Abstract
Giant clams are common across a broad geographic range and contribute important ecological functions within coral reef environments. However, giant clams are subject to considerable harvest pressure and require careful management that is underpinned by accurate data collection. The taxonomy of giant clams has undergone many changes, and recently, Tridacna noae (Röding 1798) has been resurrected as a valid species, distinct from the morphologically similar Tridacna maxima (Röding 1798). Using genetic analysis, this research confirms the presence of T. noae for the first time in the Cook Islands, extending the currently known distribution of the species by 1340 km south-east. This confirmation highlights that T. noae was possibly previously misidentified, causing overestimations of the abundance of other giant clam species. This new record improves the accuracy of identification and stock assessments, and ongoing management in the Cook Islands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Giant clams (Cardiidae: Tridacninae) occur across the Indo-Pacific region and play important roles in coral reef ecosystems, including habitat provisioning, contributing to the reef carbonate structure, food for other species and increasing water clarity (Klumpp and Griffiths 1994; Neo et al. 2015). Globally, populations of giant clams are declining due to overharvesting and habitat destruction, highlighting the need for improved management (Gilbert et al. 2006; Neo and Todd 2012; Richter et al. 2008).
Current management regimes for giant clams span trade and harvest controls that include international trade restrictions through Appendix II of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES), minimum size limits, quotas and refuge in marine protected areas (Andréfouët et al. 2018; Ramah et al. 2019; Van Wynsberge et al. 2013). Despite conservation and management efforts to halt the decline of these iconic molluscs, species misidentification undermines the efficacy of the efforts (Bortolus 2008). Misreporting or lumping species together can be problematic for populations with reported overharvest by leading to overestimation of population densities and underestimation of local extinction risk (Borsa et al. 2015b; Huelsken et al. 2013).
The taxonomy of giant clams has undergone many changes, largely due to historically divergent lines of thought between Iredale (1937) and Rosewater (1965). There has been ongoing confusion over the differentiation of two morphologically similar species: Tridacna maxima (Röding 1798) and Tridacna noae (Röding 1798). T. noae was recently resurrected as a valid species by Su et al. (2014) based on genetic distinctness and genetically confirmed occurrences in Western Australia (Penny and Willan 2014; initially referred to as Tridacna ningaloo and since synonymized with T. noae as per Borsa et al. 2015a), the Solomon Islands (Huelsken et al. 2013) and Taiwan (Su et al. 2014). Most recently, with an increasing availability of genetic material, Tan et al. (2021) have reappraised the phylogenetic structure of tridacnid species, further supporting the validity of T. noae as a species distinct from T. maxima.
The current known range of T. noae extends from the Ryuku Islands in Japan, west to Christmas Island in Australia, south to Ningaloo Reef in north-western Australia, east to American Samoa and north-east to Kiritimati in Kiribati (Borsa et al. 2015b; Kubo and Iwai 2007; Marra-Biggs et al. 2022; Neo and Low 2018). However, several regions of the Pacific are yet to be confidently surveyed for the presence of T. noae, where it may have been previously confused with T. maxima based on their similar morphology and occupation of similar reef habitat types. Observations of T. noae have been made across the Indo-Pacific, including in the Cook Islands (Marra-Biggs et al. 2022; Neo et al. 2017), but these have not been genetically confirmed.
The Cook Islands are located over 1340 km ESE of American Samoa in the Pacific Ocean. The Cook Islands exclusive economic zone is large, spanning approximately two million square kilometers. To date, T. maxima and T. squamosa (Lamarck 1819) are the only confirmed giant clam species native to the Cook Islands (Neo et al. 2017; Paulay 1987). In the early 2000s, however, observations of a unique clam with morphology distinct from these two species were made on the southern Cook Islands of Aitutaki and Rarotonga (I. Bertram, pers. comm. 2019). Based on the morphology of the clams, including mantle patterns of lighter spots bound by white rings (sensu Su et al. 2014), they were putatively identified as T. noae. In recent years, surveys by the Cook Islands Ministry of Marine Resources (MMR) have also detected potential T. noae individuals on the islands of Rarotonga and neighboring Mangaia. For subsequent reporting, however, all clams were identified to at least genus level (e.g., Tridacna sp.), but if to species level, T. noae clams were likely misreported as T. maxima or T. squamosa (Morejohn et al. 2018).
Although some morphological differences are apparent between T. noae and T. maxima (i.e., mantle ornamentation), shell characteristics cannot reliably distinguish the species (i.e., rib characters, Su et al. 2014). Hence, the resurrection of the T. noae as a valid species and its confirmed occurrence in new locations has often required confirmation through genetic analysis (Borsa et al. 2015a; Su et al. 2014; Tan et al. 2021). This study confirms the presence of T. noae clams on Rarotonga, Cook Islands, using the mitochondrial Cytochrome Oxidase I gene region (CO1). Confirmation of the occurrence of T. noae in the Cook Islands has important implications for understanding the evolutionary and biogeographic history of giant clams and will help to inform accurate stock assessments of Tridacna species in the region for their ongoing conservation and sustainable management.
Methods
Field sampling
Tissue samples were collected from clams at four sites in Rarotonga, Cook Islands, from 2019 to 2020 by the MMR. Sampling was done by reef walk, snorkel and scuba. Clams with T. noae mantle patterns (sensu Su et al. 2014) were noted and photographed. Six clams, putatively identified as T. noae, were found in multiple habitats (e.g., reef flats, lagoons and fore reefs) and in depths ranging from 0.2 to 8.8 m. The clams ranged in size from 70 to 260 mm maximum shell length. Hemostats and scissors were used to remove a 1 cm2 piece of mantle tissue from each clam. Tissue samples were taken from six clams. Non-lethal methods were used for five clams. One clam was too small for a non-lethal sample and was therefore harvested.
Tissue samples were preserved in 4-mL vials filled with 100% ethanol and stored at 4 °C. Ethanol levels were checked monthly and refreshed to ensure vials remained full. Samples were shipped at room temperature to Australia under CITES Appendix II export permit.
Laboratory methods and genetic analysis
DNA was extracted from a 20 mg tissue subsample that had been blotted dry on filter paper using a salting out procedure (Sunnucks and Hales 1996) and then purified using Monarch genomic DNA purification columns following the manufacturer’s instructions. The concentration of extracted genomic DNA was assessed using Qubit fluorometry, and 10 ng was used for PCR amplification of the mitochondrial CO1 gene. Previous studies have confirmed the utility of CO1 in distinguishing Tridacna species (including T. noae; Borsa et al. 2015a; Borsa et al. 2015b; Huelsken et al. 2013; Penny and Willan 2014; Su et al. 2014; Tan et al. 2021) and among phylogeographic clades of T. noae (Fauvelot et al. 2019). Two PCR assays were tested to generate amplicons of CO1. PCR using the primer pair CO1 Trichro Frwrd/CO1 Trichro Rev (Kochzius and Nuryanto 2008) generated inconsistent results, while the primer pair SQUAF3/SQUAR1 (DeBoer et al. 2012) was more reliable, especially when the PCR thermocycling was modified to use touchdown conditions (Hajibabaei et al. 2006). The final PCRs were performed in 50 µl reactions, using GoTaq colorless mix, 3 mM Mg2+, supplemented with 1 µl of 1 mg/ml RNAse A, and a cycling program of 94 °C for 3 min; six cycles of 94 °C for 30 s, 45 °C for 30 s, 72 °C for 75 s; followed by 36 cycles of 94 °C for 30 s, 51 °C for 30 s, 72 °C for 75 s; and a final extension at 72 °C for 5 min.
PCR products were electrophoresed in 2% agarose poured in TBE buffer, post-stained with GelRed (Biotium) and photographed using transilluminated UV light. PCR with the primers SQUAF3/SQUAR1 generated a single DNA product of approximately 600 base pairs that were sent to Macrogen, Korea, for purification and dideoxy Sanger sequencing. Primer SQUAR1 generated poor sequencing results, so the SQUAF3 primer was used for all subsequent sequencing.
DNA sequences from Macrogen were edited using GeneStudio, with the 3’ end of the sequence trimmed to remove the SQUAR1 primer. After editing the 5’ sequence, the sequences for each sample were trimmed to 455 nucleotides that could be used in alignments and for subsequent analysis.
For confirmation of species identity, sequences of the six putative T. noae sampled in the Cook Islands were compared with other Tridacna CO1 reference sequences from GenBank, including those used in the resurrected species descriptions of T. noae by Su et al. (2014). Additional sequences for T. gigas and T. derasa from other studies (Fauvelot et al. 2020; Lizano and Santos 2014; Plazzi and Passamonti 2010) were also included. Furthermore, as T. noae is comprised of three distinct mitochondrial lineages (Fauvelot et al. 2019), haplotypes representing these lineages were included to display intraspecific relationships (for details on haplotype identification see next paragraph). All sequences were imported into MEGA version 11 (Tamura et al. 2021), aligned with MUSCLE (Edgar 2004) and trimmed to a common length of 347 nucleotides. A maximum-likelihood phylogeny was then generated using the web server implementation of IQ-TREE (Nguyen et al. 2014; Trifinopoulos et al. 2016) with default settings and branch support tested with 1,000 ultrafast bootstrap replicates (Hoang et al. 2017). The best fitting substitution model (HKY + F + G4) was identified based on the Bayesian information criterion (BIC) with ModelFinder (Kalyaanamoorthy et al. 2017). The final tree was visualized with the online software iTOL (Letunic and Bork 2021).
Intraspecific relationships within T. noae were further explored through the construction of a haplotype network. All sequences listed in Fauvelot et al. (2019) and 20 T. noae sequences from Ningaloo Reef in Johnson et al. (2016) were downloaded from GenBank and MUSCLE-aligned with the six Cook Islands sequences in MEGA. The alignment was trimmed to a common length of 347 nucleotides. Two sequences (GenBank accessions: KT899619 and JX974905) containing ambiguous bases in the middle of this alignment were removed to simplify the construction of the haplotype network. Removal of these sequences had no impact on the results as, aside from an ambiguous base, they were identical to other sequences in the alignment. Sequences were then collapsed into haplotypes with DnaSP version 6.12.03 (Rozas et al. 2017). Haplotypes represented by a sequence with a GenBank accession number were identified for inclusion in the phylogeny (see previous paragraph). If the haplotype was represented by more than one sequence, one randomly selected GenBank-associated sequence was used in the phylogeny. A median-joining haplotype network (Bandelt et al. 1999) was generated in PopART version 1.7 (Leigh and Bryant 2015), with the Epsilon value set to 0. All haplotypes were assigned to lineages and geographic location following Fauvelot et al. (2019).
All sequences were deposited in NCBI (Accessions: OQ547099 – OQ547104) and metadata uploaded to the Genomic Observatories Metadatabase (GEOME, accessioned as Tridacna_CookIslands at https://n2t.net/ark:/21547/FGv2).
Results and discussion
The first records of Tridacna noae in the Cook Islands were confirmed. Based on the maximum-likelihood phylogeny, all six individuals putatively identified as T. noae during the original field collection were confirmed to reside within the clade for this species (Fig. 1). This clade comprised the two individuals used in the resurrected species description of T. noae (GenBank accessions: DQ168140 and KC456023), along with sequences from other studies since its re-establishment as a valid species, and was distinct from the clades for the two other Tridacna species native to the Cook Islands: T. maxima and T. squamosa. Both the phylogeny and haplotype network showed that the six Cook Island specimens can be placed within mitochondrial Lineage III (Figs. 1 and 2), which includes individuals from Central Polynesia (Samoa and Wallis and Futuna (Fauvelot et al. 2019)). Additionally, the haplotype network revealed the presence of four novel haplotypes for T. noae from the Cook Islands.
Maximum-likelihood phylogeny of giant clam CO1 sequences, comparing samples of putative Tridacna noae collected from Rarotonga (RAR) in the Cook Islands (highlighted in bold) with other tridacnid species and the T. noae lineages identified by Fauvelot et al. (2019). Ultrafast bootstrap support values ≥ 75% are shown on branches. GenBank accession numbers for each comparative sequence are provided after the species name
Median-joining haplotype network of Tridacna noae collected from Rarotonga in the Cook Islands, showing association with samples from other parts of its distribution and the described lineages within T. noae (following Fauvelot et al. 2019). Each circle represents a distinct haplotype, hatch marks indicate the number of mutational steps between haplotypes, and circle size is proportional to the number of individuals. Small black dots represent hypothetical or unsampled haplotypes
Field identification of all six T. noae, using mantle coloration of lighter spots bound by white rings, confirmed by subsequent genetic analysis, supports findings by Su et al. (2014) that mantle pattern may be sufficient for identification and differentiation from T. maxima. Mantle coloration of Tridacna noae observed in Rarotonga varied, including a dark brown to near-black coloration with numerous light brown ocellate spots bound by white rings (Fig. 3). Ocellate spots on the mantle edge were smaller than those medially and opened to a thin white margin on the mantle edge. Interestingly, no T. noae of the Cook Islands were observed with the vivid blue and green mantles found elsewhere (Su et al. 2014). It may be that preferential harvest of these non-cryptic color variants has removed these color morphs from Cook Islands’ populations, or that these morphs have never occurred within the Cook Islands.
Dorsal views of Tridacna noae from Rarotonga in the Cook Islands showing the brown mantle color morphology, including one of the six specimens used in the genetic analysis to confirm the species identification (specimen indicated by red dot). Other individuals were observed in the same area, were of similar size (approximately 20–27 cm) and putatively identified as T. noae. Photographs: Kirby Morejohn, Cook Islands Ministry of Marine Resources
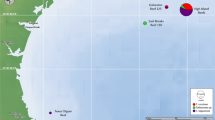
Our findings, confirmed by DNA analysis, extend the known range of T. noae, increasing the south-eastern distribution by over 1340 km (Fig. 4). Prior to our study, the south-eastern distributional limit of T. noae was determined by Kiritimati in Kiribati and Wallis in Wallis and Futuna (Borsa et al. 2015b), American Samoa (Marra-Biggs et al. 2022) and New Caledonia (Fauvelot et al. 2019). This indicates that nearby countries (e.g., French Polynesia, Tonga and Niue) may also hold undocumented populations of T. noae. Consequently, there are important implications for appropriate conservation and management of Tridacna species in those areas.
Previously known distribution of T. noae is indicated by the light gray shaded polygon. Triangles represent genetically confirmed observations and circles represent visual observations. New genetically confirmed records from the Cook Islands and expanded range are indicated by the red triangle, red line, and dark gray shading. Inset: Locations of new genetically confirmed T. noae records from Rarotonga, Cook Islands. Additional detail on known locations of T. noae throughout the region is provided in Online Resource 1
Similar to the findings of others (Huelsken et al. 2013; Borsa 2015b), the presence of T. noae in the Cook Islands also suggests that the densities of T. maxima and T. squamosa might have been overestimated because T. noae was likely included in counts of one or both species. Depending on the ratio of T. noae to the other two native Tridacna species, this overestimation could mean that T. maxima and T. squamosa populations may be smaller than previously reported. Furthermore, because T. noae had never been included in previous Cook Islands surveys, this species may already be overharvested or unknowingly extirpated from some islands. In Rarotonga, T. noae could have been afforded some protection by their cryptic brown coloration (Fig. 3) and because their habitat extends into waters deeper than those where most local clam harvests occur (K. Morejohn, unpublished data).
Although only recently confirmed as a valid species within Tridacna (Borsa et al. 2015a; Su et al. 2014; Tan et al. 2021), our study confirms that T. noae is relatively widespread. Our field observations of T. noae suggest this species occurs in a range of habitats (including reef flats, lagoons and fore reefs) and depths (0.2 to 8.8 m) and may be more tolerant of shading than co-occurring T. maxima individuals. Such ecological understanding of T. noae, however, is in its infancy and conservation and management measures will now benefit from the knowledge that their populations in the Cook Islands can be confidently identified, surveyed and monitored.
Data availability
The genetic data generated during the current study and corresponding metadata are available in NCBI (Accessions: OQ547099 – OQ547104) and the Genomic Observatories Metadatabase (GEOME, accessioned as Tridacna_CookIslands at https://n2t.net/ark:/21547/FGv2), respectively.
References
Andréfouët S, Van Wynsberge S, Kabbadj L, Wabnitz CC, Menkès C, Tamata T, Pahuatini M, Tetairekie I, Teaka I, Scha TA (2018) Adaptive management for the sustainable exploitation of lagoon resources in remote islands: lessons from a massive El Niño-induced giant clam bleaching event in the Tuamotu atolls (French Polynesia). Environ Conserv 45:30–40. https://doi.org/10.1017/S0376892917000212
Bandelt HJ, Forster P, Röhl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48. https://doi.org/10.1093/oxfordjournals.molbev.a026036
Borsa P, Fauvelot C, Andréfouët S, Chai T-T, Kubo H, Liu L-L (2015a) On the validity of Noah’s giant clam Tridacna noae (Röding, 1798) and its synonymy with Ningaloo giant clam Tridacna ningaloo Penny & Willan, 2014. Raffles B Zool 63:484–489
Borsa P, Fauvelot C, Tiavouane J, Grulois D, Wabnitz C, Naguit MA, Andrefouet S (2015b) Distribution of Noah’s giant clam, Tridacna noae. Mar Biodivers 45:339–344. https://doi.org/10.1007/s12526-014-0265-9
Bortolus A (2008) Error cascades in the biological sciences: the unwanted consequences of using bad taxonomy in ecology. Ambio 37:114–118. https://doi.org/10.1579/0044-7447(2008)37[114:ECITBS]2.0.CO;2
de Lamarck JB (1819) Histoire naturelle des animaux sans vertèbres présentant les caractères généraux et particuliers de ces animaux, leur distribution, leurs classes, leurs familles, leurs genres, et la citation des principales espèces qui s’y rapportent. Tome sixième, Librairie Verdière, Paris
Deboer TS, Baker AC, Erdmann MV, Jones PR, Barber PH (2012) Patterns of symbiodinium distribution in three giant clam species across the biodiverse Bird’s Head region of Indonesia. Mar Ecol Prog Ser 444:117–132. https://doi.org/10.3354/meps09413
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. https://doi.org/10.1093/nar/gkh340
Fauvelot C, Andréfouët S, Grulois D, Tiavouane J, Wabnitz CC, Magalon H, Borsa P (2019) Phylogeography of Noah’s giant clam. Mar Biodivers 49:521–526. https://doi.org/10.1007/s12526-017-0794-0
Fauvelot C, Zuccon D, Borsa P, Grulois D, Magalon H, Riquet F, Andréfouët S, Berumen ML, Sinclair-Taylor TH, Gélin P, Behivoke F, ter Poorten JJ, Strong EE, Bouchet P (2020) Phylogeographical patterns and a cryptic species provide new insights into Western Indian Ocean giant clams phylogenetic relationships and colonization history. J Biogeogr 47:1086–1105. https://doi.org/10.1111/jbi.13797
Gilbert A, Remoissenet G, Yan L, Andréfouët S (2006) Special traits and promises of the giant clam (Tridacna maxima) in French Polynesia. Fish Newsl South Pac Comm 118:44
Hajibabaei M, Janzen DH, Burns JM, Hallwachs W, Hebert PD (2006) DNA barcodes distinguish species of tropical Lepidoptera. Proc Natl Acad Sci 103:968–971. https://doi.org/10.1073/pnas.0510466103
Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS (2017) UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol 35:518–522. https://doi.org/10.1093/molbev/msx281
Huelsken T, Keyse J, Liggins L, Penny S, Treml EA, Riginos C (2013) A novel widespread cryptic species and phylogeographic patterns within several giant clam species (Cardiidae: Tridacna) from the Indo-Pacific Ocean. PLoS ONE 8:e80858. https://doi.org/10.1371/journal.pone.0080858
Iredale T (1937) Mollusca. In: Whitley GP (ed) The Middleton and Elizabeth Reefs. South Pacific Ocean
Johnson MS, Prince J, Brearley A, Rosser NL, Black R (2016) Is Tridacna maxima (Bivalvia: Tridacnidae) at Ningaloo Reef, Western Australia? Molluscan Res 36:264–270. https://doi.org/10.1080/13235818.2016.1181141
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14:587–589. https://doi.org/10.1038/nmeth.4285
Klumpp D, Griffiths C (1994) Contributions of phototrophic and heterotrophic nutrition to the metabolic and growth requirements of four species of giant clam (Tridacnidae). Mar Ecol Prog Ser 25:103–115
Kochzius M, Nuryanto A (2008) Strong genetic population structure in the boring giant clam, Tridacna crocea, across the Indo-Malay Archipelago: implications related to evolutionary processes and connectivity. Mol Ecol 17:3775–3787. https://doi.org/10.1111/j.1365-294X.2008.03803.x
Kubo H, Iwai K (2007) On two sympatric species within Tridacna “maxima.” Ann Rep Okinawa Fish Ocean Res Center 68:205–210
Leigh JW, Bryant D (2015) POPART: full-feature software for haplotype network construction. Methods Ecol Evol 6:1110–1116. https://doi.org/10.1111/2041-210X.12410
Letunic I, Bork P (2021) Interactive tree of life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res 49:W293–W296. https://doi.org/10.1093/nar/gkab301
Lizano AMD, Santos MD (2014) Updates on the status of giant clams Tridacna spp. and Hippopus hippopus in the Philippines using mitochondrial CO1 and 16S rRNA genes. Philipp Sci Lett 7:187–200
Marra-Biggs P, Fatherree J, Green A, Toonen RJ (2022) Range expansion and first observation of Tridacna noae (Cardiidae: Tridacninae) in American Samoa. Ecol Evol 12:e9635. https://doi.org/10.1002/ece3.9635
Neo ML, Low JK (2018) First observations of Tridacna noae (Röding, 1798)(Bivalvia: Heterodonta: Cardiidae) in Christmas Island (Indian Ocean). Mar Biodivers 48:2183–2185. https://doi.org/10.1007/s12526-017-0678-3
Neo ML, Todd PA (2012) Giant clams (Mollusca: Bivalvia: Tridacninae) in Singapore: history, research and conservation. Raffles B Zool 25:67–78
Neo ML, Eckman W, Vicentuan K, Teo SL-M, Todd PA (2015) The ecological significance of giant clams in coral reef ecosystems. Biol Conserv 181:111–123. https://doi.org/10.1016/j.biocon.2014.11.004
Neo ML, Wabnitz C, Braley RD, Gerald AH, Fauvelot C, Van Wynsberge S, Andréfouët S, Waters C, Tan AS-H, Gomez E, Costello MJ, Todd PA (2017) Giant clams (Bivalvia: Cardiidae: Tridacninae): a comprehensive update of species and their distribution, current threats and conservation status. In: Hawkins SJ, Evans AJ, Dale AC, Firth LB, Hughes DJ, Smith IP (eds) Oceanography and marine biology: an annual review. CRC Press, Boca Raton
Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ (2014) IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32:268–274. https://doi.org/10.1093/molbev/msu300
Paulay G (1987) Biology of the Cook Islands bivalves, Part I. Heterodont families. Atoll Research Bulletin
Penny SS, Willan RC (2014) Description of a new species of giant clam (Bivalvia: Tridacnidae) from Ningaloo Reef, Western Australia. Molluscan Res 34:201–211. https://doi.org/10.1080/13235818.2014.940616
Plazzi F, Passamonti M (2010) Towards a molecular phylogeny of Mollusks: Bivalves’ early evolution as revealed by mitochondrial genes. Mol Phylogenet Evol 57:641–657. https://doi.org/10.1016/j.ympev.2010.08.032
Ramah S, Taleb-Hossenkhan N, Todd PA, Neo ML, Bhagooli R (2019) Drastic decline in giant clams (Bivalvia: Tridacninae) around Mauritius Island, Western Indian Ocean: implications for conservation and management. Mar Biodivers 49:815–823. https://doi.org/10.1007/s12526-018-0858-9
Richter C, Roa-Quiaoit H, Jantzen C, Al-Zibdah M, Kochzius M (2008) Collapse of a new living species of giant clam in the Red Sea. Curr Biol 18:1349–1354. https://doi.org/10.1016/j.cub.2008.07.060
Röding PF (1798) Pars secunda continens conchylia sive testacea univalvia, bivalvia & multivalvia. In: Bolten JF (ed) Museum Boltenianum sive catalogus cimeliorum e tribus regnis naturæ quæ olim collegerat Joa. Fried Bolten. Trapp, Hamburg, viii+199 pp
Rosewater J (1965) The family Tridacnidae in the Indo-Pacific. Indo Pac Mollusca 1:347–396
Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, Sánchez-Gracia A (2017) DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol Biol Evol 34:3299–3302. https://doi.org/10.1093/molbev/msx248
Su Y, Hung J-H, Kubo H, Liu L-L (2014) Tridacna noae (Röding, 1798)–a valid giant clam species separated from T. maxima (Röding, 1798) by morphological and genetic data. Raffles B Zool 62:8925136
Sunnucks P, Hales DF (1996) Numerous transposed sequences of mitochondrial cytochrome oxidase I–II in aphids of the genus Sitobion (Hemiptera: Aphididae). Mol Biol Evol 13:510–524. https://doi.org/10.1093/oxfordjournals.molbev.a025612
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38:3022–3027. https://doi.org/10.1093/molbev/msab120
Tan EYW, Quek ZBR, Neo ML, Fauvelot C, Huang D (2021) Genome skimming resolves the giant clam (Bivalvia: Cardiidae: Tridacninae) tree of life. Coral Reefs 41:497–510. https://doi.org/10.1007/s00338-020-02039-w
Trifinopoulos J, Nguyen L-T, von Haeseler A, Minh BQ (2016) W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res 44:W232–W235. https://doi.org/10.1093/nar/gkw256
Van Wynsberge S, Andrefouet S, Gilbert A, Stein A, Remoissenet G (2013) Best management strategies for sustainable giant clam fishery in French Polynesia islands: answers from a spatial modeling approach. PLoS ONE 8:e64641. https://doi.org/10.1371/journal.pone.0064641
Acknowledgements
The authors would like to acknowledge the contributions of Ian Bertram of The Pacific Community and Richard Story and James Kora of the Ministry of Marine Resources for providing helpful advice regarding the identification of T. noae and assistance in the field. Fieldwork was undertaken in Rarotonga by staff and fisheries officers of the Ministry of Marine Resources. Tissue samples were collected with appropriate permissions from and respect for traditional owners and representatives of ocean resources in the Cook Islands.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This study was funded by the Conserving Biodiversity and Enhancing Ecosystem Function through a Ridge to Reef Approach in the Cook Islands (award ID 84399) in the United Nations Development Programme (UNDP) Global Environment Facility (GEF, project ID 5348) as part of a larger project. Co-funding was provided by the Cook Islands Ministry of Marine Resources, Macquarie University and Massey University.
Author information
Authors and Affiliations
Contributions
K.M. and L.A. conceived the research and collected new samples for analysis. M.G. and J.W. processed and sequenced genetic material. M.G., J.W., R.N. and L.L. analyzed genetic sequences. K.M. wrote the manuscript. L.A., M.G., J.W., R.N., L.L. and V.C. contributed to manuscript preparation. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
No human or animal testing was performed during this study, and no ethical approval was required. All necessary permits for sampling and international transfer of samples were obtained by the authors from the relevant authorities as described in the acknowledgements. The study is compliant with the International Convention on Biological Diversity and associated Nagoya protocol. Tissue samples were exported to Australia under CITES Appendix II permit (permit number: PWS-AU-001494).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Morejohn, K., Ainley, L., Williamson, J. et al. Genetic confirmation of Tridacna noae (Röding 1798) in the Cook Islands. Coral Reefs 42, 1343–1350 (2023). https://doi.org/10.1007/s00338-023-02432-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-023-02432-1