and ecological role of non-scleractinian corals (Antipatharia and Alcyonacea) on scleractinian cold-water coral mounds. Front Mar Sci 6:184. https://doi.org/10.3389/fmars.2019.00184
Deidun, A., Andaloro, F., Bavestrello, G., Canese, S., Consoli, P., Micallef, A., & Bo, M. (2015). First characterisation of a Leiopathes glaberrima (Cnidaria: Anthozoa: Antipatharia) forest in Maltese exploited fishing grounds. Italian Journal of Zoology, 82(2), 271–280. https://doi.org/10.1080/11250003.2014.986544
Fernandez-Gonzalez V, Navarro-Mayoral S and Sanchez-Jerez P (2021) Connectivity Patterns for Direct Developing Invertebrates in Fragmented Marine Habitats: Fish Farms Fouling as Source Population in the Establishment and Maintenance of Local Metapopulations. Frontiers in Marine Science. 8:785260. https://doi.org/10.3389/fmars.2021.785260
Fraser KM, Lefcheck JS, Ling SD, Mellin C, Stuart-Smith RD, Edgar GJ (2020) Production of mobile invertebrate communities on shallow reefs from temperate to tropical seas. Proc R Soc B 287(1941):20201798. https://doi.org/10.1098/rspb.2020.1798
Freiwald A, Helge Fosså J, Grehan A, Koslow, T, Roberts JM (2004) Cold-water coral reefs: out of sight - no longer out of mind. UNEP-WCMC Biodiversity Series, 22. Cambridge, UNEP/WCMC. ISBN 92-807-2453-3. 84 pp
Galil BS (1987) The adaptive functional structure of mucus-gathering setae in trapezid crabs symbiotic with corals. Symbiosis 4:75–86
Gray, D. J. E. (1857). Synopsis of the families and genera of axiferous zoophytes or barked corals. In Proceedings of the Zoological Society of London (Vol. 25, No. 1, pp. 278–294). Oxford, UK: Blackwell Publishing Ltd.
Guerra-García JM, De Figueroa JT, Navarro-Barranco C, Ros M, Sánchez-Moyano JE, Moreira J (2014) Dietary analysis of the marine Amphipoda (Crustacea: Peracarida) from the Iberian Peninsula. J Sea Res 85:508–517. https://doi.org/10.1016/j.seares.2013.08.006
Gurjanova, E. (1938). Amphipoda, Gammaroidea zalikov Siaukhu I Sudzukhe (Yaponskoe More). [Amphipoda, Gammaroidea of Siaukhu Bay and Sudzukhe Bay (Japan Sea)]. Reports of the Japan Sea Hydrobiological Expedition of the Zoological Institute of the Academy of Sciences USSR in 1934, 1, 241–404.
Hall-Spencer JM, Tasker M (2006) Report of the Working Group on Deep-water Ecology (WGDEC), 4–7 December 2005, Miami. Report of the Working Group on Deep-water Ecology (WGDEC), USA
Harrison, X. A., Donaldson, L., Correa-Cano, M. E., Evans, J., Fisher, D. N., Goodwin, C. E., ... & Inger, R. (2018). A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ, 6, e4794. https://doi.org/10.7717/peerj.4794
Havermans C, Smetacek V (2018) Bottom-up and top-down triggers of diversification: A new look at the evolutionary ecology of scavenging amphipods in the deep sea. Prog Oceanogr 164:37–51. https://doi.org/10.1016/j.pocean.2018.04.008
Herler J (2007) Microhabitats and ecomorphology of coral-and coral rock-associated gobiid fish (Teleostei: Gobiidae) in the northern Red Sea. Mar Ecol 28:82–94. https://doi.org/10.1111/j.1439-0485.2007.00165.x
Hoang DT, Vinh LS, Flouri T, Stamatakis A, von Haeseler A, Minh BQ (2018) MPBoot: fast phylogenetic maximum parsimony tree inference and bootstrap approximation. BMC Evol Biol 18:1–11
Horton, T.; Lowry, J.; De Broyer, C.; Bellan-Santini, D.; Coleman, C.O.; Corbari, L.; Costello, M.J.; Daneliya, M.; Dauvin, J.-C.; Fišer, C.; Gasca, R.; Grabowski, M.; Guerra-García, J.M.; Hendrycks, E.; Hughes, L.; Jaume, D.; Jazdzewski, K.; Kim, Y.-H.; King, R.; Krapp-Schickel, T.; LeCroy, S.; Lörz, A.-N.; Mamos, T.; Senna, A.R.; Serejo, C.; Sket, B.; Souza-Filho, J.F.; Tandberg, A.H.; Thomas, J.D.; Thurston, M.; Vader, W.; Väinölä, R.; Vonk, R.; White, K.; Zeidler, W. (2022). World Amphipoda Database. Stenothoidae Boeck, 1871. Accessed through: World Register of Marine Species at: https://www.marinespecies.org/aphia.php?p=taxdetails&id=101409 on 2022–06–15.
Jacobucci, G. B., Tanaka, M. O., & Leite, F. P. P. (2009). Temporal variation of amphipod assemblages associated with Sargassum filipendula (Phaeophyta) and its epiphytes in a subtropical shore. Aquatic Ecology, 43(4), 1031–1040. https://doi.org./https://doi.org/10.1007/s10452-009-9230-2
Jiménez Prada P, Hachero Cruzado I, Guerra García JM (2015) Importancia de los anfípodos en la dieta de especies de interés acuícola del litoral andaluz. Zoologica Baetica 26:3–29
Kalyaanamoorthy S, Minh BQ, Wong TK, Von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14(6):587–589
Karlson K, Bonsdorff E, Rosenberg R (2007) The impact of benthic macrofauna for nutrient fluxes from Baltic Sea sediments. AMBIO: J Human Env 36(2):161–167. https://doi.org/10.1579/0044-7447(2007)36[161:TIOBMF]2.0.CO;2
Krapp-Schickel G (1993) Do algal-dwelling amphipods react to the ‘critical zones’ of a coastal slope? J Nat Hist 27(4):883–900
Krapp-Schickel T (2000) Thaumatelsonine stenothoids (Crustacea, Amphipoda), Part 1. Mem Mus Vic 58(1):89–124. https://doi.org/10.24199/j.mmv.2000.58.5
Krapp-Schickel, T. (2006). Thaumatelsonine stenothoids (Crustacea, Amphipoda). Part 2. Zootaxa, 1165(1), 1–31.
Krapp-Schickel, T. (2011). On the Austral-Antarctic stenothoids Proboloides, Metopoides, Torometopa and Scaphodactylus (Crustacea Amphipoda). Part 2: the genus Proboloides, with description of two new genera and the transfer of two nominal species to Metopoides. Zookeys, (86), 11. https://doi.org/10.3897/zookeys.86.785
Krapp-Schickel T (2013) On Austral-Antarctic stenothoids (Amphipoda), part 3: Torometopa, Scaphodactylus, and two new genera. Crustaceana 86(7–8):829–852
Krapp-Schickel, T. (2015). Minute but constant morphological differences within members of Stenothoidae: The Stenothoe gallensis group with four new members, keys to Stenothoe worldwide, a new species of Parametopa and Sudanea n. gen. (Crustacea: Amphipoda). Journal of natural history, 49(37–38), 2309–2377. https://doi.org/10.1080/00222933.2015.1021873
Krapp-Schickel T, Vader WJM (2015) Stenothoids living with or on other animals (Crustacea, Amphipoda). Zoosystematics and Evolution 91(2):215–246. https://doi.org/10.3897/zse.91.5715
Kuznetsova, A., Brockhoff, P. B., & Christensen, R. H. (2017). lmerTest package: tests in linear mixed effects models. Journal of statistical software, 82, 1–26. https://doi.org/10.18637/jss.v082.i13
Latreille, P. A. (1806). Genera crustaceorum et insectorum secundum ordinem naturalem in familias disposita, iconibus exemplisque plurimis explicata. Amand Koenig, Parisiis et Argentorati (Paris & Strasbourg). 1: xviii + 303 pp., pls. 1–24
Latreille PA (1816) Amphipoda. In : Nouveau dictionnaire d’Histoire naturelle, appliquée aux Arts, à l’Agriculture, à l’Économie rurale et domestique, à la Médecine, etc. Par une société de Naturalistes et d’Agriculteurs. Vol. 1. 2nd Edn. Deterville, Paris, 467–469.
Lavelle, K.A., 2012. Epifaunal Assemblages on Deep-Water Corals in Roatan, Honduras. Master’s thesis. Texas A&M University–Corpus Christi.
Lee YJ, Park WG (2021) Population dynamics of Stenothoe valida Dana, 1852 (Amphipoda, Stenothoidae) at Cheongsapo beach of Busan. Republic of Korea Crustaceana 94(4):413–429
Lewis JB (1992) Abundance, distribution and behavior of a commensal amphipod Stenothoe valida Dana on the hydrocoral Millepora omplanate Lamarck. Bull Mar Sci 51(2):245–249
Love MS, Yoklavich MM, Black BA, Andrews AH (2007) Age of black coral (Antipathes dendrochristos) colonies, with notes on associated invertebrate species. Bull Mar Sci 80(2):391–399
Lowry JK, Myers AA (2013) A phylogeny and classification of the Senticaudata subord. Nov. (Crustacea: Amphipoda). Zootaxa 3610(1): 1–80. https://doi.org/10.11646/zootaxa.3610.1.1
Mann KH, Lazier JR (2013) Dynamics of marine ecosystems: biological-physical interactions in the oceans. Blackwell Scientific Publiations, Boston, MA, USA, p 446
Marin, I. N., & Sinelnikov, S. Y. (2017). Amphipod assemblage found on sublittoral hydroids in the White Sea with the special remarks to symbiotic association of stenothoid Metopa alderi with hydroid Tubularia larynx. Ukrainian Journal of Ecology, 7(4), 473–479. https://doi.org/10.15421/2017_146
Martins I, Maranhão P, Marques JC (2002) Modelling the effects of salinity variation on Echinogammarus marinus Leach (Amphipoda, Gammaridae) density and biomass in the Mondego estuary (Western Portugal). Ecol Model 152(2–3):247–260. https://doi.org/10.1016/S0304-3800(02)00012-1
Matamoros-Calderón, W., Lara, M., & Breedy, O. (2021). Bosques de coral negro del Área de Conservación Guanacaste, Costa Rica: especies y distribución. Revista de Biología Tropical, 69, 208–218. https://doi.org/10.15517/rbt.v69is2.48317
Minh, B. Q., Schmidt, H. A., Chernomor, O., Schrempf, D., Woodhams, M. D., Von Haeseler, A., & Lanfear, R. (2020). IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution, 37(5), 1530–1534.
Molodtsova T, Opresko D (2023) World List of Antipatharia. Antipatharia. World Register of Marine Species, Accessed through
Moore, P. G., Rainbow, P. S., & Vader, W. (1994). On the feeding and comparative biology of iron in coelenterate-associated gammaridean Amphipoda (Crustacea) from N. Norway. Journal of experimental marine biology and ecology, 178(2), 205–231.
Navarro-Mayoral S, Fernandez-Gonzalez V, Otero-Ferrer F, Tuya F (2020) Spatio-temporal variability of amphipod assemblages associated with rhodolith seabeds. Mar Freshw Res 72(1):76–83. https://doi.org/10.1071/MF19360
Navarro-Mayoral, S., Tuya, F., Prado, P., Marco-Méndez, C., Fernandez-Gonzalez, V., Fernández-Torquemada, Y., ... & Martínez-Crego, B. (2023). Drivers of variation in seagrass-associated amphipods across biogeographical areas. Marine Environmental Research, 186, 105918.
Neuparth T, Costa FO, Costa MH (2002) Effects of temperature and salinity on life history of the marine amphipod Gammarus locusta. Implications for Ecotoxicological Testing Ecotoxicology 11(1):61–73. https://doi.org/10.1023/a:1013797130740
Orejas C, Gori A, Iacono CL, Puig P, Gili JM, Dale MR (2009) Cold-water corals in the Cap de Creus canyon, northwestern Mediterranean: spatial distribution, density and anthropogenic impact. Mar Ecol Prog Ser 397:37–51. https://doi.org/10.3354/meps08314
Osman RW (1977) The establishment and development of a marine epifaunal community. Ecol Monogr 47(1):37–63
Otero-Ferrer, F., Mannarà, E., Cosme, M., Falace, A., Montiel-Nelson, J. A., Espino, F., & Tuya, F. (2019). Early-faunal colonization patterns of discrete habitat units: A case study with rhodolith-associated vagile macrofauna. Estuarine, Coastal and Shelf Science, 218, 9–22. https://doi.org/10.1016/j.ecss.2018.11.020
Ren X, Huang L (1991) Studies on gammaridea and caprellidea (Crustacea: Amphipoda) from the northwest waters off the Antarctic Peninsula. Studia Marina Sinica 32(10):185–323
Riera R, Tuya F, Ramos E, Rodríguez M, Monterroso Ó (2012) Variability of macrofaunal assemblages on the surroundings of a brine disposal. Desalination 291:94–100. https://doi.org/10.1016/j.desal.2012.02.003
Rossi, S., Bramanti, L., Gori, A., & Orejas, C. (2017). An overview of the animal forests of the world. Marine animal forests: the ecology of benthic biodiversity hotspots, 1–28. https://doi.org/10.1007/978-3-319-17001-5_1-1
RStudio Team. (2022). RStudio: Integrated development for R. RStudio, PBC.
Ruffo S (1998) The amphipoda of the Mediterranean. Memoires De L’institut Oceanographique De Monaco 13:959
Sainte-Marie B (1991) A review of the reproductive bionomics of aquatic gammaridean amphipods: variation of life history traits with latitude, depth, salinity and superfamily. Hydrobiologia 223(1):189–227. https://doi.org/10.1007/BF00047641
Sano M, Omori M, Taniguchi K (2003) Predator-prey systems of drifting seaweed communities offthe Tohoku coast, northern Japan, as determined by feeding habitanalysis of phytal animals. Fish Sci 69(2):260–268
Schellenberg, A., (1931). Gammariden und Caprelliden des Magellangebietes, Sudgeorgiens und der Westantarktis. Further Zoological Results of the Swedish Antarctic Expedition, 1901–1903 2(6): 1–290.
Sedano F, de Figueroa JT, Navarro-Barranco C, Ortega E, Guerra-García JM, Espinosa F (2020) Do artificial structures cause shifts in epifaunal communities and trophic guilds across different spatial scales? Marine Env Res 158:104998
Spalding M, Ravilious C, Green EP (2001) World Atlas of coral reefs. Univ of California Press.
Stebbing, T. R. R. (1888). Report on the Amphipoda collected by HMS Challenger during the years 1873–1876. Report on the Scientific Results of the Voyage of HMS Challenger during the Years 1873–1876, Zoology, 29, pls-I.
Tandberg AHS, Vader W, Berge J (2010) Studies on the association of Metopa glacialis (Amphipoda, Crustacea) and Musculus discors (Mollusca, Mytilidae). Polar Biol 33:1407–1418
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38(7):3022-3027
Tazioli S, Bo M, Boyer M, Rotinsulu H, Bavestrello G (2007) Ecological observations of some common antipatharian corals in the marine park of Bunaken (North Sulawesi, Indonesia). Zoological Studies 46(2):227–241
Tuya F, Png-Gonzalez L, Riera R, Haroun R, Espino F (2014) Ecological structure and function differ between habitats dominated by seagrasses and green seaweeds. Mar Environ Res 98:1–13. https://doi.org/10.1016/j.marenvres.2014.03.015
Tuya, F., Aguilar, R., Espino, F., Bosch, N. E., Meyers, E. K., Jiménez‐Alvarado, D., ... & Haroun, R. (2021). Differences in the occurrence and abundance of batoids across an oceanic archipelago using complementary data sources: Implications for conservation. Ecology and Evolution, 11(23), 16704–16715. https://doi.org/10.1002/ece3.8290
Vader W (1983) Associations between amphipods (Crustacea: Amphipoda) and sea anemones (Anthozoa, Actiniaria). Memoirs of the Australian Museum 18:141–153
Vader, W., & Krapp-Schickel, G. (1996). Redescription and biology of Stenothoe brevicornis Sars (Amphipoda: Crustacea), an obligate associate of the sea anemone Actinostola callosa (Verrill). Journal of Natural History, 30(1), 51–66. https://doi-org.bibproxy.ulpgc.es/https://doi.org/10.1080/00222939600770041
Vázquez-Luis, M., Sanchez-Jerez, P., & Bayle-Sempere, J. T. (2013). Does the invasion of Caulerpa racemosa var. cylindracea affect the feeding habits of amphipods (Crustacea: Amphipoda)?. Journal of the Marine Biological Association of the United Kingdom, 93(1), 87–94.
Vermaat, J. E., & Verhagen, F. C. (1996). Seasonal variation in the intertidal seagrass Zostera noltii Hornem.: coupling demographic and physiological patterns. Aquatic Botany, 52(4), 259–281.
Vytopil, E., & Willis, B. (2001). Epifaunal community structure in Acropora spp. (Scleractinia) on the Great Barrier Reef: implications of coral morphology and habitat complexity. Coral Reefs, 20(3), 281–288. https://doi.org/10.1007/s003380100172
Wagner D, Luck DG, Toonen RJ (2012) The biology and ecology of black corals (Cnidaria: Anthozoa: Hexacorallia: Antipatharia). Adv Mar Biol 63:67–132. https://doi.org/10.1016/B978-0-12-394282-1.00002-8
Wee, S. Y. C., Sam, S. Q., Sim, W. T., Ng, C. S. L., Taira, D., Afiq-Rosli, L., ... & Chou, L. M. (2019). The role of in situ coral nurseries in supporting mobile invertebrate epifauna. Journal for Nature Conservation, 50, 125710. https://doi.org/10.1016/j.jnc.2019.125710
Acknowledgements
We acknowledge Alvaro Roldán (Liquid Planet) and José Manuel Golderos Tena for their assistance in technical diving.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study has been produced with the financial support from the LIFE Programme of the European Union, the French Office for Biodiversity (OFB), and the French Development Agency (AFD) through the LIFE4BEST Programme. The contents of this document are the sole responsibility of B-CHARMED (The Black Coral forests as unexplored Biodiversity Hotspots in the MAcaronesian Region: omplanat functions and sErvices analyzed) project and can under no circumstances be regarded as reflecting the position of the European Union nor of the OFB and AFD. Lucas Terrana was funded by the association Fonds Léopold III pour l’Exploration et la Conservation de la Nature ASBL. DNA sequencing was financed by the Belgian Fond de la Recherche Scientifique—FNRS via “Projet de Recherches” grant n◦ T.00078.23 to Jean-François Flot.
Author information
Authors and Affiliations
Contributions
Conceptualization was performed by FOF, LB, SNM; method design by FOF, LB, SNM; investigation by FOF, LB, SNM, LT, FE, RH; analysis by SNM, FT; interpretation of data by SNM, FT, FOF, LB; taxonomy work by BG, SNM, VFG; drawings and taxonomic preparation by BG; DNA extraction, PCR amplification, and nanopore sequencing by NL; assembly and analysis of DNA sequences by JFF; funding acquisition by FOF, LT, JFF; supervision by FOF, FT; writing—original draft preparation by SNM; writing—review and editing by all authors.
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. The contents of this document are the sole responsibility of the authors and can, under no circumstances, be regarded as reflecting the position of the EU, nor of the OFB and AFD.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Navarro-Mayoral, S., Gouillieux, B., Fernandez-Gonzalez, V. et al. “Hidden” biodiversity: a new amphipod genus dominates epifauna in association with a mesophotic black coral forest. Coral Reefs 43, 655–672 (2024). https://doi.org/10.1007/s00338-024-02491-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-024-02491-y