Abstract
Establishment of the coral–algal symbiosis begins during early ontogeny when juveniles acquire a mix of algae from their environment that often differs from the adults’ algal assemblages. Despite the importance of the type of Symbiodiniaceae to this symbiosis, it is largely unknown how coral host identity and environment affect symbiosis establishment and is affected by the genetic composition of the symbionts. Here, we reciprocally transplanted planulae of the octocoral Rhytisma fulvum (Forskål, 1775) across depths and monitored the algal assemblages in the developing juveniles for 11 months. We then compared these to adult assemblages using ITS2 metabarcoding. Juveniles were consistently dominated by Symbiodinium, in addition to multiple Cladocopium species, which shifted in dominance with the juvenile age but maintained high similarity across depths. The type of Symbiodiniaceae environmentally available thus likely contributes to the algal symbionts that are initially acquired, while host identity may play a significant role in selecting for symbionts that are maintained during juvenile development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Octocorals are highly abundant on many reef ecosystems, providing essential habitat and nutrition services to a variety of reef dwellers, as well as filtering nutrients and maintaining water quality (Fabricius 2005; Fabricius and Klumpp 1995). Most shallow Indo-Pacific octocorals establish mutualistic endosymbioses with intracellular photosynthetic dinoflagellates of the family Symbiodiniaceae (Fabricius and Klumpp 1995; LaJeunesse et al. 2018; Goulet et al. 2008). Previous studies have shown that genetically different Symbiodiniaceae assemblages can lead to distinct responses of their hosts under diverse environmental conditions (DeSalvo et al. 2010; Sampayo et al. 2008). In most coral species, the algal symbionts must be acquired anew from the environment each generation (i.e., horizontal transmission: Baird et al. 2009). This implies a potential for environmental adaptation by means of flexibility in algal symbiont uptake, even though the high genetic diversity of algal communities during the initial acquisition stage is eventually winnowed in favor of relatively stable and less diverse assemblages in adult corals (Abrego et al. 2009; Poland and Coffroth 2017). Examining the effects of prevailing environmental conditions on the symbiont acquisition process and their winnowing during the highly dynamic stage of juvenile development is critical to our understanding of the possible effects of external cues on this symbiosis and its trajectory under climate change (Coffroth et al. 2022; Cumbo et al. 2013; Poland and Coffroth 2017; Quigley et al. 2017).
Mesophotic coral ecosystems (MCEs, 30–150 m depth) extend the shallow-water coral reefs down to the lower edge of the photic zone (Hinderstein et al. 2010). MCEs are subjected to light attenuation, thus affecting coral community structure and reliance on photoautotrophic carbon production (Kahng et al. 2019). While most coral species have a limited vertical distribution, some species are able to thrive across a wide depth range (i.e., “depth-generalists”: Bongaerts et al. 2015). Certain coral species may host different Symbiodiniaceae assemblages across depth that is considered to be more acclimatized to the deeper water environment (Andras et al. 2011; Bongaerts et al. 2015; Cooper et al. 2011; Kirk et al. 2009; Pochon et al. 2015; Prada et al. 2014), whereas others maintain similar algal assemblages across depth (Ziegler et al. 2015; Liberman et al. 2022a, b). In contrast with studies on symbiont assemblages in adult colonies, there are very few studies on algal symbiont acquisition and identity in MCE juvenile corals.
The octocoral Rhytisma fulvum (Forskål, 1775) is a surface-brooder that releases azooxanthellate planula larvae whose algal symbionts are acquired during early metamorphosis (Benayahu and Loya 1983). In the Gulf of Aqaba (GoA), R. fulvum hosts similar Cladocopium sp. symbionts across its entire depth of occurrence (0–50 m: Liberman et al. 2022a, b). The previous studies have shown the critical role of the environment in shaping octocoral-Symbiodiniaceae associations during early life stages of the host (Abrego et al. 2012; Coffroth et al. 2001; McIlroy et al. 2019). However, other findings on scleractinians have revealed that host genetics may have a role in determining symbiont associations (McIlroy and Coffroth 2017; Quigley et al. 2017; Yamashita et al. 2014). Despite rather extensive research on symbiont acquisition across spatial scales, comparisons across depth ranges, such as between shallow reefs and MCEs, are lacking. To address this gap, we examined the symbiont associations in R. fulvum juveniles from shallow waters to MCEs in the GoA. We addressed the question of whether depth affects the identity of algal symbionts present in the juveniles over time. We further assessed whether depth affects the winnowing process during the 1st year of the juveniles’ development. The findings from examining symbiont dynamics from shallow to mesophotic depths are expected to provide an ecological context concerning the role played by the host and its environment during early establishment of the octocoral–algal partnership across depth gradients.
Materials and methods
Study site and sampling
Collection of R. fulvum planulae took place at the reef across from the Interuniversity Institute for Marine Sciences (IUI) in Eilat (Israel), from both the shallow reef (5–10 m) and an upper mesophotic reef (40–42 m), at a short horizontal distance from one another (see Supplementary Information). Upon sampling, the planulae were cleansed of mucus by washing them in filtered seawater. They were then introduced into 50-ml transparent PVC chambers (40 planulae per chamber, 80 chambers) (see also Liberman et al., 2020). Each chamber contained two preconditioned terracotta tiles (2 × 2 cm each) and was sealed with a 100 µm mesh. The terracotta tiles had been preconditioned on the Eilat reef for 7–8 months at the shallow (8 m) and the upper mesophotic (40 m) depth prior to the experiment (November 2018). The tiles were then placed into the chambers according to their preconditioning history (i.e., shallow-conditioned tiles in all chambers deployed in shallow and MCE-conditioned tiles in all chambers deployed in MCE). The chambers were transferred to the reef and placed on artificial structures at both shallow (8 m) and upper MCE (40 m) locations (20 chambers from each sampling depth at each depth). The planulae successfully settled on the tiles, metamorphosed into primary polyps, and subsequently developed into juvenile colonies (hereafter “juveniles”) that were later retrieved at five time points: 1 week and 2, 5, 7, and 11 months after placement on the reef, in order to determine any changes in Symbiodiniaceae identity as the coral development progressed. To ensure sufficient DNA quantities from R. fulvum juveniles, samples comprised of primary polyps with 1–4 polyps were pooled together into individual samples (between 2 and 8 juveniles per sample), while larger juveniles, comprised of more than 4 polyps, were sampled individually (see Table S1).
ITS2- and psbA-based algal symbiont typing
DNA isolation was performed on a total of 49 samples using the Qiagen DNeasy Blood and Tissue kit, with minor adjustments. Briefly, the ethanol used to preserve the samples was removed, and the samples were left to air-dry to remove any residual ethanol. Following this, 180 μl of ATL buffer was added, and each sample was ground with a pestle to rupture the algal cells. Next, 20 µl of proteinase K was added, and the samples were incubated at 56 °C for 1.5 h. DNA extractions were then performed according to the manufacturer’s instructions, with a final elution volume of 40 μl in molecular-grade water. DNA concentrations were quantified by Qubit (Invitrogen). Amplification of the ITS2 region was done using the primers SYM_VAR_5.8S2 and SYM_VAR_REV, according to Hume et al. (2018), with unique 8-mer barcodes at their 5’ ends. To confirm successful amplification, 1 µl of each PCR product was run on a 1% agarose gel. Samples were cleaned using ExoProStar (GE Healthcare) and normalized using the SequalPrep Normalization Plate Kit (Thermo Fisher Scientific). Samples were paired-end sequenced (2 × 250 bp) on the NovaSeq 6000 platform at the Novogene Sequencing Centre (Cambridge, England).
Samples were further analyzed by amplifying the non-coding region of the plastid psbA minicircle, following Lajeunesse and Thornhill (2011). To accommodate for samples comprising more than one algal symbiont community, PCR products were run on a 2% TBE gel for 2 h. Samples comprising more than one band were then extracted directly from the gel (QIAquick Gel Extraction Kit). For samples featuring a single band, the DNA was sequenced directly from the PCR product. Samples were Sanger-sequenced at Eurofins Genomics with internal primer, following Hume et al. (2015).
Data analysis
Demultiplexed ITS2 paired reads from each sample were analyzed using the SymPortal framework (Hume et al. 2019). The prediction of Symbiodiniaceae ITS2 profiles (putative symbiont genotypes) was based on the presence and abundance of the ITS2 sequences within the SymPortal database. Additionally, we merged ITS2 profiles by using a clustering approach to consider highly similar profiles as the same distinct entity. For example, all profiles featuring A1-A1bw-A1bf were clustered into “A1-A1bw-A1bf,” while other profiles from this genus were named “Other Symbiodinium.”
To resolve cases in which more than a single Symbiodiniaceae genus was present in an individual coral sample, psbA marker sequences were used (Davies et al. 2023). Sequence chromatograms for the psbA region were visually inspected for accuracy in base calling using Geneious, and the edited sequences were aligned together with sequences from Hume et al. (2015) and Lajeunesse and Thornhill (2011). Phylogenetic relationships were reconstructed under the maximum-likelihood (ML) criterion, as implemented in the program IQ-TREE version 2.1.3 (Nguyen et al. 2015).
Results and discussion
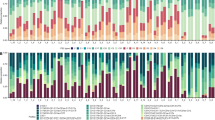
To assess algal symbiosis establishment and their assemblage dynamics, also in relation to the host environment, R. fulvum planulae were removed from their shallow-water and mesophotic environments and reciprocally transplanted into each other’s collection depth (Fig. 1). Overall, the algal symbiont profiles observed across the first 11 months of development in R. fulvum juveniles differed across time, but remained similar across depth, although some exceptions did occur (Fig. 1).
Algal symbiont assemblage dynamics in octocorals collected from shallow and mesophotic reefs and transplanted to reciprocal depths. Normalized relative abundance of ITS2-type profiles in Rhytisma fulvum juveniles and adult colonies in shallow and mesophotic depth environments. The different squares represent different parental and transplanted depth environments. N indicates the total number of primary polyps, juveniles, and colonies sampled and analyzed at each time point at a given depth
Three profiles corresponding to Cladocopium were detected in R. fulvum juveniles at both transplanted depths (8 and 40 m). The clustered profile “C1-C. thermophilum mix” accounted for 4–31% and 21–31% of the relative abundance in 1-week and 2-month-old juveniles, respectively (Table S2). One exception was detected in the 2-month-old juveniles that had been collected and developed in the MCE, and which mostly hosted Cladocopium symbionts of the clustered profile “C64a/C64b” (30% of relative abundance, Table S2). While the clustered Cladocopium profile “C64a/C64b” dominated the symbiont community of 5-month and 7-month-old juveniles (30–59% and 29–59% of the relative abundance, respectively), this profile was absent from juveniles that had been collected from the MCE environment and developed in the shallow transplanted environment (Fig. 1 and Table S2). Later in the experiment, the relative abundance of the clustered Cladocopium profile C64a/C64b in 11-month-old juveniles remained high (45% and 30% of juveniles that had been collected and transplanted in the shallow and MCE depths, respectively). In addition, the clustered Cladocopium profile “C1-C1dw” dominated the shallow in shallow symbiont community of 11-month-old juveniles, whereas its relative abundance in juveniles that were collected and transplanted in the MCE was considerably lower (47% and 12%, respectively). Surprisingly, the latter profile also dominated the symbiont composition of 1-week-old juveniles that had been collected from the MCE and transplanted to the shallow environment (61% of the relative abundance).
Clustered profiles corresponding to genera that had not been previously detected in adult colonies of R. fulvum dominated the juveniles across depth (Fig. 1). For example, Symbiodinium algal symbionts of the clustered profile “A1-A1bw-A1bf” were detected in all sampled juveniles. Their relative abundance remained high throughout the experiment, ranging from 30 to 74%, with the exception of two time points (9% in 1-week-old juveniles collected from the MCE and developed in shallow-water; and 5% in 11-month-old juveniles collected from shallow-water and developed in a similar (shallow) transplanted environment (Table S2)). Notably, Symbiodinium is commonly found in the early ontogeny of some coral recruits and has been previously shown to be one of their first colonizers, although its presence may disappear in the adult stage (Coffroth et al. 2001, 2022; Gómez-Cabrera et al., 2008; Yamashita et al. 2013). Shallow Stylophora pistillata adult colonies may release algal symbionts into the surrounding seawater and could potentially serve as a source of Symbiodinium spp. in the reef environment (Mass et al. 2007). It is also noteworthy that in the northern Red Sea, Symbiodinium have also been detected in vertically-transmitting octocoral species (Barneah et al. 2004; Liberman et al. 2022a, b). Moreover, other profiles of the genus Symbiodinium were acquired by R. fulvum juveniles during the first 2 months of ontogeny (Fig. 1). A low incidence of Durusdinium algal symbionts of the clustered profile “D1-D4” (1–12%) was occasionally detected in juveniles of R. fulvum from week 1 to month 11.
A considerable number of studies have assessed the effect of light intensity and symbiont availability on symbiont composition in horizontally-transmitting corals at the planula and juvenile stages (Coffroth et al. 2022; Cumbo et al. 2013; Howells et al. 2013; Quigley et al. 2017). Although it is commonly assumed that a well-lit environment significantly affects algal symbiont assemblages (Rowan et al. 1997; Rowan and Knowlton 1995), our findings may suggest that light environment (depth) had only a minor effect on the symbiont composition in juveniles of R. fulvum. It has also been posited that it is not fully clear whether Symbiodiniaceae associations during larval stages already represent mutualistic interactions, which would further explain the here-observed pattern (Mies et al. 2017). In addition, the previous studies have suggested that if the symbionts in the environment are similar, then that pool that is likely where the similarities originate from (Andras et al. 2011; Coffroth et al. 2022). By preconditioning the settlement tiles used here at similar depths to those of the transplanted depths, we sought to simulate the symbiont composition that is found at the respective environmental depth. Thus, the observed similarity in symbiont composition between most juveniles at the early time points across depth (Fig. 1) may be a reflection of comparable Symbiodiniaceae populations in their adjacent environment (i.e., on the settlement tiles or in the water). Moreover, the similarity of algal compositions across depth at the later stages of some juveniles is likely due to host selection during the winnowing process. However, in the future studies, juveniles would need to be followed for a longer time period to unequivocally confirm this. Further studies elucidating the relative effect of environmental symbiont availability and selection by host identity may provide insights into the intriguing question of how these drivers interact to shape host-symbiont early life stage associations from shallow to mesophotic depths.
Our current findings indicate that 5- and 7-month-old juveniles were dominated by algal symbionts (C64a/C64b) that are rarely found in adult colonies and were absent from most of the juveniles sampled at earlier time points. In addition to this profile, 11-month-old juveniles were also found to harbor two distinct Cladocopium profiles; C64a/C64b and an additional profile not found in adults (“C1-C1dw”; Figs. 2 and 3). Thus, the findings indicate a marked disparity between the composition of Cladocopium algal symbionts in R. fulvum juveniles at various ages and those found in the respective adult colonies, which is likely a combination of the locally predominant algal symbiont pool and symbiont competition. Such disparity challenges the prediction of identity of algal symbionts by means of exclusively identifying those of adult colonies or those of juveniles from a single time point in their 1st year of development.
Principal coordinate analysis (PCoA) based on Bray–Curtis distances of ITS2-type profiles. The PCoA shows the grouping of Rhytisma fulvum juveniles/adults based on associated algal symbiont ITS2 sequences for the three most dominant genera throughout the study: A Cladocopium, B Symbiodinium, and C Durusdinium. Color indicates juvenile age at the time of collection, and shape indicates their transplanted depth. The percentage variation explained by each PC1 and PC2 is indicated
Little is known regarding the duration of the winnowing process in corals, in particular with regard to the time required for juveniles to establish a stable-state symbiosis with those complementary symbionts that are eventually present in the adult colonies. The previous studies have indicated that such a process in juvenile corals can be extensive and may differ among species (3–4 yrs.: Abrego et al. 2009; Poland and Coffroth 2017). The current results indicate that, at 11 months, R. fulvum juveniles still host a different Cladocopium algal community from that found in adult colonies, while also hosting Symbiodinium and Durusdinium D1–D4 symbionts. These findings are in line with the previous studies (Gómez-Cabrera et al., 2008), also reinforcing the notion that the duration of the winnowing process in R. fulvum juveniles is extensive. Thus, it is likely that the period of our experiment was not sufficiently long to capture the entire winnowing process until host adulthood.
Using both data from the ITS2 marker and the psbA non-coding region, the current results indicate an unexpected presence of C. thermophilum in 1-week and 2-month old juveniles (Figs. 1 and 3). This is the first evidence of this thermally-tolerant algal species in the GoA, confirming that it has a wider geographic distribution than previously described (Hume et al. 2016). In addition, it is the first observation of the heat-stress-tolerant D. trenchii (Silverstein et al. 2014; Stat and Gates 2010), in the very northern tip of GoA, although it has been previously recorded in the Arabian Peninsula (Hume et al. 2016; Osman et al. 2020; Ziegler et al. 2017). Accelerated warming in the northern Red Sea, compared to global averages (Chaidez et al. 2017; Fine et al. 2013), coupled with the Symbiodiniaceae potential for rapid, long-distance dispersal (LaJeunesse et al. 2014), may facilitate the establishment of these heat stress-tolerant symbiont species in the GoA reef environment. By sampling the octocoral juveniles, however, we uncovered the regional Symbiodiniaceae diversity, including the presence of cryptic and previously overlooked taxa. To verify whether these findings represent a true range expansion of the different symbionts, further analyses of both juveniles and adults across their host species are required. As ocean warming accelerates globally (IPCC 2021), insights into the flexibility of coral juveniles to acquire and maintain diverse photosymbionts, including potential stress-tolerant taxa, are critical for predicting and mitigating climate change impacts.
Data availability
The Illumina sequences of the ITS2 marker are available under NCBI BioProject PRJNA953952 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA953952). SymPortal ITS2 abundance files and scripts used in this work can be found on GitHub (https://github.com/ronenliberman/Symbiodiniaceae-in-juvenile-octocoral).
References
Abrego D, Van Oppen MJH, Willis BL (2009) Onset of algal endosymbiont specificity varies among closely related species of Acropora corals during early ontogeny. Mol Ecol 18(16):3532–3543. https://doi.org/10.1111/j.1365-294X.2009.04276.x
Abrego D, Willis BL, van Oppen MJH (2012) Impact of light and temperature on the uptake of algal symbionts by coral juveniles. PLoS ONE 7(11):e50311. https://doi.org/10.1371/journal.pone.0050311
Andras JP, Kirk NL, Drew Harvell C (2011) Range-wide population genetic structure of Symbiodinium associated with the Caribbean Sea fan coral. Gorgonia Ventalina Molecul Ecol 20(12):2525–2542. https://doi.org/10.1111/j.1365-294X.2011.05115.x
Baird AH, Guest JR, Willis BL (2009) Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu Rev Ecol Evol Syst 40(1):551–571. https://doi.org/10.1146/annurev.ecolsys.110308.120220
Barneah O, Weis V, Perez S, Benayahu Y (2004) Diversity of dinoflagellate symbionts in Red Sea soft corals: Mode of symbiont acquisition matters. Mar Ecol Prog Ser 275:89–95. https://doi.org/10.3354/meps275089
Benayahu Y, Loya Y (1983) Surface brooding in the red sea soft coral parerythropodium fulvum fulvum (Forskål, 1775). Biol Bull 165(2):353–369. https://doi.org/10.2307/1541201
Bongaerts P, Carmichael M, Hay KB, Tonk L, Frade PR, Hoegh-Guldberg O (2015) Prevalent endosymbiont zonation shapes the depth distributions of scleractinian coral species. Royal Soc Open Sci 2(2):140297. https://doi.org/10.1098/rsos.140297
Chaidez V, Dreano D, Agusti S, Duarte CM, Hoteit I (2017) Decadal trends in Red Sea maximum surface temperature. Scientific Rep. https://doi.org/10.1038/s41598-017-08146-z
Coffroth M, Santos S, Goulet T (2001) Early ontogenetic expression of specificity in a cnidarian-algal symbiosis. Mar Ecol Prog Ser 222:85–96. https://doi.org/10.3354/meps222085
Coffroth MA, Leigh NJ, McIlroy SE, Miller MW, Sheets HD (2022) Genetic structure of dinoflagellate symbionts in coral recruits differs from that of parental or local adults. Ecol Evol 12(9):e9312. https://doi.org/10.1002/ece3.9312
Cooper TF, Ulstrup KE, Dandan SS, Heyward AJ, Kühl M, Muirhead A, O’Leary RA, Ziersen BEF, Van Oppen MJH (2011) Niche specialization of reef-building corals in the mesophotic zone: Metabolic trade-offs between divergent Symbiodinium types. Proc Royal Soc Biol Sci 278(1713):1840–1850. https://doi.org/10.1098/rspb.2010.2321
Cumbo VR, Baird AH, Van Oppen MJH (2013) The promiscuous larvae: flexibility in the establishment of symbiosis in corals. Coral Reefs 32(1):111–120. https://doi.org/10.1007/s00338-012-0951-7
Davies SW, Gamache MH, Howe-Kerr LI, Kriefall NG, Baker AC, Banaszak AT, Bay LK, Bellantuono AJ, Bhattacharya D, Chan CX, Claar DC, Coffroth MA, Cunning R, Davy SK, del Campo J, Díaz-Almeyda EM, Frommlet JC, Fuess LE, González-Pech RA, Parkinson JE (2023) Building consensus around the assessment and interpretation of Symbiodiniaceae diversity. PeerJ 11:e15023. https://doi.org/10.7717/peerj.15023
del C. Gómez-Cabrera, M., Ortiz, J. C., Loh, W. K. W., Ward, S., & Hoegh-Guldberg, O. (2008) Acquisition of symbiotic dinoflagellates (Symbiodinium) by juveniles of the coral Acropora longicyathus. Coral Reefs 27(1):219–226. https://doi.org/10.1007/s00338-007-0315-x
DeSalvo MK, Sunagawa S, Fisher PL, Voolstra CR, Iglesias-Prieto R, Medina M (2010) Coral host transcriptomic states are correlated with Symbiodinium genotypes. Mol Ecol 19(6):1174–1186. https://doi.org/10.1111/j.1365-294X.2010.04534.x
Fabricius KE (2005) Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar Pollut Bull 50(2):125–146. https://doi.org/10.1016/j.marpolbul.2004.11.028
Fabricius KE, Klumpp D (1995) Widespread mixotrophy in reef-inhabiting soft corals: the influence of depth, and colony expansion and contraction on photosynthesis. Mar Ecol Prog Ser 125:195–204. https://doi.org/10.3354/meps125195
Fine M, Gildor H, Genin A (2013) A coral reef refuge in the Red Sea. Glob Change Biol 19(12):3640–3647. https://doi.org/10.1111/gcb.12356
Goulet T, Simmons C, Goulet D (2008) Worldwide biogeography of Symbiodinium in tropical octocorals. Mar Ecol Prog Ser 355:45–58. https://doi.org/10.3354/meps07214
Hinderstein LM, Marr JCA, Martinez FA, Dowgiallo MJ, Puglise KA, Pyle RL, Zawada DG, Appeldoorn R (2010) Theme section on “Mesophotic Coral Ecosystems: characterization, ecology, and management.” Coral Reefs 29(2):247–251. https://doi.org/10.1007/s00338-010-0614-5
Howells EJ, Willis BL, Bay LK, van Oppen MJH (2013) Spatial and temporal genetic structure of Symbiodinium populations within a common reef-building coral on the Great Barrier Reef. Mol Ecol 22(14):3693–3708. https://doi.org/10.1111/mec.12342
Hume BCC, D’Angelo C, Smith EG, Stevens JR, Burt J, Wiedenmann J (2015) Symbiodinium thermophilum sp. Nov., a thermotolerant symbiotic alga prevalent in corals of the world’s hottest sea, the Persian/Arabian Gulf. Scientific Rep 5(1):8562. https://doi.org/10.1038/srep08562
Hume BCC, Voolstra CR, Arif C, D’Angelo C, Burt JA, Eyal G, Loya Y, Wiedenmann J (2016) Ancestral genetic diversity associated with the rapid spread of stress-tolerant coral symbionts in response to Holocene climate change. Proc Natl Acad Sci 113(16):4416–4421. https://doi.org/10.1073/pnas.1601910113
Hume BCC, Smith EG, Ziegler M, Warrington HJM, Burt JA, LaJeunesse TC, Wiedenmann J, Voolstra CR (2019) SymPortal: a novel analytical framework and platform for coral algal symbiont next-generation sequencing ITS2 profiling. Mol Ecol Resour 19(4):1063–1080. https://doi.org/10.1111/1755-0998.13004
Kahng SE, Akkaynak D, Shlesinger T, Hochberg EJ, Wiedenmann J, Tamir R, Tchernov D (2019) Light, temperature, photosynthesis, heterotrophy, and the lower depth limits of mesophotic coral ecosystems. In: Y Loya, KA Puglise, TCL Bridge (eds), Mesophotic Coral Ecosystems (pp 801–828). Springer International Publishing. https://doi.org/10.1007/978-3-319-92735-0_42
Kirk NL, Andras JP, Harvell CD, Santos SR, Coffroth MA (2009) Population structure of Symbiodinium sp. Associated with the common sea fan, Gorgonia ventalina, in the Florida Keys across distance, depth, and time. Mar Biol 156(8):1609–1623
LaJeunesse TC, Thornhill DJ (2011) Improved resolution of reef-coral endosymbiont (Symbiodinium) species diversity, ecology, and evolution through psbA non-coding region genotyping. PLoS ONE 6(12):e29013. https://doi.org/10.1371/journal.pone.0029013
LaJeunesse TC, Parkinson JE, Gabrielson PW, Jeong HJ, Reimer JD, Voolstra CR, Santos SR (2018) Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Current Biology 28(16) 2570-2580.e6. https://doi.org/10.1016/j.cub.2018.07.008
LaJeunesse TC, Wham DC, Pettay DT, Parkinson JE, Keshavmurthy S, Chen CA (2014) Ecologically differentiated stress-tolerant endosymbionts in the dinoflagellate genus Symbiodinium (Dinophyceae) Clade D are different species. Phycologia 53(4):305–319. https://doi.org/10.2216/13-186.1
Liberman R, Benayahu Y, Huchon D (2022a) Octocorals in the Gulf of Aqaba exhibit high photosymbiont fidelity. Front Microbiol 13:1005471. https://doi.org/10.3389/fmicb.2022.1005471
Liberman R, Shlesinger T, Loya Y, Benayahu Y (2022b) Soft coral reproductive phenology along a depth gradient: Can “going deeper” provide a viable refuge? Ecology 103(9): e3760. https://doi.org/10.1002/ecy.3760
Mass T, Einbinder S, Brokovich E, Shashar N, Vago R, Erez J, Dubinsky Z (2007) Photoacclimation of Stylophora pistillata to light extremes: metabolism and calcification. Mar Ecol Prog Ser 334:93–102. https://doi.org/10.3354/meps334093
McIlroy SE, Coffroth MA (2017) Coral ontogeny affects early symbiont acquisition in laboratory-reared recruits. Coral Reefs 36(3):927–932. https://doi.org/10.1007/s00338-017-1584-7
McIlroy SE, Cunning R, Baker AC, Coffroth MA (2019) Competition and succession among coral endosymbionts. Ecol Evol 9(22):12767–12778. https://doi.org/10.1002/ece3.5749
Mies M, Sumida PYG, Rädecker N, Voolstra CR (2017) Marine invertebrate larvae associated with Symbiodinium: A mutualism from the start? Front Ecol Evolut 5:56. https://doi.org/10.3389/fevo.2017.00056
Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ (2015) IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32(1):268–274. https://doi.org/10.1093/molbev/msu300
Osman EO, Suggett DJ, Voolstra CR, Pettay DT, Clark DR, Pogoreutz C, Sampayo EM, Warner ME, Smith DJ (2020) Coral microbiome composition along the northern Red Sea suggests high plasticity of bacterial and specificity of endosymbiotic dinoflagellate communities. Microbiome 8(1):8. https://doi.org/10.1186/s40168-019-0776-5
Pochon X, Forsman ZH, Spalding HL, Padilla-Gamiño JL, Smith CM, Gates RD (2015) Depth specialization in mesophotic corals (Leptoseris spp.) and associated algal symbionts in Hawai’i. Royal Soc Open Sci 2(2):140351. https://doi.org/10.1098/rsos.140351
Poland DM, Coffroth MA (2017) Trans-generational specificity within a cnidarian–algal symbiosis. Coral Reefs 36(1):119–129. https://doi.org/10.1007/s00338-016-1514-0
Prada C, McIlroy SE, Beltrán DM, Valint DJ, Ford SA, Hellberg ME, Coffroth MA (2014) Cryptic diversity hides host and habitat specialization in a gorgonian-algal symbiosis. Mol Ecol 23(13):3330–3340. https://doi.org/10.1111/mec.12808
Quigley KM, Bay LK, Willis BL (2017) Temperature and water quality-related Patterns in sediment-associated Symbiodinium communities impact symbiont uptake and fitness of juveniles in the genus Acropora. Front Marine Sci 401. https://doi.org/10.3389/fmars.2017.00401
Rowan R, Knowlton N (1995) Intraspecific diversity and ecological zonation in coral-algal symbiosis. Proc Natl Acad Sci 92(7):2850–2853. https://doi.org/10.1073/pnas.92.7.2850
Rowan R, Knowlton N, Baker A, Jara J (1997) Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 388(6639): 265–269. https://doi.org/10.1038/40843
Sampayo EM, Ridgway T, Bongaerts P, Hoegh-Guldberg O (2008) Bleaching susceptibility and mortality of corals are determined by fine-scale differences in symbiont type. Proc Natl Acad Sci 105(30):10444–10449. https://doi.org/10.1073/pnas.0708049105
Silverstein RN, Cunning R, Baker AC (2014) Change in algal symbiont communities after bleaching, not prior heat exposure, increases heat tolerance of reef corals. Glob Change Biol 21(1):236–249. https://doi.org/10.1111/gcb.12706
Stat M, Gates RD (2010) Clade D Symbiodinium in scleractinian corals: a “Nugget” of hope, a selfish opportunist, an ominous sign, or all of the above? J Marine Sci 2011:e730715. https://doi.org/10.1155/2011/730715
Yamashita H, Suzuki G, Hayashibara T, Koike K (2013) Acropora recruits harbor “rare” Symbiodinium in the environmental pool. Coral Reefs 32(2):355–366. https://doi.org/10.1007/s00338-012-0980-2
Yamashita H, Suzuki G, Kai S, Hayashibara T, Koike K (2014) Establishment of coral–algal symbiosis requires attraction and selection. PLoS ONE 9(5):e97003. https://doi.org/10.1371/journal.pone.0097003
Ziegler M, Roder CM, Buchel C, Voolstra CR (2015) Mesophotic coral depth acclimatization is a function of host-specific symbiont physiology. Front Marine Sci 2:4. https://doi.org/10.3389/fmars.2015.00004
Ziegler M, Arif C, Burt JA, Dobretsov S, Roder C, LaJeunesse TC, Voolstra CR (2017) Biogeography and molecular diversity of coral symbionts in the genus Symbiodinium around the Arabian Peninsula. J Biogeogr 44(3):674–686. https://doi.org/10.1111/jbi.12913
Acknowledgements
The study was supported by the Israel Academy of Sciences and Humanities, Batsheva de Rothschild Fund: Aharon and Ephraim Katzir Fellowship to RL. Benjamin CC Hume was supported by the Sequencing Analysis (SequAna) Core Facility of the University of Konstanz. We thank the staff at the Interuniversity Institute for Marine Sciences in Eilat (IUI) for their support and hospitality throughout the study period. We acknowledge D. Huchon for her invaluable support through manuscript preparation. We also thank Jessica Bellworthy and Gabriela Perna for technical assistance, and Naomi Paz for editorial assistance.
Funding
Open access funding provided by Tel Aviv University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no financial or personal interests that may have influenced the research work or its outcome. They have no conflict of interest to disclose and have provided an unbiased analysis and interpretation of the data presented in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liberman, R., Voolstra, C.R., Hume, B.C.C. et al. Juvenile octocorals acquire similar algal symbiont assemblages across depths. Coral Reefs 43, 489–496 (2024). https://doi.org/10.1007/s00338-024-02470-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-024-02470-3